Simultaneous Determination of the 6-Methyl-5-hepten-2
advertisement

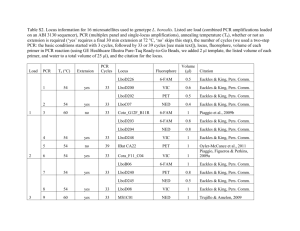
Simultaneous Determination of Four Major Components in Essential Oil from Litsea cubeba / Asian Journal of Traditional Medicines, 2007, 2 ( 2 ) Simultaneous Determination of the 6-Methyl-5-hepten-2-one, Limonene, Linalool and Citral in Essential Oil from Litsea cubeba (Lour.) Pers by Capillary Gas Chromatography Shuanghui Yu, Xiaohui Chen, Ling Tong, Kaishun Bi * School of Pharmacy, Shengyang Pharmaceutical University, Shenyang 110016, China A simple and fast Capillary Gas Chromatography (CGC) method was established for the simultaneous determination of four major components, namely 6-methyl-5-hepten-2-one, limonene, linalool and citral in essential oil from Litsea cubeba (Lour.) Pers. The CGC analysis was performed on a 30 m × 0.50 mm i.d. SE-54 capillary column, and was carried out with temperature programs by using nitrogen as carrier gas. The good linear regression relationship was obtained over the range of 0.030–1.502 mg·mL-1 (r = 0.9999) for 6-methyl-5-hepten-2-one, 0.018–0.894 mg·mL-1 (r = 0.9997) for Limonene, 0.016–0.809 mg·mL-1 (r = 0.9997) for Linalool , 0.079–3.962 mg·mL-1 (r = 0.9999) for citral, respectively. The method is accurate with overall intra-day variation and repeatability less than 5 % and the recovery more than 95 %. The contents of sample from different regions varied greatly. CGC was proved to be a quick and informative tool for analysis of essential oil from Litsea cubeba (Lour.) Pers. The method was successfully applied to analyze four major components. Key words: Litsea cubeba(Lour.)Pers; 6-Methyl-5-hepten-2-one; limonene; linalool; citral; CGC Litsea cubeba (Lour.) Pers is well-known traditional Chinese medicine (TCM) that has been used for warming and dispersing coldness as well as relieving pain for more than thousands of years. According to Pharmacopoeia of china, Litsea cubeba (Lour.) Pers was derived from the ripe and dried fruitage. It is widespread in the southern regions of China, and mainly produced in Guangxi, Zhejinang, Sichuan Provinces and so on. Photochemical and pharmacological studies proved that essential oil and fatty acid were the bioactive constituents, and the former ones have the functions of antiasthma, eliminating phlegm, anti-bacteria, anticongregation of platelet, anti-myocardial infarction, and anti-hypersusceptibility [1]. 6-Methyl-5-hepten2-one, Limonene, Linalool and Citral are the main constituents of essential oil from Litsea cubeba (Lour.) Pers. In the past documents, although the methods for characterization of active compositions in essential oil extracted from the Litsea cubeba (Lour.) Pers have well been reported using GC-MS, there is no reports on simultaneous determination of compositions in essential oil. Therefore, it is necessary to develop a new approach for the quality control of essential oil from Litsea cubeba (Lour.) Pers. The aim of the present study is to develop a direct and rapid CGC with the internal standard method for simultaneous determination of the four major components, 6-methyl-5-hepten-2-one, limonene, linalool and citral, and to produce a suitable method of quality control. Materials and Methods Chemicals and materials n-Hexane and ether of analytical grade were purchased from Yu-Wang Chemical Company (Shangdong, China). 6-Methyl-5-hepten-2-one and limonene were supplied from J & K Chemical (Beijing, China). Linalool was obtained from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Citral was purchased from Sigma Chemical Company (St. Louis, MO, USA). Drug samples of Litsea cubeba (Lour.) Pers were collected from different regions in China and authenticated as Litsea cubeba (Lour.) Pers by Prof. Qishi Sun (Shenyang Pharmaceutical University). Apparatus and conditions * Author to whom correspondence should be addressed. Tel.: +86-24-23928487; E-mail: bikaishun@yahoo.com The analyses were performed on a Shimadzu 2010 66 Simultaneous Determination of Four Major Components in Essential Oil from Litsea cubeba / Asian Journal of Traditional Medicines, 2007, 2 ( 2 ) Gas Chromatograghy (GC) system equipped with FID detector. Data was processed by Lab solution 2.1 workstation. Separation was carried out on a 30 m× 0.50 mm i.d.× 0.32 µm film SE-54 capillary column (Danlian, Zhonghuida, China). The non-polar was 75 ˚C programmed to 100 ˚C at 5 ˚C·min -1, then to 140 ˚C at 2 ˚C·min-1, and then to 230 ˚C at 6 ˚C·min-1; Split injection was conducted with split radio of 1:1; Flow rate was 5.5 mL·min-1; Nitrogen was used as carrier gas; injector temperature, 250 ˚C; flame ioinization detection temperature, 250 ˚C; and injected volume, 1 µL. Phenylethylketone was used as internal standard. Some individual components could be identified by co-injection of pure compounds and comparison of the retention time. obtained, and was added into 10 mL volumetric flasket, then 0.5 mL internal standard solution (2.060 mg·mL-1) was added, and was dilute with n-hexane, then shaken. Results and Discussion Calibration curves and quantification limits The reference standards of the target compounds, namely mehty heptenone, limonene, linalool and citral were accurately weighed and dissolved in n-hexane to produce the stock mixed standard solution, then diluted to a series of appropriate concentration for construction of calibration curves and determination of the limit of quantification (LOQ). A 0.5 mL (2.060 mg·mL-1) volume of the internal standard solution was added in each concentration solution. Calibration curves were calculated with six different concentrations by plotting the peak area rations of analyte to internal standard versus analyte concentration. All the target compounds showed good linearity over a relatively wide concentration range. The results are expressed as the values of the correlation coefficient (r) in Table 1. and the chromatograms are shown in Fig. 1. Sample pretreatment 10 g of Litsea cubeba (Lour.) Pers was weighed accurately. A ten fold mass of distilled water was added and refluxed for 6 hours with essential oil’s extractor after being soaked for 5 hours according to appendix XD in Chinese Pharmacopoeia (2005). When cooled down to the room temperature, extracted for three times by ether, and evaporated to dry, then re-dried with sodium sulfate anhydrous, essential oil was Table 1 Calibration curves of four compounds and limits of quantitation­­ Calibration curve r Linear range (mg·mL-1) LOQ (mg·mL-1) 6-Methyl-5-hepten-2-one Y =10.878 X­­­­­­­−0.0638 0.9999 0.030–1.502 0.030 Limonene Y =15.158 X−0.133 0.9997 0.019–0.894 0.019 Linalool Y =11.530 X−0.0919 0.9997 0.016–0.809 0.016 Citral Y =11.288 X−0.132 0.9999 0.079–3.962 0.079 Compound X denotes the concentration and Y denotes peak area rations Precision, repeatability and accuracy linalool and 4.5 % for citral. The recovery was determined by spiking with the mixed standard solution at high, medium and low concentration levels in samples. The ratios of measured and added amounts were calculated to reflect the recovery. The results are shown in Table 2. and demonstrate that the developed method is reproducable and accurate. The intral-day variability of the content was used for assessing the precision of the developed method by performing six duplicated injection of the same solution in a single day. The relative standard derivation (RSD) were 2.6 %, 2.8 %, 2.7 %, and 1.8 % for 6-methyl-5-hepten2-one, limonene, linalool and citral, respectively. The repeatability of the total procedure was tested using six processed samples of the essential oil from Litsea cubeba (Lour.) Pers, and the RSD (n = 6) were 1.9 % for 6-Methyl-5-hepten-2-one, 2.6 % for limonene, 4.5 % for Application Sample solution was obtained according to the sample pretreatment method, then 1 μL of each 67 Simultaneous Determination of Four Major Components in Essential Oil from Litsea cubeba / Asian Journal of Traditional Medicines, 2007, 2 ( 2 ) Table 2 Method recoveries (n=3) Compound Basic value (mg) 6-Methyl-5-hepten-2-one 0.542 Limonene 0.299 Linalool 0.357 Citral 0.465 Amount (mg) Added 0.300 0.601 0.901 0.179 0.358 0.536 0.162 0.324 0.485 0.792 1.585 2.377 Recovery (%) Found 0.844 1.153 1.464 0.478 0.644 0.806 0.516 0.679 0.842 2.221 3.044 3.850 Average recovery (%) RSD (%) 101.5 2.1 96.9 3.0 99.6 3.6 98.6 4.3 100.7 101.0 102.7 99.9 95.9 95.0 99.9 98.5 100.3 96.5 98.7 100.6 Table 3 Results of the determination in 6 samples (mg·g-1 ) Region 6-Methyl-5-hepten-2-one Limonene Linalool Citral Sichuan Guangdong Jiangxi Zhejiang Hubei Guangxi 0.092 0.187 0.053 0.317 0.159 0.108 0.039 1.363 0.225 2.129 0.731 0.060 0.040 0.222 0.097 0.446 0.127 0.071 0.296 0.289 0.354 1.463 0.750 0.294 listed as follows: 75 ˚C programmed to 100 ˚C at 5 ˚C ·min-1, then to 140 ˚C at 2 ˚C·min-1, and then to 230 ˚C at 6 ˚C·min-1; Split injection was conducted with split radio of 1:1; Flow rate was 5.5 mL·min-1; Nitrogen was used as carrier gas; injector temperature, 250 ˚C; flame ionization detection temperature, 250 ˚C; and injected volume, 1 µL. The results revealed that four components could be separated successfully on the SE-54 capillary columns with temperature programs. For the selection of internal standard compound, eicosane, nonadecane and octadecane were once considered, however, under the present conditions, their retention time were lagged to 20 minutes, and their peaks were tailing with bad symmetry. The phenylethylketone mentioned in the previous document was still selected as the internal standard. Good reproducability and accuracy for the quantification of four major bioactive terpenoid of the essential oil from Litsea cubeba (Lour.) Pers were permitted. solution was injected to GC-FID analysis (Fig. 1). The content of each component was calculated from the corresponding calibration curve. The results are shown in Table 3. The contents of sample from different regions vary greatly. Chromatographic condition To assay components of the essential oil from Litsea cubeba (Lour.) Pers with good resolution and a reasonable retention time, capillary columns of different types and temperature programs were investigated. Thus, we analyzed the performance of DB-1, SE-54, DB-17, DB-WAX capillary column at the same temperature, in the case of the DB-1, 6-methyl-5-hepten-2-one could not be completely separated from impurities; in the case of DB-17 or DB-WAX, citral could not be completely separated from impurities. It was demonstrated that optimal chromatographic condition were obtained by using SE-54 column. Hence, the SE-54 column was applied to analyze the essential oil from Litsea cubeba (Lour.) Pers as shown in Fig. 1. The optimal conditions for achieving a good chromatographic resolution were Essential oil extraction condition Vapor distilment was a common way of extracting essential oil, which was introduced in the Chinese 68 Simultaneous Determination of Four Major Components in Essential Oil from Litsea cubeba / Asian Journal of Traditional Medicines, 2007, 2 ( 2 ) Pharmacopoeia, and also applied in our study. Consequently, we have acquired that granularity affects greatly on the contents of limonene and linalool. When traditional Chinese medicine (TCM) was reduced into thin powder, the contents of limonene and linalool from Litsea cubeba (Lour.) Pers were hardly detected. It was likely that they were apt to volatilize. The repeatability of the total procedure is satisfactory by using thick powder. The results indicated that there were great differences in essential oil of Litsea cubeba (Lour.) Pers from various areas. Compared with the previous study[4], they made also sharp distinctions in citral content. In addition, the content and component of essential oil were greatly influenced by the environment such as collecting time, ways of storage, duration of storage and detecting apparatus and so on. In conclusion, the established CGC-FID method A B Fig. 1 (A) Chromatogram of a standard mixtuere containing 6-methyl-5-hepten-2-one (1), Limonene (2), I.S. (3) Linalool (4), Citral (5, 6) and (B) chromatogram of essential oil from Litsea cubeba(Lour.)Pers is reproducable with excellent resolution, recovery and reproducability for simultaneously quantitative analysis of terpenoid in Litsea cubeba (Lour.) Pers. It provided a suitable quality control method for Litsea cubeba (Lour.) Pers samples and could be readily utilized for the determination of the major biologically active ingredients in Litsea cubeba(Lour.)Pers. Meanwhile,essential oil from Litsea cubeba (Lour.) Pers namely 6-methyl-5-hepten-2-one, limonene, linalool and citral were simultaneously determined by this method for the first. on Essential Oil from Litsea cubeba(Lour.)Pers in Guizhou by GC-MS. Journal of Guizhou University (Natural Sciences), 2001, 18 (1): 45-47 [4] Li BY, Li YZ, et al. Chromatographic Quantification of Citral in Essential Oil from Litsea cubeba(Lour.)Pers. Chemical World, 1998, 2: 99-100 References [1] Song LR, Hong X, Ding XL, et al. Modern Dictionary of Chinese Pharmacy. Beijing: People's Medical Publishing House, 2000, 2397 [2] Zhou RH, Wang LS, Liu XM, et al. Analysis of Guangxi Litsea cubeba(Lour.)Pers by GC-MS. Journal of Chemical Industry of Forest Products, 2003, 37 (1): 19-21 [3] Zhou X, Mo BB, et al. Analysis of chemical components 69