Energy System PDF
advertisement
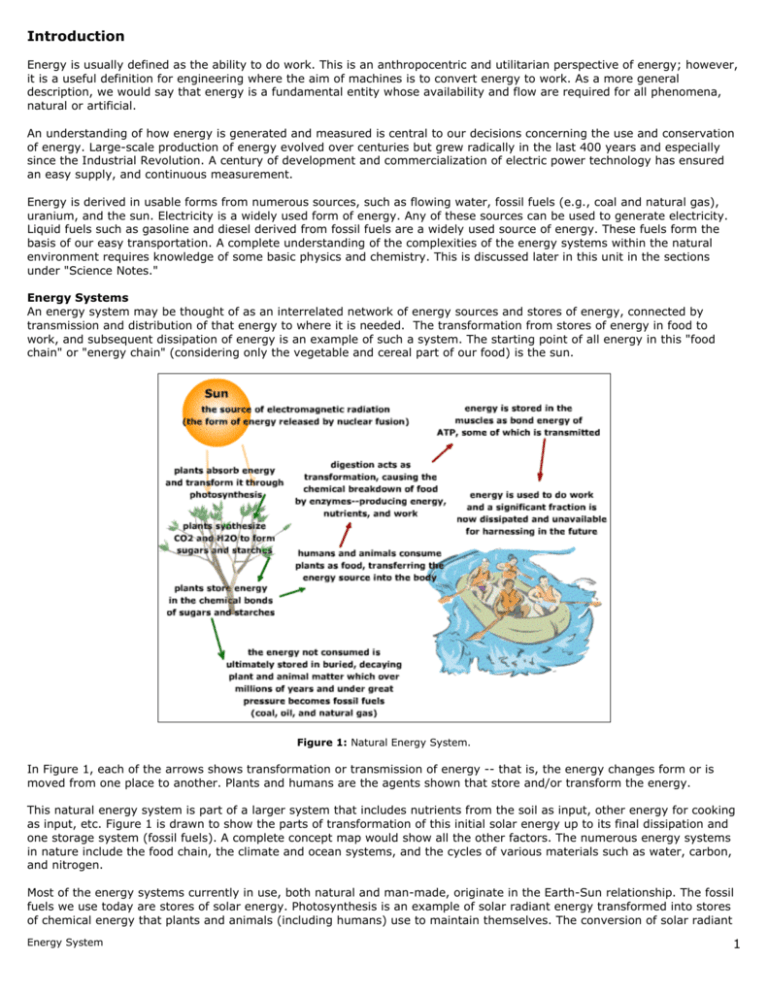
Introduction Energy is usually defined as the ability to do work. This is an anthropocentric and utilitarian perspective of energy; however, it is a useful definition for engineering where the aim of machines is to convert energy to work. As a more general description, we would say that energy is a fundamental entity whose availability and flow are required for all phenomena, natural or artificial. An understanding of how energy is generated and measured is central to our decisions concerning the use and conservation of energy. Large-scale production of energy evolved over centuries but grew radically in the last 400 years and especially since the Industrial Revolution. A century of development and commercialization of electric power technology has ensured an easy supply, and continuous measurement. Energy is derived in usable forms from numerous sources, such as flowing water, fossil fuels (e.g., coal and natural gas), uranium, and the sun. Electricity is a widely used form of energy. Any of these sources can be used to generate electricity. Liquid fuels such as gasoline and diesel derived from fossil fuels are a widely used source of energy. These fuels form the basis of our easy transportation. A complete understanding of the complexities of the energy systems within the natural environment requires knowledge of some basic physics and chemistry. This is discussed later in this unit in the sections under "Science Notes." Energy Systems An energy system may be thought of as an interrelated network of energy sources and stores of energy, connected by transmission and distribution of that energy to where it is needed. The transformation from stores of energy in food to work, and subsequent dissipation of energy is an example of such a system. The starting point of all energy in this "food chain" or "energy chain" (considering only the vegetable and cereal part of our food) is the sun. Figure 1: Natural Energy System. In Figure 1, each of the arrows shows transformation or transmission of energy -- that is, the energy changes form or is moved from one place to another. Plants and humans are the agents shown that store and/or transform the energy. This natural energy system is part of a larger system that includes nutrients from the soil as input, other energy for cooking as input, etc. Figure 1 is drawn to show the parts of transformation of this initial solar energy up to its final dissipation and one storage system (fossil fuels). A complete concept map would show all the other factors. The numerous energy systems in nature include the food chain, the climate and ocean systems, and the cycles of various materials such as water, carbon, and nitrogen. Most of the energy systems currently in use, both natural and man-made, originate in the Earth-Sun relationship. The fossil fuels we use today are stores of solar energy. Photosynthesis is an example of solar radiant energy transformed into stores of chemical energy that plants and animals (including humans) use to maintain themselves. The conversion of solar radiant Energy System 1 energy through photosynthesis is a fundamental natural energy system. The food chain is an example of a natural, solarbased energy system that has sustained human life on Earth. Often we take for granted that energy will always be available for us to use. We fail to recognize the complexities of the energy systems that drive these environmental phenomena and sustain life on Earth. We are intricate parts of the system as end users, completing the dissipation of energy to forms that are so spread out that it is impossible to use that energy again. Fossil fuels (coal, oil, gas) result from a transformation of plant and animal material over millions of years. The solar energy originally stored in the plant or animal is eventually converted into energy stored in carbon and hydrogen bonds of the fossil fuel. The fuels that took millions of years to make are being used at an enormously rapid rate. Figure 2 is a representation of the use of fossil fuels over time, including an estimation of how long they might last. Figure 2: Fossil Fuel Timeline. Source: Clark, Mary E. Ariadne's Thread. St. Martin's Press, New York, 1989. Reprinted with permission of Macmillan Ltd.. Fossil fuels and fuels like uranium are "spent" once they are used to obtain energy. These are called non-renewable sources of energy. Although new plants can be planted that eventually turn to coal, the process takes millions of years and that is why coal and other fossil fuels are considered non-renewable. Solar and wind energy arrive or circulate air on the Earth everyday. These sources are called renewable. Wood and trees used as fuel are called renewable, because they can be replanted. However, when we use them so that the rate of use far exceeds the rate of replenishment (trees take time to grow), referring to these sources as "renewable" can be a misnomer! Energy use in each human activity has grown exponentially since the early days of human civilization. For example, technological capabilities enable us to travel more and process more food. Figure 3 shows the amount of energy (in calories) we spend for each calorie of food we get. It shows that technologies have mechanized and made large production systems of cultivation and fishing. These systems involve large expenditures of energy, as seen in Figure 3. The figure shows that for wet rice production in Asian countries, it takes between 0.02 and 0.1 calories of energy to produce 1 calorie worth of rice as food. Large-scale food production consumes enormous amounts of energy. For example, it takes over 2 calories of energy input to produce 1 calorie worth of eggs in large-scale farms, and it takes 10-15 calories of input for every calorie worth of beef produced in the U.S.. Note how the intensity of energy consumption for U.S. food production has grown almost ten-fold in the 20th century! Add to this the fact that for every calorie of energy our body gets, we have to take in over 5 calories worth of food! Energy System 2 Figure 3: Summary of the energy required for various types of food production. Source: Clark, Mary E. Ariadne's Thread. St. Martin's Press, New York, 1989. Reprinted with permission of Macmillan Ltd.. Table 1 shows that as we become more industrialized, each human consumes more calories daily as well. This, along with population increase, has resulted in an enormous increase in the daily calories consumed by humans. Economic systems Years Ago Maximum global population* (approx.) Daily calories/ person† Global daily calories consumed by human population Hunter-gathering (before cooking) 1,000,000 to 500,000 1 million 3000 3 x 109 Hunter-gathering (after cooking) 500,000 to 10,000 10 million 8000 8 x 1010 Early agriculture 10,000 to 2000 300 million 15,000 4.5 x 1012 Middle Ages 1000 500 million Europe 10% Rest 90% Today North America Eur, USSR, Japan Third World 0 ~ 8 x 1012 23,000 15,000 2.8 x 1014 5000 million 5% 18% 77% 314,000 157,000 15,000+ Notes: *R. Leakey and R. Lewin, Origins (New York: E.P. Dutton, 1977) p. 143; J. Weeks, Population: An Introduction to Concepts and Issues, 2nd edn(Belmont, CA: Wadsworth, 1981) p. 46 †Harrison Brown, The Human Future Revisited (New York: W.W. Norton, 1978) pp. 30-3, with per capita figures for industrialized nations upgraded from 1970 to 1980 levels. Table 1: Per capita and global energy consumption for different types of human economies. Source: Clark, Mary E. Ariadne's Thread. St. Martin's Press, New York, 1989. p. 102. Reprinted with permission of Macmillan Ltd.. Energy System 3 History of the Energy System In the Beginning: Pre-Industrialization The muscle power of human beings and animals was the first application of energy by humans and the food chain was the energy system in use. Humans have long "designed" energy systems with the goal of producing the most work possible with the least amount of human effort to generate the energy. Pre-Industrial society depended primarily on muscle power and biomass for their energy needs. Biomass consisted primarily of wood or peat and its energy delivery had a low efficiency. Amory Lovins, an expert on energy, states, "Most of the energy generated by wood or peat went up in the chimneys rather than into the room or cooking pot of pre-industrial societies." Animal power in the form of horse mills, wind power in the form of windmills, and water power with the use of a water wheel were major energy sources harnessed until the 19th century; especially for "industrial uses." Wood and charcoal were the main fuels for cooking, heating, and other domestic uses, but coal and oil were available as well. "In the Middle East crude oils have been known for millennia from natural seepage and pools, but they were used only rarely as fuels, and more frequently as protective coatings."1 Coal has its origin in "the lithification of peats produced by accumulations of dead plant matter in wetlands. Difference in original vegetation and, more importantly, in magnitudes of durations of transforming temperatures and pressures, have produced a large variety of coals."2 As early as the 13th century, coal pits were mined and coal energy was used specifically for the forcing and smelting of metals. In the 1600's, England experienced an energy crisis due to a shortage of wood and began using coal as a substitute fuel source for domestic purposes. Even in the 1700's, wood was the major fuel source in colonial America. The Industrial Revolution The quest for more powerful energy sources was propelled by the inventions and discoveries of the Industrial Revolution. As sophisticated mechanical inventions were made, a large reliable and seemingly inexhaustible source of energy became necessary for industrial uses, and transportation. The need for large quantities of accessible, dependable, and transportable energy encouraged the exploration of energy sources. The inventions of the Industrial Revolution provided the equipment to further mine or drill the already visible deposits of coal and oil. Steam power was developed in the 1600's in conjunction with coal mining to help pump water out of the mines. It had been known since ancient times that heat could be used to produce steam, which could then do mechanical work. However, it was only in the late eighteenth century that commercially successful steam engines were invented. The first commercially successful steam engine was invented by Thomas Savery (1650-1715), an English military engineer. In 1712, this engine was refined by Thomas Newcomen (1663-1729), another Englishman. The Newcomen engine was widely used in Britain and Europe throughout the eighteenth century, but had very low energy efficiency. A greatly improved steam engine was designed and built in 1763 by James Watt who was asked to repair a Newcomen engine. Watt built and then sold or rented his engines to mining companies, charging them for the "power" in the rate of work the engine produced. Today, the unit for power is called a Watt. The sun was also studied as an energy source in the 18th century. In 1767, the first solar thermal collector was developed by the Swiss scientist Horace de Saussure. Solar thermal power was used in the American west as an energy source for cooking until oil and natural gas became a more reliable way to generate energy. For simple cooking solar energy was absorbed by black cast iron pots. Solar thermal collectors were also used in the form of hot boxes to cook food. In 1839, Alexandre Becquerel discovered that an electric current could be generated when certain elements were exposed to light. The scientific explanation of this phenomenon by Albert Einstein, called photoelectricity (light-induced electricity), came much later in 1905. Photoelectricity is the basis of the photovoltaic cells, now used to convert light into electricity. Despite the century and a half since it discovery, photovoltaic means of generating electricity have not been developed with enough vigor for it to become a major source of electricity. This is because the material technology for photovoltaic panels developed slowly. As coal and other fossil fuels were easier to use, and available in plenty, not much effort has gone into photovoltaic research. Until the early 1800's our understanding of the science of energy was not well developed. The theory at that time was the caloric theory, which said that heat is a substance called "caloric" that flowed from hotter to colder bodies. In the 1840's the English physicist James Prescott Joule did a long series of experiments that showed that heat is a form of energy. Joule found the relationship between a unit of mechanical energy and a unit of heat. This helped Joule finalize what chemists and natural philosophers had come to believe--that the total energy in the universe is constant, although energy is continuously changing forms. 1 Smil, Vaclav. Energies: An Illustrated Guide to the Biosphere and Civilization. The MIT Press: Cambridge, MA, 1999. Nye, David E. Consuming Power: A Social History of American Energies. The MIT Press: Cambridge, MA, 1999. Energy System 2 4 The study and invention of the heat engine and steam power established and confirmed the Laws of Thermodynamics. From 1840-1880, Joule, Lord Kelvin, and James Clark Maxwell in England; Sadi Carnot and Rudolf Clausius in France; and Ludwig Boltzmann in Austria formulated a theory of heat engines, laying the foundations of Thermodynamics, literally the science of "motion from heat." (Thermo=heat and dynamics=motion). In 1820, the advances in mechanical and materials engineering made the railroad the most efficient and fastest means of transportation. Coal and wood were used as the primary fuel source for the steam engine. The locomotive also changed society's perception of travel and transportation. Wind energy was developed on a large scale in the United States as an energy source for farms and railroad stations, using tall windmills to pump water from underground wells. There were specific design developments that made these windmills more efficient, although they still generated relatively little power. The height of these windmills helped to ensure they caught the wind and a tailfin generally kept the fan facing the wind. Another result of the Industrial Revolution was an energy distribution infrastructure built into cities that promoted domestic convenience. As early as 1816, natural gas was piped into cities for domestic uses such as cooking, home illumination, and street lighting. The steam engine was used to pump water into homes and sewage away from homes. The city was undergirded with networks that usually began with water pipes and gas lines and gradually expanded to include sewers, electrical conduits, and telephone lines.3 In 1859, when petroleum was drilled in Titusville, Pennsylvania, an apparently plentiful energy source began to replace coal. Oil was distilled into kerosene (referred to as coal oil) and used as a lamp oil. It replaced dwindling supplies of whale oil used for lamps. There were many reasons oil became a more desirable fuel source than coal: it was easy to obtain and transport; it emitted less particulate pollution than coal; it replaced scarce whale oil as a fuel for lamps; and coal had become an unreliable fuel source because of the labor issues surrounding the mining of coal. Miners were striking for safer work environments and more money, which affected the amount of coal available to the consumer. But the most significant use of crude oil was as the liquid fuel for the internal combustion engine, designed in 1861 by Nikolaus August Otto. The internal combustion engine became one of the most influential inventions of the Industrial Revolution. Although this engine is low in efficiency, it could produce enough work to move a large metal vehicle far distances. The fuel of the internal combustion engine was also easier to use than, for example, shoveling coal into a furnace to power a locomotive. This was the beginning of the use of liquid fuel to advance transportation. In 1879, Thomas Edison invented the incandescent light bulb -- a major step in the human use of storable energy leading eventually to large-scale electrification. Electricity is similar to a liquid fuel in that it can be transported easily (although not efficiently) from one place to another. One of Edison's goals was to make electricity affordable for all homes. Edison began with the distribution of electricity through a direct current (DC). This meant that electrons would flow one way through a wire to bring electricity to a home; however, a good portion of the energy was lost as the electrons moved through the wire. This loss of energy using direct current to move electricity meant that power plants had to be built close to the homes the plant serviced and was eventually considered impractical. Nikola Tesla, an inventor employed by Edison, discovered that electrons would alternate or travel back and forth on a wire and travel longer distances with less energy loss. This was called alternating current (AC) and had an advantage because AC could be more easily generated. Edison had so much money invested in his DC power plants that he discredited Tesla's alternate current as dangerous -- thus beginning a "war of the currents." Tesla eventually joined forces with George Westinghouse and began developing power plants using alternating current (AC). In the late 19th and early 20th centuries the steam turbine, using coal as a fuel, was developed as a cheap power source that generated electricity. In 1882, the first functional steam turbine was designed by Charles Parsons, an English engineer. He used the high pressure of steam to hit the blades of a rotor. The principle of the turbine was a major step toward today's production of electricity. In 1893, Westinghouse demonstrated a "universal system" of generation and distribution at a Chicago exposition. The universal system meant that power or energy could be used in a variety of ways at many different voltages. Westinghouse, using Tesla's invention of the transformer and the electric motor, as well as steam turbines, transformed Niagara Falls into one of the first hydroelectric plants in the world In 1910, Henry Ford opened the 60-acre Highland Park automotive plant with a moving assembly line. This was the beginning of an eventually enormous use for fossil fuels. Fossil fuels were used not only to propel the automobiles that were made at the plant, but also to generate electric power for the automotive plant. Energy technologies developed rapidly during the 20th century. Although the current version for solar thermal collectors was designed in 1908, they were not developed well enough for mass distribution. In the 1920's, 30's, and 40's, there was large-scale construction and development of hydroelectric plants/dams to support increasing population in the Southwest. 3 Nye, David E. Consuming Power: A Social History of American Energies. The MIT Press, 1999. (p. 94) Energy System 5 In 1938, Otto Hahn and Fritz Strassman demonstrated nuclear fission and within four years (1942), Oak Ridge, Tennessee, was chosen as the site for the first functional nuclear reactor plant, and for the preparation of uranium and plutonium used to the create the atomic bomb at Los Alamos. The first nuclear chain reactor was demonstrated at the University of Chicago in December 1942. In July 1945, the testing of the first atomic bomb at Alamogordo, New Mexico, demonstrated the technology used to release nuclear energy on a large scale. In 1957, the first commercial nuclear power plant opened in Shippingport, Pennsylvania. The first large scale use of photovoltaic (PV) solar energy in conjunction with satellite technology developed in the 1950's. The United States Vanguard I was the first PV-powered satellite. By the early part of the 20th century, crude oil and its products had become an indispensable part of the industrial economy. James Young had patented a process in England in 1850 to distill oil from coal and shale. Oil refining is not just about gasoline. The distilled chemicals from crude oil have many purposes -- for example, petroleum is used for plastics manufacturing. Young's process of fractal distillation forms the basis of the world's oil refining industry. Figure 4 shows the oil reserves that we know for sure as of 1987. Figure 4: Proven Oil Reserves as of 1987 (billions of barrels) Source: Energy, National Academy Press, pg 268 While a large amount of oil occurs in many parts of the world, the largest stores are located in the regions governed by the Arab countries. The Oil and Petroleum Economic Cartel (OPEC) is the economic coalition of these countries that control the flow of that oil. In the 1970's, OPEC placed an embargo on their oil sales. This "energy crisis" brought energy scarcity to the consciousness of all nations -- and especially the U.S., with its higher dependence on imported oil. This crisis began to generate interest in the exploration of renewable energy sources for large-scale generation of electricity and other energy needs. Energy System 6 Human Energy Needs Figure 5: Composite of satellite images showing the extent of outdoor lighting in the continental United States. Courtesy of the Defense Meteorological Satellite Program (DMSP). Our energy consumption has led us to develop new energy sources and technologies. In a century, liquid fuels and electricity have improved our standard of living and provided us with more mobility than people in any other era. This section reviews our human energy needs, how we currently meet them, and what the future may have to offer. Energy is essential for all we do as individuals and as societies. Energy production, use, and distribution also cause some of the most pressing environmental problems. Figure 6 shows the overall picture of human energy needs, the ways in which we meet our energy needs, and the impacts. Figure 6: Flowchart of Energy Needs. Although industrialized countries use the most energy at present, newly industrialized countries are increasing their rate of use. Figure 7 graphs the projected energy needs of industrialized countries, developing countries, and Eastern Europe and the former Soviet Union. Many environmental and economic issues arise from the escalating energy use all over the world. Understanding the science and technology driving the energy system enables us to better understand our relationship to the environment. Energy System 7 Figure 7: Projected Energy Needs. Energy Use and Sources - Data The daily energy needs -- especially of industrialized countries -- are vast. The United States used 3,236 billion kilowatthours (kWh) in 1999 for a population of approximately 300 million. This is more than a million kWh per person per year! (This includes all sectors of energy used.) Table 2 gives the billions of kilowatt-hours of electricity used by various regions. History Region Industrialized Countries United States EE/FSU Developing Countries Developing Asia China India South Korea Other Developing Asia Central and South America Total World Projections 1990 1999 2005 2010 2015 2020 Average Annual Percent Change, 1999-2020 6,385 7,517 8,580 9,352 10,112 10,888 1.8 2,817 1,906 3,236 1,452 3,761 1,622 4,147 1,760 4,484 1,972 4,804 2,138 1.9 1.9 2,258 3,863 4,988 6,191 7,615 9,203 4.2 1,259 551 257 93 2,319 1,084 424 233 3,088 1,533 545 294 3,883 2,035 656 333 4,815 2,635 798 386 5,856 3,331 949 437 4.5 5.5 3.9 3.0 357 578 716 858 996 1,139 3.3 449 684 844 1,035 1,268 1,552 4.0 10,549 12,833 15,190 17,303 19,699 22,230 2.7 Note: EE/FSU = Eastern Europe and the former Soviet Union. Sources: History: Energy Information Administration (EIA), International Energy Annual 1999, DOE/EIA-0219(99) (Washington, DC, January 2001). Projections: EIA, World Energy Projection System (2001). Table 2: World Net Electricity Consumption by Region, 1990-2020 (Billion Kilowatt-hours) The factors that affect energy use most are the level of industrialization, the climate of the region, and the population. These factors are not independent of each other. All of these factors influence energy choices, production, distribution, and usage. The average use of energy per person in different countries varies widely. Table 3 highlights world energy consumption for electricity generation by region and fuel source. It is evident in these tables that industrialized countries use more energy. Energy System 8 Region and Fuel Industrialized Oil Natural Gas Coal Nuclear Renewables EE/FSU Oil Natural Gas Coal Nuclear Renewables Developing Oil Natural Gas 1995 77.1 5.7 9.7 27.7 19.4 14.7 26.4 2.8 10.6 7.4 2.5 3.1 38.1 5.1 4.8 History 1999 83.8 6.5 11.6 29.5 20.6 15.6 23.8 2.4 10.3 5.4 2.7 3.0 40.9 5.7 6.0 2005 91.6 5.4 15.6 32.1 20.9 17.5 25.9 3.1 11.1 5.4 3.2 3.2 52.3 6.9 8.4 Projections 2010 2015 97.2 103.5 5.3 5.5 18.3 23.1 33.4 34.0 20.9 20.5 19.4 20.4 27.0 28.9 3.5 4.2 12.3 14.4 4.5 3.3 3.1 3.1 3.5 4.0 63.1 75.0 8.3 10.0 11.0 13.6 2020 108.0 5.9 27.4 34.3 19.1 21.3 30.8 4.7 15.9 2.8 2.8 4.5 86.6 12.0 16.4 Coal Nuclear Renewables Total World Oil Natural Gas Coal Nuclear Renewables 16.8 1.4 10.1 141.7 13.6 25.1 51.9 23.3 27.9 15.8 1.9 11.5 148.4 14.6 27.9 50.7 25.3 30.0 20.4 2.6 14.1 169.8 15.4 35.2 57.8 26.7 34.8 24.7 3.4 15.8 187.3 17.0 41.7 62.5 27.4 38.7 32.6 5.1 20.5 225.4 22.5 59.7 69.7 27.1 46.4 29.2 4.1 18.2 207.4 19.7 51.0 66.5 27.7 42.5 Note: EE/FSU = Eastern Europe and the former Soviet Union. Sources: History: Derived from Energy Information Administration (EIA), International Energy Annual 1999, DOE/EIA-0219 (99) (Washington, DC, January 2001). Projections: EIA, World Energy Projection System (2001). Table 3: World Energy Consumption for Electricity Generation by Region and Fuel, 1995-2020 (Quadrillion Btu) Information describing our energy use often can be evasive. Figure 8 shows the main heating fuels the U.S. uses for residential home heating. Electricity use for heating is on the rise; however, electricity is not a direct fuel source like the others in this figure. It is a form of energy. Different sources, such as coal and uranium, are used to generate electricity. Therefore, if electricity use is on the rise, the use of coal and uranium are also on the rise. Figure 8: Main Heating Fuels. We are all affected by and pay for the life cycle of electricity. As end users of electricity, understanding the process of electricity generation gives a clearer picture of what you are paying for and helps in your decision making as a consumer. Transportation as an Energy Sector Liquid fuels such as gasoline have made our lives more mobile. Desire to maintain our current ability to travel and the fact that crude oil is a non-renewable energy source, have encouraged the development of new technologies are being developed. Meanwhile, our current travel habits are fast depleting the crude oil stores on Earth. Energy use for transportation is the least efficient use of fossil fuels. The automobile loses far more energy than it uses. As shown later (in the Energy Transformation section), for every 20 gallons put into a car we only get about 2 gallons worth of Energy System 9 actual work. The rest is dissipated as heat. The other inherent inefficiency is in the fact that to move one person, we have to spend energy moving over a ton of extra material (the automobile itself). Figure 9: Average Annual Residential Vehicle Fuel Consumption by Region, 1994. Source: Energy Information Agency Use of crude oil is escalating as developing countries emulate industrialized mobility. Figure 10 shows the use of crude oil for transportation needs. This increase in oil consumption specifically for transportation not only impacts the environment, it also further depletes the limited oil reserves in the Earth's core. Increased consumption of oil for transportation could also affect a variety of industries (i.e. plastics), which in turn affects the economy. Figure 10: Use of Crude Oil for Transportation Needs. A review of the science supporting our current energy systems can be found in the section "Science Notes." Science Notes: Energy Transformation The definition of energy as the ability to do work came from the 19th century as steam engines and other work-producing machines were developed. The first engines converted heat (thermal energy) into motion (dynamics). The science of heat engines developed by Lord Kelvin in England and Joule and Clausius in France founded the science of thermodynamics. They showed that two rules always held when energy was used to produce motion or work. These are called the two Laws of Thermodynamics. It was noticed that when engines performed work, heat was always produced in addition to the work, and that this represented wasted energy. The Two Laws of Thermodynamics One of our observations about energy is that the total quantity of mass and energy combined in the universe is always the same. Energy can change forms and mass and energy may even change into each other, but the total quantity remains the same. This fact is called the principle of Conservation of Energy, or the First Law of Thermodynamics. For most reactions that are studied here the total energy remains constant. Energy System 10 Conservation of energy was an idea proposed by numerous people. Julius Robert Mayer, a German physician, proposed in 1844 that energy was conserved by observing physical processes involving heat and respiration, but with no quantitative measurements. The first formal statement was in a paper by a young physicist, Hermann von Helmholtz, in 1847. (Helmholtz is known as one of the greatest physicists of the 19th century). Joule's experiments from 1843 and his paper in 1849 publicizing his most precise experiments stimulated gradual acceptance of the principle. However, it met with a lot of skepticism early on because it seemed speculative. Even as late as 1858, William Barton Rogers, the founder of Massachusetts Institute of Technology (MIT), wrote to his friend that the principle of conservation of energy was "mysticism"! This is an example of both the slow pace of science and of the fact that great discoveries are often a leap of speculation, although based on observations. The First Law of Thermodynamics states that energy cannot be created or destroyed. Simply stated: energy can change forms; mass and energy may even change into each other (this happens only in special reactions within nuclei of atoms) but the total energy in the universe remains constant. The first law says that you can convert energy into work, and into the simultaneous production of heat. If the total energy is E to start with, and all of it is spent doing an amount of work (W), with a production of heat (Q), the First Law states: E=W+Q More correctly, we are talking of the change of energy, work, and heat in a system. Using the symbol "∆" to denote the changes, the first law is written as: ∆Q non useful work heat and noise ∆Q1 + ∆W = ∆E + work done = change in energy of the system + mechanical energy = + ∆E1 ∆W1 = = transformation from chemical energy to mechanical energy ∆Q2 + ∆E2 ∆E2 All the descriptions above are equivalent. As energy changes forms, the energy becomes more and more spread out and inaccessible to us. For example, the energy that is stored in a compact form within a gallon of gasoline in a car tank becomes transformed into work in moving the car, and dissipated into the energy of the surrounding air and road. As the molecules are heated they spread over a much larger area. This energy, now spread out all over, is not destroyed but has become dissipated and therefore unavailable for us to do more work with it. In this process, matter (such as the heated molecules in the air surrounding the car) has also become more disordered. These two facts combined are known as the Second Law of Thermodynamics. This law states that the unavailable energy in the universe increases, or equivalently, that the disorder (also called entropy) in the universe increases (as energy is used). The second law then simply states that within each process of producing work, we are increasing the unavailable energy and the disorder in the universe. This means that even though the total energy in the universe is constant, we are decreasing the "quality" of the energy. We are decreasing the amount of available energy -- the energy that we can use to produce work. We cannot harness all the energy coming out of a process of energy transformation. We define efficiency as: Efficiency N = Energy System work done energy spent OR work output energy input 11 EXAMPLES OF EFFICIENCY: How efficient is an automobile? In other words, how much of the energy in the gasoline results in kinetic energy or energy of motion of the automobile? 12% if well maintained, 8-10% if not maintained What are the implications of this inefficiency? Of the 20 gallons you of gasoline you put in your car, how much actually moves the car to your destination? 1.6-2.4 gallons the rest is transformed to waste heat and noise How efficient is a coal-fired power plant? transformation by steam turbine: 30-40% How efficient is a hydroelectric plant? transformation by water driven turbine: 80% (the difference from above is because no conversion to steam is involved) How efficient is a nuclear power plant? the nuclear reaction is 90% efficient, however the same combustion process (steam turbines) is used to generate electricity as with the coal-fired plant so the net efficiency: 30-40%. How efficient is the human body? conversion of the energy in the food to muscular and other kinds of work 20% efficient Based on the First Law of Thermodynamics, we neither create nor destroy energy. Whenever we say that we are producing energy, what we really mean is that we are transforming energy from one form to another that is more usable. Energy that is the result of work usually manifests as change in position or as the motion of an object. Energy that is stored is called potential energy -- energy that has the 'potential' to do work. A second form of energy -- energy of motion -- is called kinetic (meaning "moving") energy. Both kinetic and potential energy can be transformed into work. Later in this unit we present the physical basis of energy and work. Without going into the details that will come then, let us discuss two transformations of energy: a waterfall and a pendulum. Every gallon of water in the fall has potential energy at the top of the waterfall by virtue of its position. At the bottom of the waterfall, this gallon of water travels faster and has gained kinetic energy at the expense of potential energy. As a pendulum swings back and forth, the energy changes from potential energy at the top position to kinetic energy at the lowest position and potential energy again as it goes to the top. We will explain the deeper meanings of potential and kinetic energy later in the unit. We begin by reviewing some fundamentals of physics and chemistry relevant to understanding the basic principles of energy transformation. In particular, we focus on concepts related to the fundamental aspects of energy: Matter, Force, and Energy and the Fundamental Forces of Nature. Then we describe the physics and chemistry of Measuring Energy, Work, and Power. Chemical reactions and energy release are then described to understand more clearly how the chemical combustion of fossil fuels produces the carbon dioxide and other products that cause environmental problems. Science Notes: Measuring Matter, Force, and Energy Matter, force, and energy are common terms that have scientific meanings close to the common use of the words. Matter is the stuff of which everything is composed, and force is something that is capable of changing the motion of an object. Energy is a property that tells us how much work we can get out of the object that possesses that energy. In keeping with the fact that science is based on measurements and observations, all of these entities are described in terms that we can observe and measure. Therefore, we first discuss these entities by examining and measuring their effects. Matter and force are the two fundamental entities of which the universe is composed. All that exists can be classified in these terms. All environmental phenomena occur because of the interactions between matter and transformations of matter in space and time. As the arrangements between forces and masses change, the change is manifested in terms of energy. Table 4 gives the abbreviations for the physical qualities and their definition and units. Energy System 12 Physical Quantity mass m distance d time t speed, velocity, v acceleration, a force F Work, energy W, E Definition, Unit quantity of matter, kilogram linear dimension of space, meter dimension of time, second distance per time, m/sec 2 velocity change per time, m/sec 2 mass times acceleration, kg. m/sec = Newton, nt force times distance, nt.m = Joule, J Table 4: Summary of physical quantities and units. Matter is discussed in terms of the quantity of matter. This is called the mass. The unit we will use to measure mass is the kilogram (gram, milligram) in the MKS system that is globally adopted as the scientific system of units. For most engineering applications, and for common trade in the United States, the British system is used, in which the unit of mass is the pound. Force is a more complicated concept. We experience or observe force through its action on matter (as a push or pull). When a force acts on matter, it changes the way that quantity of matter is moving (Newton's first law). So we measure force by its capacity to produce acceleration in a mass. Acceleration is a measure of change of motion, measured by how much speed (m/sec) changes in a second. So the units for acceleration are meter per second per second, or, m/sec2. (Newton's second law: F = ma). Thus the unit to measure force is a composite of the measure of mass, and of 2 acceleration. The unit is called a newton (kg.m/sec ), written as NT or N. So a one-newton force can change the 2 acceleration of a 1 kg mass by 1m/ sec . When a force (F) is applied to an object and moved through a distance (d, measured in meters, m) in the direction of the force, we define the work (W) done as: Work = Force * Distance moved in the direction of the force, or symbolically, W= F· d (Joules, J = NT · m) When F is measured in newtons (NT, or N) and distance in meters, the resulting quantity of work is expressed in Joules. So, a force capable of producing an acceleration of 2.3 m/sec2 by acting on a mass of 3 kilograms is a 6.9 NT force. The weight of an object of mass m on Earth is the force due to Earth’s gravitational pull on that object. The gravitational acceleration of the Earth is about 9.8 m/sec2. (This means that the gravitational force exerted by the Earth on a 1 kg object is 9.8 nt.). 2 Thus the weight of a 5-kilogram object on Earth is 5 kg * 9.8 m/sec , or 49.0 nt. A 2.5 NT force moving something through a distance of 4 m (meters) in its direction does a work of 2.5 nt * 4 m, or 10 Joules. When a 5 kg object falls a distance of 4 m, the work is done by the Earth’s gravitational force. As the gravitational force on 5kg is 49 NT, the work done by the Earth on the 5 kg object in pulling it down by 4 m is 49 nt * 5 kg = 245 nt-m = 245 Joules. Measuring Energy: Work, Energy, Heat, and Power All phenomena involve transformations of energy between potential and kinetic forms. We discuss some transformations and calculations involving energy in the next section. Before we do that, we need to understand some definitions of different means of measuring energy. Due to historical reasons, different measures of energy were developed and used in physics and chemistry. In the early times, physics dealt mainly with motion -- of bodies such as planets and stars, as well as smaller masses. Thus forces and motion were the focal points of early physics4. Physics measured energy by means of the force required to change the state of motion. The units of physics dominated the emerging fields of engines as well, where forces were used to produce motion. How much energy could be produced every second was the question in designing engines. The amount of energy per unit of time is defined as power. Thus power has the units of Joules (energy) per second, also known as a watt. One watt is one Joule per second. We also have the Horsepower, which is the unit in the British system. It is understandable that with the horse as one of the important “animal engines,” the early engines were compared to the power of a horse to move things. Chemistry started as the study of changes in the nature of substances5. Heat was a common method used to change substances. Temperature, or the feel of heat, was used to measure the amount of heat in a substance. Thus heat energy was measured by chemists in terms of the energy required to change the temperature of a common substance - water. Thus 4 Physics derives its name from the Greek word "Physis," meaning the nature of things; and the field was given its name by Aristotle. Chemistry derives its name from "Cheo," which means "to pour." Energy System 5 13 the unit of energy (heat) most used by chemists was the calorie, defined as the amount of heat required to change the temperature of one gram of water by one degree Celsius. Of course, there are also two different measures of temperature depending on whether you follow the British or the Metric system. We will confine ourselves to the Metric system, and hence to degrees celsius (or centigrade). Count Rumford (Benjamin Thompson) and James Joule, scientists in the eighteenth century, were among the earliest to show that mechanical energy and heat could be changed into each other, primarily by noting that when mechanical work is done, the friction produces heat. Joule in fact determined that 4.18 Joules of mechanical work is equivalent to 1 calorie of heat6. In practice, a calorie is a very small amount of heat, and so a kilocalorie, also written as Calorie (or kcal) is used. A kilocalorie is therefore 4,180 Joules. The units of energy, heat, and power are summarized in Table 5. Because of the different origins of the ways of measuring energy, and the numerous manifestations of energy, there are several units for measuring energy. Units also vary depending on the practices in different fields, and on the type of energy being measured. This can be confusing at times. The tables below summarize most of the units and contexts. Physical Quantity and Definition Energy, work (mechanical) = force x distance Energy (chemical, heat) = energy to change temperature Power = work/energy per unit time Energy = power * time Metric system nt.m = J calorie (cal) J/sec = Watt 1000 Watts = 1 KW kilowatt x hour = (kwh) kilowatt hour Units British system ft-lbs British thermal unit (BTU) 1BTU = 778 ft-lbs horse-power (HP) 1 HP = 550 ft.lbs/sec 1 HP = 746 watts no equivalent Table 5: Physical Quantity, Definition and Units. Work and energy transform into one another. They are measured in the same units. So a "Joule" is also a unit for measuring energy. As described in more detail later, all energy is either stored (potential energy) or is in the process of causing motion of an object (kinetic energy). Thus we can represent the energy work relationship in a continuous state of mutual transformation in a system, including the energy that is "lost" (has become unavailable) Potential Energy Work Kinetic Energy + Unavailable Energy Depending on the context, the different units above are used in physics or mechanical engineering: • • • • Physics/mechanical: work = force * distance = lb * ft2/ sec2, kg * m2/ sec2 (Joule) electrical energy: kilowatts * hour = kilowatt hour (KWh) hydraulics/fluids: energy head = equivalent distance in feet, or meters chemical process energies: energy head and calorific content = calorie For physics, mechanics, and engines, the work is typically stated in terms of moving something in a unit of time, e.g., energy per second, with power (watts) in terms of joules per second. For chemistry, the work is changing the nature of substances, therefore the units are in terms of the amount of heat needed to change the temperature of water (calorie). Name of Unit kilocalorie Symbol kcal or Cal Value in calories 1000 Value in Joules 4184 calorie British Thermal Unit cal BTU 1 252 4.184 1054 Joule J .24 1 Kilowatt-hour kwh 8.6 · 105 3.6 · 106 Quad Measures Scientific work unit for nutritional requirements and heat Scientific work Engineering Technology, heating, a/c Standard unit especially for mechanical energy Standard Unit for Electrical Engineering Used for large quantities of Energy Table 6: Units of Energy and conversion factors. 6 There is an anecdote that Joule did this experiment for the first time by noting the temperature difference between the bottom and top of a beautiful waterfall in Switzerland while he was on his honeymoon there! Energy System 14 Exercises 1. Look at the following appliances to get an idea of the power they generate: a hair dryer, a toaster, a clothes dryer, a light bulb. For frame of reference, we receive about 1,400 watts of solar energy for every square meter of the Earth. 2. Each kilogram of water at Niagara Falls falls through a height of 184 ft. (or approximately 56 m). What is the amount of work done by the force of gravity on the 1 kg of water? What happens to this work? 3. You use a force of 19.6 nt to raise a rock (of mass 2 kg) vertically through a distance of 3 m from the ground. • • • • Why vertically? What force are you working against? What is providing the energy for that work? How? How many Joules of work is done? Now, assume the rock is kept at the level of 3 m above ground. How many Joules of (stored) potential energy does it have? Potential energy will be released if the rock is allowed to fall. For example, suppose the rock falls squarely on the top of a nail head held vertically on a piece of wood, and the nail has to overcome a force of friction of 9.8 nt to be driven into the wood. • • How far can the rock theoretically drive the nail in? What assumptions have you made in answering this? 4. A block is pushed 1 meter along a horizontal surface by a horizontal force of 60 nt. The opposing force of friction is 10 nt. • How much work is done by the 60 nt force? • What is the work of the friction force? • Where does the work go? • What is the role of gravity in this situation? 5. Power is the energy released (work done) per unit time, or the rate of releasing energy (or, doing work). It is measured in watts (Joules per second). Thus a coal burning power plant of 1000 megawatts (MW) releases __________ Joules every second. Where does the power come from? If the power plant were a hydroelectric plant instead, generating power using the Niagara waterfalls, how much water (in kilograms) will have to fall every second if the water falls through the 56m to release the same amount of energy? In the operation of the Niagara power plant, 102,000 cu.ft. of water falls through 56m every second. If all this energy could be converted to electricity, how many megawatts of power would be produced? 6. A skier who weighs 700 nt skis down a hill that is 60 m long and 20 m high. If 1000 J of energy is lost in overcoming friction, what is his kinetic energy at the bottom of the hill? Science Notes: Energy Accounting and Balance Once we understand the various transformations of energy that are possible, an energy balance can be used to track energy through a system, and is a very useful tool for determining resource use and environmental impacts. The idea is to use the First and Second laws to determine how much energy is needed at each point in the system and in what form that energy is. The accounting system keeps track of energy in, energy out, and non-useful energy versus work done, and transformations within the system. An energy balance diagram is used. Non-useful work is what is often responsible for environmental problems. Energy System 15 Example: We wish to determine how much coal is needed to produce 1 kWh of electricity. Assume the power plant is 33% efficient, with 85% of waste heat to cooling tower, and 15% to stack. Assume you can get 24 kJ of energy from 1 gram of coal. Note that 1 kW of electricity is equivalent to 1 KJ/s of electricity. What are the environmental issues? Figure 11: Coal Fired Power Plant. 1 KJ/s kWh * 3600 s/hr = 3600 KJ per hour If the power plant is 33% efficient, then need 3600 * 3 = 10,800 KJ 10,800 KJ * 1/24 KJ/gram = 450 grams coal = approximately 1 lb = 454 grams Therefore, we need 450 grams coal for 1 KWh of electricity How much electrical energy do we use worldwide? 10 EJ (1990), 1 ExaJoules = 1018 Joules, or 27.8 * 1015 kWh. A home in the US may average 500 kWh over a month. What happens to non-useful energy in this example? 0.85*7200 = 6120 KJ to cooling water (note that we use vast amounts of water to cool waste heat; this cooling water produces thermal pollution of water bodies) Also, 0.15*7200 = 1080 KJ goes to stack as waste heat, which carries impurities in the form of air pollution. Environmental issues for this example are nonrenewable natural resource consumption, air pollution, water pollution, and solid waste. Science Notes: Fundamental Forces of Nature All forces in nature may be classified into four types. The gravitational force holds together the universe at large, plus the atmosphere, water, and us to the planet Earth. The electromagnetic force governs atomic level phenomena, binding electrons to atoms, and atoms to one another to form molecules and compounds. The strong nuclear force holds the nucleus together. The fourth force, the weak nuclear force, is responsible for certain types of nuclear reactions and has little bearing on energy sources today. Table 7 shows the four forces, the property on which each acts, and examples of each force. Gravitation and electromagnetism are the two forces with which we will be primarily concerned, as these are the two forces that operate at the macroscopic level of environmental systems. These also currently form the basis of our most prevalent sources for energy technologies. The strong nuclear force is the strongest of the forces. Nuclear fusion reactions on the surface of the sun are the result of the nuclear strong force. FORCE Gravitational RELEVANT PROPERTY Mass Electromagnetic Electric charge Strong Nuclear Isotopic spin* Weak Nuclear Spin* EXAMPLES Weight of object near a planet; force that keeps planets in their orbits around the sun Force that keeps an electron in its orbit around the atomic nucleus; (i.e., attraction or repulsion between a “charged” plastic comb and a strand of hair) Force that keeps protons and neutrons together in a nucleus Force responsible for certain types of nuclear reactions Table 7: The four fundamental forces (or, interactions) and the properties on which they act. *Spin and isotopic spin are properties of elementary particles that we will not define here. Energy System 16 Exercise: Find four “sources” of energy and identify the force responsible for the energy transformation. For example, a hydroelectric plant has a waterfall for its source, and the force responsible is gravitation. What fundamental force is responsible for (a) wind, (b) solar, (c) tidal, and (d) nuclear energy? Explain your reason. Every force that we experience belongs to one of these four categories, even when the connection is hard to see. The force of friction, for example, is an electromagnetic force. The force of the explosion of a chemical explosive is also electromagnetic in origin. The energy of an atomic bomb (more correctly, a nuclear bomb) is released as a result of the action of strong nuclear forces. Energy changes occur when matter changes position or matter changes state in the presence of these forces. What we call energy “production” is really energy transformation -- that is, energy is converted from a potential form to a form available to us for use. Each type of force acts on a specific property of an object. The specific property refers to the aspect of an object that is necessary for that object to be influenced by the force-- or “to feel” the force. For example, gravitation acts on the mass of an object. Strictly speaking, as an object fall towards the Earth, the Earth is falling towards the object. Because of the mass difference, the total effect of the Earth on the object is much more than that of the object on the Earth. However, the gravitational force on each kg of Earth is the same as on each kg in the object. In the case of the electromagnetic force, the object must have an electric charge. But if that object has no net electric charge, then an outside source of electromagnetic force cannot exert a force on that object. The space in which a force is felt is called the "field" of the force. Thus all objects on Earth are in Earth's gravitational field, the planets and moons are in the sun's gravitational field, as well as in their mutual gravitational field. Potential and Kinetic Energy Whenever we say that we are producing energy, what we really mean is that we are transforming energy from one form to another that is more usable. For example, water at the top of a waterfall has more gravitational potential energy than when is at the bottom of the waterfall, because the water at the top is further from the center of the Earth than at the bottom. So, if the water is allowed to fall from the top to the bottom, (that is, the Earth’s gravitational force does work on the water moving it), then the energy stored as potential energy at the top becomes transformed into the kinetic energy of this water and we can use it to do work. This is the principle behind the production of hydroelectric power. Exercise: In each of the following cases, trace the chain of energy transformations from the sun to the energy in its final form: a. b. c. A pot of water is boiled on an electric stove. A 100 nt automobile accelerates from rest on a level road, climbs a hill at constant speed, and comes to stop at a traffic light. A windmill pumps water out of a flooded field Potential energy, therefore, is the energy associated with different positions in the force field. The water at the top of a waterfall has higher gravitational potential energy than at the bottom because of the different positions in the gravitational field. Consider two points (A and B) in the Earth's gravitational field (g) where B is h meters higher than A. Then a mass (m) has a potential energy mgh higher than its potential energy at A. At a point 2h above A, the mass has a potential energy of 2 mgh. So height is a measure of the potential energy. Thus, an analogy with water and gravitational potential energy gives us a way to represent energy levels showing the potential energy state of a system in terms of horizontal lines. Thus we could say that the 100m point above the lowest level in a waterfall has 980 Joules gravitational potential energy per kg of water above the lowest point. m*g*h=E (Energy) 1 kg * 9.8m/sec2 * 56m = 549 J Energy System 17 Figure 12: ENERGY LEVELS DIAGRAM (gravitational). These formulas also demonstrate that potential energy is a representation of the position of a system in a field of force. The 1 kg of water in our example has higher potential energy when it is further away from the center of force (center of the Earth). At point A, the water is more "bound" (to the Earth) than at point C. We will use this idea later to draw the analogous levels to represent chemical potential energy. Science Notes: Energy and Chemical Stability Natural systems left to themselves move towards states of lower potential energy. For example, water flows down a hill or a ball rolls down a hill, if free to do so. States of lower potential energy are more stable. As a rule, the lower the potential energy of a system, the more stable it is. As a result, left to themselves, systems attempt to reach the configuration with the lowest energy possible under a given set of constraints. To change the state of a system from lower to higher potential energy, one must therefore supply energy to the system. less stable higher potential energy less bound ⇒ ⇒ ⇒ more stable lower potential energy more bound As more stable states have lower potential energy, we can get energy for use by moving a system to a lower potential energy. This is the basis for all energy transformation technologies. Chemical Stability Chemicals and their reactions are the medium through which nature stores and transforms energy. This energy is partly derived from the sun’s pure electromagnetic energy that reaches the Earth as solar radiation, and partly from the energy stored in chemicals as potential energy in the chemical bonds. Recall the food chain diagram from the Introduction. Photosynthesis is an example of how nature stores and transforms energy via chemical bonds. Chemical bonds are essentially the phenomenon that atoms of elements stay close to each other, forming a compound, because that puts them in a lower (more stable) state of total energy. These lower energy configurations of elements happen when the elements get an electronic configuration similar to the nearest inert gas. Electronic configurations of inert (noble) gases are the most stable in a given period (horizontal segment) of the Periodic Table. In fact, that is why these specific elements do not "need" to react with anything and are, therefore, chemically inert (or “noble”). These elements have no "need" to combine because their electron shells are completely filled with electrons. The noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Chemical activities of the elements are simplest to predict when they are close to the inert gases in the periodic table. Look at the first few periods of the Periodic Table in Figure 13. The activity can be described as a result of elements "wanting" to complete their shell. Although this is an anthropomorphic description, it is a helpful analogy. Energy System 18 Figure 13: First three periods in the Periodic Chart. Lithium and sodium would tend to "lose" one electron to become more stable (more like the closest inert element). They can do this, for example, by combining with elements that can gain stability by adding one electron to their shell -- elements such as F and Cl. These pairs therefore favor ionic bonds, in which the electron is actually transferred. When similar atoms such as H or O or N come together they can gain stability by sharing electrons in a covalent bond. Shared electrons spend time with either of the H atoms in H2 for equal amounts of time, for instance. In a case of a covalent bond such as those in H2O, however, the electrons spend more time on the average in the neighborhood of the oxygen. The water molecule, which has an angle of 105° between the two H-O bonds, is therefore a polar molecule, being more negative at the oxygen end of the molecule, because the negatively charged electrons spend more time near the oxygen atom. Nitrogen, hydrogen and oxygen atoms go to more stable configurations by forming the diatomic gases, N2, H2, and O2 respectively rather than remain in the atomic form: N, H, and O. When hydrogen atoms are produced in any reaction, pairs of these hydrogen atoms form covalent bonds with each other so that each has the helium (nearest inert gas) configuration at least a fraction of the time. Hydrogen has one electron and needs a total of two to be like He. So two hydrogen atoms share a pair of electrons, each belonging to one of the original atoms, thus forming H2. Schematically, it could be written as HxxH (x representing an electron). This schema is represented by H-H where the single line represents a bond consisting of two shared electrons. Again, the same can be said for oxygen and nitrogen. Oxygen has an atomic number of 8, and has four electrons in the outermost shell. It needs two more to be like Neon (nearest inert gas). EXERCISE: Nitrogen has __ electrons in the outer shell, needs __ to be more like _____ Carbon has __ electrons in the outer shell, needs __ to be more like _____ Oxygen has __ electrons in the outer shell, needs __ to be more like _____ Phosphorus has __ electrons in the outer shell, needs __ to be more like _____ Hydrogen has __ electron in the outer shell, needs __ to be more like _____ Following this logic, we can figure out the most frequent bond configurations for carbon, nitrogen, oxygen, phosphorus, hydrogen, with 4, 3, 2, 3 and 1 bonds respectively, as schematically in Figure 14. Figure 14: Shows the bond configurations for carbon, nitrogen, oxygen, phosphorus, and hydrogen. Energy System 19 Energy transformations using chemical sources consist of changing the mutual configurations of these compounds accompanied by the release of energy, which we can then use for something. Chemical bonds “contain” energy that may be released when the bonds are made. Representation of Bonds Each atom has a number of bonds coming out of it equal to the number of electrons it shares in covalent bonds. So the line of a bond represents two electrons in activity, one from each of the two atoms it bonds. Thus for H2, H-H is really H:H, with each hydrogen atom contributing one electron to the bond. Let's look at the example of nitrogen (atomic number = 7). Nitrogen, being 3 electrons short of its nearest inert gas (neon, atomic no. = 10), tries to bond so as to share 3 of it's electrons with other atoms bonding with it, in order to get a complete shell of 10 whenever possible. Thus it may share electrons with another N atom (forming N2), or with hydrogen (forming NH3). Figure 15: Representations of compound using the bond scheme. Similarly carbon can bond with four hydrogens to form CH4 (a gas called methane), or with two oxygen atoms to form CO2 as depicted above. Note that we always have four bonds coming out of carbon, one out of hydrogen, and one out of oxygen. Look at their position in the periodic table to see why this is so. Atoms in the middle of the Periodic Table and their bonding become more complicated, and we will not need to deal with them here. Carbon is the basis for all our life forms. It is a very versatile atom because of its capability to form four bonds. Depending on the amount of hydrogen available to bond, and the temperature and pressure conditions, carbon can form a plethora of compounds with hydrogen alone. One such family is the hydrocarbons, important in our context because they are the basis of fossil fuels. Note how some of these compounds have double and triple bonds between carbons. This happens when carbon and hydrogen combine under circumstances in which there is not enough hydrogen to satisfy all four bonds of each carbon. For example, if there is plenty of hydrogen to combine with carbon, we get CH4 or C2H6 (Ethane), with all single bonds. With less hydrogen we get C2H4 or C2H2 (less hydrogen for the same number of C atoms). C2H4 has a double bond between the carbons, and C2H2 has a triple bond. Compounds with double and triple bonds are called unsaturated, while single bond compounds like C2H6 are said to be saturated. Unsaturated compounds are more reactive than saturated compounds because not all the C atoms are bonded to four other atoms. Hydrocarbons are not the only compounds that can be unsaturated. Carbon monoxide is a good example of an unsaturated compound, "looking" for another oxygen atom to form CO2, a more saturated compound. When carbon (in coal or wood, for example) burns in an environment with insufficient oxygen, it forms CO which is deadly when breathed in. This is the reason to ensure plenty of access to fresh air when we have a fireplace or running car engine. Note that many representations are two-dimensional, and that in actuality, the electrons forming the bonds are distributed in three dimensions. In a compound like CH4, the carbon is in the middle of a tetrahedron with the 4 H atoms at the vectors. Energy System 20 Figure 16: Linear hydrocarbons. Similarly there can also be C3H8 (propane), C4H10 (butane), C5H12 (pentane) and so on. When a formula is written as CH4 just showing the proportion of atoms, it is called an empirical formula. When the bonds are shown as in Figure 15, it is called a structural formula. A single empirical formula may represent different compounds because the structures may be different for the same number of atoms combining. Try drawing propane, butane, and pentane. Note that there are always four bonds coming from carbon. The linear structures are called aliphatic hydrocarbons. In addition to the linear hydrocarbon molecules, hydrocarbons may also be formed into ring structures. The ring structure possesses the property that enables us to smell these compounds! So they are called aromatic hydrocarbons. The simplest aromatic hydrocarbon is C6H6, benzene. The structure of benzene was long a puzzle in chemistry, with chemists wondering what the structural formula for C6H6 could be. They knew the empirical formula was C6H6. It is said that the great organic chemist Kekulé, who had been wondering about this, dreamed one night of a snake swallowing its tail and was inspired to draw the ring structure! Note the alternating single and double bonds, a clever way of ensuring four bonds from each carbon shell. The versatility of carbon in forming bonds, ring structures and various configurations is the basis of life on our planet. The chemistry of carbon compounds is therefore called organic chemistry. More complicated carbons compounds are described in the Ecological System and Materials System. For now let us look at some additional aromatic and aliphatic compounds, and note some aspects that are relevant to energy storage and release. Aliphatic hydrocarbons are the basis of fossil fuels. All saturated hydrocarbons react with oxygen at high temperatures to form carbon dioxide and water, and give off energy. This oxidation reaction is the basis of the internal combustion engine. Gasoline normally contains hydrocarbons from C6 to C18, a mixture of over 100 compounds! An example reaction of the combustion of a hydrocarbon is: C7H16 + 11O2 ⇒ 7CO2 + 8H2O + energy "Burning" (or a combustion reaction) consists of combining with oxygen at high temperatures. The combustion reaction of acetylene (C2H2) with oxygen gives off such a large amount of energy that it is used as a welder's torch. Ring compounds do not play as large a role in energy production but often occur as byproducts or waste products. These polyaromatic hydrocarbons (PAH's) pose a serious pollution problem. Ring compounds, based on the benzene ring, are so common in biochemistry that we just draw to represent C6H6. Adding one more carbon and two hydrogens to the benzene ring gives us C7H8 which is methyl benzene or toluene: Ring compounds can get very complicated. Several organic compounds playing an important role in our physiology are shown in the unit on Ecological Systems. Energy System 21 Chemical Energy Release and Bond Energies The amount of energy released when a bond is formed between atoms is called the bond energy. Bond energies represent a state of potential chemical energy. We can get energy from a system as it moves from a state of higher potential energy to one of lower potential energy (e.g. water falling). Chemical reactions in which the compounds formed after a reaction (called products) have lower total bond energy than the reactants can release chemical energy. Such reactions in which energy is given off are called exothermic (or more correctly, exoergic) reactions. Conversely, reactions that absorb energy are said to be endothermic. Table 8 gives the energies for bonds we will commonly encounter. The table defines these energies in units of kcal/mole. A mole is an abbreviation for "gram-molecular weight" of a substance. BOND H-H C-H C-C C=C C C O-O O=O O-H C-O C=O Energy (kcal/mole) 104 99 83 146 200 35 119 111 86 177 BOND H-F H-Cl H-Br H-I N-N N N N-H CL-CL Br-Br I-I Energy (kcal/mole) 135 103 87 71 39 225 93 58 46 35 Table 8: Bond Energies. (the bond energy is expressed in kcal/mole.) Let us see what the energy values in Table 8 mean. The bond energy of H-H is 104 kcal/mole. This means that when hydrogen atoms combine to form molecular hydrogen H2, represented by the reaction H + H → H2, for every mole (2g) of H2 formed, 104 kcal of energy are released. Conversely it takes 104 kcal to break apart a mole (6 x 1023) of hydrogen molecules. From this we can draw a simple chemical energy level diagram for the above reactions, analogous to Figure 12 of gravitational potential energy. Figure 17: Energy Level Diagram of H2. One mole of an H2 (2g) molecule has 104 kcal total potential energy less than 2 g of H atoms. This is why when H atoms are formed in a reaction, and these atoms are the only atoms available, they combine to form H2 (roll down the potential energy "hill" towards a more stable state). In forming the H-H bond, 104 kcal of energy are released for every 2 g (6.02 x 1023 molecules) of hydrogen gas (H2) formed. Similarly oxygen, and nitrogen exist as O2 and N2 rather than in the atomic form as O and N. So whenever we say hydrogen, oxygen, or nitrogen gas, we mean H2, O2, N2. For H, O, N we specifically say atomic hydrogen, atomic oxygen, and atomic nitrogen. Science Notes: Chemical Formations Formation of Ammonia (NH3) as Example Let us look at a more complicated example of the formation of a molecule. Just as the energy released by water falling can be captured, we can find ways to capture chemical energy. Let us look at a more complicated reaction: the formation of ammonia. Nitrogen gas (N2) and hydrogen gas (H2) can be made to combine to form NH3, or ammonia gas. As we proceed, look for the answers to these questions: Is the reaction exothermic? How many kilograms of hydrogen are needed to produce one kilogram of ammonia? The reaction is N2 + 3H2 Energy System ⇒ 2NH3 22 This equation says that one mole of N2 requires three moles of H2 for a complete reaction, and this would then yield two moles of NH3. Note that we can also say that one molecule of N2 reacts with three molecules of H2 to yield two molecules of ammonia (NH3). But the table gives energies in units of kcal/mole, which is why it is easier to work with moles. The energy involved in the reaction involving just one or two molecules of ammonia is too small. The following table contains the variety of ways in which you can write the reaction that forms ammonia. All the descriptions below are equivalent. The atomic masses are rounded off values from the Periodic Table. Nitrogen + Hydrogen N2 N≡N + 3H2 3 H-H 1 mole of N2 28 g of N + + + 3 moles of H2 6 g of H ⇒ ⇒ ⇒ ⇒ ⇒ Ammonia 2NH3 2NH3 2 moles of ammonia 34 g ammonia What are the energies of the reactions? In order to get the molecular structure of ammonia, we have to break one N ≡ N bond and 3 H - H bonds. This requires that we supply energy to break the bonds. From Table 8, we get the bond energies: N≡N H-H N-H 225 kcal/mole 104 kcal/mole 94 kcal/mole To form each molecule of NH3, we break one bond of N2 and three of H2, and then 3 N - H bonds form to make NH3. To calculate energy released (or absorbed) in the reaction, we have to calculate the energy needed to break the bonds of N2 and H2, and the energy released when the atoms rearrange to form NH3. N2 3H2 : : Energy required to break the bonds of N2, 3H2 1 mole x 225 kcal/mole = 3 moles x 104 kcal/mole = total energy absorbed = 225 312 537 kcal 2NH3 : : Energy released forming the bond 2NH3 2 moles x (3 x 93 kcal/mole) = 2 moles x 279 kcal/mole = 558 total energy released = 558 kcal 558 kcal - 537 kcal = 21 net kcal released for 2 moles of NH2 formed, so the net energy released is 10.5 kcal/mole of ammonia formed. More energy is released than absorbed in the formation of ammonia, so this reaction is exothermic. We can also say that 10.5 kcal are released when 17g of ammonia are formed. The energy level diagram here is more complex than that for the H + H = H2 reaction because of the steps involved. Figure 18: Energy Level Diagram for the formation of Ammonia (NH3). For this reaction, we had to put in some energy to "activate" the reaction, which was the energy required to break the N2 and H2 bonds. N2 and H2 brought together with no addition of energy would not spontaneously react. This is analogous to our striking a match to start the burning of coal. The energy required to start the reaction is called activation energy. Then, left to themselves, the N and H form bonds to release 568 calories. What is a corresponding example with the gravitational force? Energy System 23 Formation of Water (H2O) as Example Hydrogen and oxygen combine to form water. Which is more stable -- hydrogen and oxygen gas separately, or in combination as water? Write equation and balance: 2H2 + O2 ⇒ 2H2O amounts molecules element element 2 molecule + 1 molecule oxygen hydrogen moles 2 moles + 1 mole oxygen hydrogen grams 4 g hydrogen + 32 g oxygen + kilograms 32 kg O2 4kg H2 1. Is the energy release - exothermic or endothermic? yields compound 2 molecules of water 2 moles of water yields yields 36g of water 36 kg of water yields 2. Which is more stable, hydrogen gas and oxygen gas separately or combined chemically as water? Explain. Structural formula: 2 H-H + O=O ⇒ 2 H-O-H (drawn as linear although molecule is not) Bonds: break old (spend energy), recombines to form new bonds (release energy) Break 2 H-H, break O=O and in recombination, 4 H-O bonds are made and release energy. Using the bond energies of H-H 104 kcal/mole, O-O 119 kcal/mole, and H-O 111kcal/mole from Table 8, we can see how much energy is released when bonds are broken and formed. BONDS BROKEN # of Energy Bonds bonds Required H-H 2 208 kcal O=O 1 119 kcal 327 kcal Total: required BONDS FORMED # of Energy Bonds bonds Released H-O 4 444 kcal Total: 444 kcal released Figure 19: Energy level diagram for formation of water. Energy released is greater than energy required to break bonds so there is a net energy release. The reaction is exothermic. The amount of energy released is 444 kcal minus 327 kcal which equals 117 kcal per mole of oxygen burnt or: • • • • • 444 kcal - 327 kcal = 117 kcal per mole of oxygen burnt 58.5 kcal/mole of hydrogen burnt or 58.5 kcal/mole water formed. 117 kcal for 36g of oxygen; OR 58.5 kcal per 2 g hydrogen; OR 58.5 kcal released when 18 g water formed Energy System 24 • • 58.5 x 1000 = 5,850 kcal of energy released when 18kg water formed 5,850/18 = 325 kcal per kg water formed. The energy released (or absorbed) per mole of the product is called "the heat of reaction." Thus the heat of reaction of water is above 58.5 kcal/mole (all the above statements equivalent) The energy released per unit mass may also be calculated in Joules. When we talk about released energy as the output of a power plant, Joules are the customary units; therefore it is often necessary to convert the energy from kcal to Joules. Kilocalories are units of heat. 4,190 kilojoules make 1 kilocalorie. So, multiply kilocalories by 4,190 to convert to kilojoules. This is necessary to convert heat (or chemical energy) units to work (or mechanical energy) units. Recall that these two systems of units evolved separately. Heat units were used by chemists and chemical engineers and mechanical units by physicists and mechanical engineers. 325 x 4,190 = 1,361,750 kJ of energy is released per kg water formed. This reaction can theoretically do 1,361,750 kJ of work. We usually get less useful work because of Second Law of Thermodynamics--some energy is lost as heat. EXERCISE: Chemical Bond: Elements form stable combinations In each of the following reactions: 1. 2. 3. 4. 5. name the product balance the reaction write the moles of each component say whether the reaction is endothermic or exothermic say whether the system is stable before or after the reaction Reaction 1. H2 + O2 ⇒ H2O 2. N2 + O2 ⇒ NO2 3. C + H2 ⇒ C2H6 4. C + H2 ⇒ C3H8 5. N2 + H2 Heat of Reaction (kcal/mole) -58.5 +21.6 -20.2 -24.8 -11.0 ⇒ NH3 The ( - ) sign means that there is a net energy release when the compound is formed; that is, the final product is lower in energy or the reaction is exothermic. Science Notes: Chemistry of Fossil Fuels Combustion and Energy Release The chemistry principles previously described can be used to describe the burning of methane (CH4, marsh or natural gas), or of carbon in coal. Combustion involves combinations of the fuel with oxygen. Thus, C + O2 ⇒ CO2 CH4 + 2O2 ⇒ CO2 + 2H2O We can show that these reactions release energy. The basic reaction of the burning of C is the basis of our largest energy source -- fossil fuels of various types, including coal, natural gas, and oil. Recall the energy in these bonds came originally form the solar energy captured by plants and then "processed" for millions of year (transformed over millions of years) under the pressure in the Earth. Burning of Coal Coal is mainly carbon, water, some hydrogen, and oxygen. There are many different kinds of coal. In addition to H and O, coal also contains some small amounts of nitrogen, sulfur, and some other minerals. Most of the carbon in coal is bound so that there is only one C-C bond for every C atom. Thus, for calculating the energy release of C + O2 ⇒ CO2 in the case of coal, one assumes only the breaking of one C-C bond. Following the previous example, C (1 mole) Energy System + O2 (1 mole) ⇒ CO2 (1 mole) 25 C-C + O=O ⇒ O=C=O Energy required to break bond: 83 kcal/mole + 119 kcal/mole = 202 kcal/mole Energy Released = 2 x 177 kcal/mole = 354 kcal/mole net release = 152 kcal/mole of carbon Thus 12 g of carbon yields 152 kcal of energy provided sufficient oxygen is available for complete combustion. 1 kg of carbon therefore gives approximately 11,000 kcal 152 kcal x 1000 g = 12666 kcal 12g As coal contains other ingredients, it works out that the active yield of 1 kg of coal is about 700 kcal. Byproducts from Coal Combustion As seen in the equation, CO2 is the main byproduct of coal combustion -- 44g of CO2 is produced for every 23g of C burnt. The contribution of CO2 to global climate change is one of the fundamental problems of our fossil fuel economy. Other products of coal burning originate from the sulfur and nitrogen present in coal. The nitrogen is usually released as N2 or NO2 gas. The sulfur forms SO2, which is one of the gases that causes acid rain (discussed in detail in the Atmospheric System). Insufficient oxygen supply during combustion -- as for example, burning coal (or wood) in a closed environment such as a room without adequate ventilation or a fireplace without a proper chimney -- produces carbon monoxide. As the oxygen is depleted, the reaction C + CO2 ⇒ 2CO becomes possible. Carbon monoxide is a colorless, odorless, and very poisonous gas. When breathing in CO, the CO takes the place of O2 in the hemoglobin molecules in the blood supply in the lungs, causing asphyxiation. Energy Use, Efficiency, and the Future Garrett Hardin who originated the idea of the Tragedy of the Commons, summarizes the two laws of Thermodynamics in terms of human significance as: "You can't win, you are sure to lose; and - you can't get out of the game."7 Whether we can get more "out of the game" is the central question of energy production. The examples above show what a small fraction of the input potential energy is actually used as output work. Designing for energy efficiency means in order to get a higher ratio of output, the input needs to become a central concern of intelligence design and practice. Most of the energy planning is done by looking at the supply side. We examine how we can increase the supply of the resource in question, rather than by asking how the demand side (all our uses of energy) can be managed. Energy availability and use are good indicators of the standard of living in our technological world. In the U.S. the "average consumption per capita" is 55 barrels of oil. In the poorer countries, the consumption is 6 barrels per year. Figure 20 shows the projections of world energy supplies from 1970-2020. The increased coal supply is based on mining coal that is harder (and hence more costly) to extract. Figure 20: World Energy Consumption by Fuel Type, 1970-2020. Sources: History: Energy Information Administration (EIA), Office of Energy Markets and End Use, International Statistics Database and International Energy Annual 1997, DOE/EIA-0219 (97) (Washington, DC, April 1999). Projections: EIA, World Energy Projection System (2000). 7 Hardin, Garret. Filters Against Folly: How to Survive Despite Economists, Ecologists, and the Merely Eloquent. Viking Press, 1985. Energy System 26 Demand-side management instead of or in addition to supply side management would mean a focus on increasing efficiency of use and considerations of how to reduce the demand. The CAFE (Corporate Average Fuel Economy) standards legislated in the U.S. in 1980's required a certain level of fuel efficiency of U.S. automobiles. By demanding that the corporations figure out an overall fuel rating for all their fleets, the decisions on design and distribution of big and small cars in the total fleet were left to the industry, as long as the total corporate fuel economy goals were met. There are also some efforts to recapture some of the "waste energy" from the processes of energy generation. Cogeneration described in Figure 21 is an example of industries working together to see how exchanges of energy and materials could minimize waste. Figure 21: Co-generation. An Example of "Waste Power" Use An unusual example of such a partnership network in Denmark is show in Figure 22. It is the result of 10 years of planning, and involves exchange of water, steam, gas, and gypsum. Figure 22: Industrial Ecosystem. from Allenby and Graedel, "Defining the Environmentally Responsible Facility." Measures of Environmental Performance and Ecosystem Condition. National Academy Press: Washington, D.C. Energy System 27 Four companies (a power plant, a refinery, a gypsum facility for producing wall board, and a pharmaceutical plant) effect the exchange shown in Figure 22. The "waste" products from the power station (including heat in the form of warm water) are used to warm the greenhouse and other facilities. Such a co-generation system provides an industrial ecosystem with a much higher efficiency for overall energy use than if any of the organizations had organized independently for their material and energy needs. The ten years of planning required shows that such processes take time to explore the possibilities, develop the relationships, then plan and execute. Energy Sources, Technologies, and Impacts Historical, geographical, and political contexts have led to the adoption of different fuels and related technologies to produce energy. As described in the history section, we have progressed from using above ground, easily accessible sources of energy, such as wood and direct solar energy, to fuels such as coal and oil that require large infrastructures and energy to mine and process before extracting energy from them. Table 9 at the end of this section outlines different energy sources and the information relevant to the environmental impact of these sources. As described before, over 95% of the world's energy requirement is currently met by fossil fuels -- coal, oil, and natural gas. In various technologies, they release energy by the process of combustion. Major byproducts are carbon dioxide and various residuals such as fly ash. The environmental problems relating to fossil fuel use are described in detail in the Atmospheric System. Environmental pollution, especially air, global climate change, and resource depletion are the greatest drawbacks of heavy fossil fuel use. Another problem (particularly for the U.S.) is dependence on foreign resources. Development of oil in the Atlantic has been a response to the U.S. need for fuel independence. Alaskan oil exploration involves destruction of pristine land and unique natural habitats. Coal burning, while a simple technology, has numerous side effects in addition to carbon dioxide emission noted above. Coal occurs in combination with sulfur in many places. High sulfur coal, when burned, produces sulfur dioxide, which is the source of acid rain. Countries like the U.S. regulate the amount of sulfur that can be in coal used for power production; therefore high sulfur coal must be cleaned before it can be burned. Coal burning also produces large amounts of particulates in the form of fly or bottom ash, which must be disposed of or recycled. Currently, coal power plants with the encouragement of government and to the liking of environmental groups, are developing ways to burn coal and reduce the large amount of coal byproducts. Coal-gasification is one such technology. Coal is pulverized, mixed with water and then combined with gases such as nitrogen and oxygen. The gaseous mixture is then heated and a synthetic gas is produced as the particulates or ash falls to the bottom of the burner. As the gas cools, sulfur particulates are separated and used to make sulfuric acid. This is an example of getting more than one product from a fuel source. The gas is then used to produce steam that spins turbines and generates electricity. The energy in the bonds within the nucleus may be released through nuclear fission or nuclear fusion reactions. The technology used to produce nuclear power is based on nuclear fission. The fuel for fission reactions are heavy nuclei, particularly uranium, thorium, and plutonium (a material that no longer occurs in nature, but of which the U.S. and states of the former Soviet Union have enormous supplies because of the bomb programs). The U.S. led the world in nuclear power production, producing about 728 billion kilowatt-hours. France ranked second, with 375 billion kilowatt-hours, and Japan was third, at about 309 billion kilowatt-hours produced.8 In 1999, the nuclear share of total electricity generation for France was 75%, for Japan was 33%, and for the U.S. was 20%.9 Nuclear energy is often considered the desirable alternative to coal, because it does not release carbon dioxide. The materials involved in nuclear power are, however, heavily radioactive varying from uranium, the starting material, to the various byproducts during all phases of the energy production cycle, and the last byproduct, generally termed "radioactive waste." These byproducts are "radioactive," that is, they emit particles of radiation and high-energy electromagnetic radiation, such as gamma rays. This quality makes the materials dangerous. People and other parts of nature exposed to this radiation can suffer serious long-term damage. Many of the radioactive materials are also very long-lived, continuing to emit radiation for hundreds of years or more. Nuclear fission power technologies have been designed with numerous safeguards and extreme caution as to be "safe." However, if the global economy were to depend predominantly on nuclear power, radioactive material transport over air, land, and water could pose a very large exposure risk. Radioactive waste disposal is also a challenging problem, as it has to be kept isolated for thousands to millions of years! Nuclear fusion is the reaction responsible for the production of energy in the sun. Hydrogen is the main fuel for energy source. In fact, the sun is a huge nuclear fusion reactor. But the extreme high temperature and pressure needed for fusion to take place has been a formidable obstacle to designing fusion systems on any usable scale. Nuclear fusion would not produce many radioactive wastes like fission but will produce radioactive tritium (an isotope of hydrogen) for which we would have to design safeguards. 8 Source: Energy Information Administration, International Energy Annual 1999, DOE/EIA-0219(99) (Washington, DC, January 2001.) Source: Energy Information Administration, International Energy Outlook 2001, DOE/EIA-0484(2001) (Washington, DC, March 2001.) Energy System 9 28 Hydropower, wind, direct solar radiation, and geothermal power are all renewable resources. Of these, hydropower is the best developed. Starting with water wheels that converted the kinetic energy of running water into various kinds of motion, to vast projects like the Hoover Dam, the technology for conversion of hydropower to electricity has long been explored. While it produces no byproducts, hydroelectric power requires waterfalls or dams with a large volume of flow. In the case of dams built for hydropower, the devastation of land and distinctive ecological niches can be large. The conflict between development of cheap hydroelectricity, preservation of habitat, and economic interests are encapsulated in the example of the endangerment of salmon in the Pacific Northwest, due to extensive dam building on the Columbia River. Hydropower can also come from hot water springs, where the kinetic energy of water comes from the heat at the Earth's core. The Sun and Energy Except for nuclear energy, geothermal energy from the Earth's hot core, and energy from running water accelerated by the Earth's gravitation naturally (waterfalls) or artificially (dams), all other energy on the Earth comes from the sun. The sun's energy also plays a principal role in hydropower by driving the water cycle. The nature of solar radiation -- electromagnetic energy from the sun -- is described in detail in the Atmospheric System. YOU ARE HERE On average, one square meter on the side of the Earth facing the sun receives 1400 W (Joules per second). In a 24-hour period the total amount of energy reaching the upper atmosphere is 14.4 million calories. One-third of it is reflected back into space by cloud cover, and the rest, traveling through the atmosphere, powers the wind and water cycle, and drives the Earth's climate. The total sunshine entering our atmosphere every year is equivalent to 500,000 billion barrels of oil or 800,000 billion metric tons of coal! On a bright sunny day in the northern latitudes, when the sun is at the highest point, about 1000 Watts/ m2 reaches the ground. On cloudy days, it can be as low as 200 Watts/m2. The main problem with solar energy for high levels of use comes from the fact that it is so diffuse and spread out, and has to be collected over large areas. In effect, this is what the foliage of plants does, storing some of the energy through photosynthesis. It is important to note that only a small fraction of the solar spectrum -- in the violet and some in the red region -- is used for photosynthesis. Sunlight has a large quantity of energy in the green and yellow regions, most of which is reflected by the leaves. However, it is the capture of energy through photosynthesis, combined with elements like carbon, oxygen, and hydrogen from the Earth and its atmosphere, which results in biomass immediately. This biomass eventually results in a favorite fuel of today (coal) after millions of years of "processing" by the Earth. Oil and natural gas are similarly produced from organisms buried for millions of years under rock foundations. Ancient humans used the gentle, spread out solar energy and biomass for drying, cooking, and heating. Today's needs demand much larger quantities concentrated in space and time. This tendency promotes rapid depletion of the solar "capital" invested into coal formation over millions of years. One of the less thoughtful energy uses in our convenience-dominated society is the use of "high-quality" and concentrated energy even when "low-quality" spread-out energy would suffice. A good example is our use of fossil fuel energy driven clothes dryers, even in the summer when clothes could just dry on a clothesline from direct solar energy. In the ideal use of energy, we would distinguish between the needs requiring high or low "quality" energy. Using energy at the appropriate level and from renewable resources is what is referred to by Amory Lovins as a "soft energy path." One soft technology philosophy argues that we adapt our life styles to suit the energy available to us. Figure 23 is derived from the proceedings of UNERG, the United Nations Conference on New and Renewable Sources of Energy held in Nairobi, Kenya, in 1981. The figure shows the different grades of energy derived from direct solar energy. Passive collectors are static and collect heat energy that falls on them. Active collection involves mechanisms for storing and/or following the direction of the sun's rays to receive the maximum energy. Many ancient civilizations, including the Egyptians, Pueblo and Anastasi Indians, and Greeks, built their houses to take maximum advantage of the sun's apparent movement in the sky. However, as more technological energy systems developed, building with the sun's position in mind became a lost art, especially in industrialized countries. Energy System 29 Figure 23: Sun-trapping options. Source: UNERG, but slightly modified. Photon collection refers to collecting the energy through natural or artificial chemicals -- in the form of biomass (firewood, animal dung, and waste materials) or in water or chemicals such as certain salts, which hold the heat. To convert solar radiation into electricity, we use photovoltaic cells. Photovoltaic cells are based on the phenomenon of photoelectricity -- that light can release electrons from certain materials. Using these materials, light can be converted directly into electricity. This phenomenon was discovered in the late 1800's by George May in Ireland. Rudolf Hertz in Germany produced the first photoelectric cell soon thereafter using the element selenium and Albert Einstein explained the physics of photoelectricity in 1905. However, the first practical solar cells were only developed in the 1950's by the Bell Telephone Company. The early version of the cell cost thousands of dollars per watt of electricity yielded. The first large scale testing occurred in space on the NASA satellite Vanguard in 1958. The conversion efficiency (amount of electrical energy output per input of light energy) of the largest photoelectric cells is still below 20%. This means we have to collect sunlight over a large area for any useful application. For example, if the conversion efficiency is 15%, we need a 6.5 square feet of photovoltaic material to power a 100-watt light bulb. Solar technologies have made the largest inroads in space applications. Collection of solar energy by satellites for Earth's applications have been long considered. The basic idea is that if the collection were in a geosynchronous orbit around the Earth (35,890 km or about 22,500 miles above the equator), we could capture the energy before so much of it was absorbed by clouds and the atmosphere. Various technical difficulties have essentially halted this SPS (Solar Power Satellite) project. If we followed a philosophy of ecologically friendly design, the best use of solar would be in the passive or active collection, rather than the conversion to electricity. However, as a society, we have chosen to use electricity as the form of energy for almost all applications and a large source of our environmental problems -- pollution, resource depletion, habitat loss -- lies in this societal choice. Wind is derived from solar energy moving large masses of air. The two basic phenomena that are responsible for wind patterns are a large global circulation and local effects. Cool polar air is drawn towards the tropics to replace lighter, warmer air that rises and moves towards the poles. This creates areas of high and low pressure and circulation patterns are set up differently in the northern and southern hemispheres because of the Earth's rotation. This sets up the global patterns, such as the trade winds. Locally, the circulation depends upon whether the air is over land or water. Air over oceans and large bodies of water is cooler than that over land, and cooler air is drawn toward the land as the warmed air rises. Together these two patterns produce movements of enormous complexity. The wind represents kinetic energy of air arising from the thermal energy of sunlight. A large part of this energy is lost in various functional forces, and only a small portion can be captured by windmills. High tech windmill designs have been developed by various aircraft companies, because of their expertise in wind dynamics. Energy System 30 In summary, the sun it still our most valuable source, powering most of our energy sources. Our survival may depend upon a wise and judicious use of the numerous, versatile sources the sun provides. Life Cycle of Electricity Generation Electricity is a form of energy that has become the core of our industrial societies. The ease with which it can be transported, stored, controlled, and used has changed the fabric of society. This section looks at the generation of electricity from various common fuel sources. Electrical energy is the combined potential and kinetic energy of electrons in materials. Materials that have electrons that are mobile, rather than being confined to orbits around the atomic nucleus, can conduct electricity. Metals are prime examples of conductors. A discovery by Michael Faraday of England in 1831 is the cornerstone of our large-scale electricity generation. Faraday discovered that when a conductor moves in a magnetic field, an electric current is produced in the conductor. This Faraday's Law, and the fact that we can make large magnets is the basis of an electric generator. If we can use an energy source to move a conducting coil of wire that is placed in a magnetic field, the current produced in the wire can then be transported to deliver electric energy. Figures 24.A-D demonstrate the sequence of electric power production and distribution from four sources: running water, coal, nuclear fission, and wind. Energy System 31 Figure 24.A-D: Electricity Generation Methods A power plant consists essentially of a huge generator -- a gigantic coil of wire capable of rotating in the space between the poles of an enormous horseshoe magnet. The motion of the coil is caused by a shaft connecting it to a turbine, whose rotation spins the coil. In all power plants then, the energy obtained from the sources has to cause the turbines to turn, which then rotates the coil. This energy is delivered directly to the turbines by the falling water in a hydroelectric power plant, and by the wind in a wind farm. In the case of coal and nuclear fission, the primary energy is used to transform water into steam or high-pressure water, which then drives the turbines. Figures 24.A-D illustrate the steps prior to this, and show the similarity of the final steps of electricity distribution for all sources. The current from the coil is then carried to the final place of use through transmission and distribution lines. Some of the energy is lost along the wires. To minimize this loss, the electricity is transmitted at very high voltages (thousands of volts) along transmission lines and the voltage "stepped down" near the location of use using transformers. Electricity is then carried over smaller distribution lines to homes and businesses for use. The huge steel towers typical of transmission lines are a familiar sight, as are the distribution lines -- the smaller wires near buildings attached to "telephone poles." Transformers are the ceramic structures on local distribution line poles. Impacts of Energy Production and Use Energy production and use produce some of the most lasting and significant environmental effects. Some of these are discussed in detail in the Atmospheric System. Each source of energy brings with it some impacts. Here we summarize the overall nature of the impacts. Fossil fuels cause some of the largest impacts. In order for a typical 500-Megawatt plant to produce about 158 Terawatthours (tera = 1012) of electricity per year, it takes 1.5 million tons of coal and 0.15 million tons of limestone. It produces emissions to the air of 1 million tons of carbon as carbon dioxide, plus 10000 tons of ash and 193000 tons of scrubber sludge -- both of which contain large quantities of sulfur. (check numbers) Global climate change, resulting from atmosphere increases in CO2, is described in detail in the Atmospheric System. Even seemingly slight temperature changes can cause changes in weather patterns, climate, melting of polar ice caps, and sea-level rise. Energy System 32 Figure 25: Industry Emissions, in thousands of short tons. Source: Energy Information Agency (http://www.eia.doe.gov/neic/brochure/elecinfocard.html) Gasoline combustion releases pollutants that, under certain conditions, give rise to photochemical smog and high levels of atmospheric ozone. Impacts from oil drilling include destruction of ecosystems. The many impacts of extensive fossil fuel use is discussed in detail in the Atmospheric System. From the mining and processing of the fuels to the production stage, nuclear power requires the handling of radioactive material. The potential for accidental release of these materials and exposure to people, and the problem of long-term disposal of radioactive wastes, are the main environmental concerns of nuclear power. Hydroelectric power causes disturbances in ecosystems from dams and large land use. A striking example of the loss of biodiversity is the rapidly declining populations in the remaining species of salmon in the Pacific Northwest. The Three Gorges Dam project currently underway in China requires the displacement of one million people, in addition to the devastation of land and ecosystems. But the People's Republic of China has made rapid industrialization a national priority, and this requires an enormous development of power production systems. Alternative energy sources, such a wind and solar energy, also have large land use implications. Energy System 33 Energy Source Force of Origin Energy production Oil, Petroleum Electromagnetic forces in atomic bonds Non renewable Natural Gas Coal Biomass: Wood and organic waste including societal waste Hydroelectric Electromagnetic forces in atomic bonds Electromagnetic forces in atomic bonds Electromagnetic forces in atomic bonds Gravitational force of water • 38% of world's consumption in 2000 • Easily transported • Large portion in transportation industry • 20% of world's consumption in 2000 • Flexible for use in industries, transportation, power generation Non renewable Refining and consuming produce air, water, and solid waste pollutants Produces fewer pollutants than oil and coal, and less CO2 Primary resource for electricity Produces CO2 and other air, water and solid waste pollutants • Renewable • In terms of timber, it is easily harvested and abundant in certain areas; but it takes a long time to grow a tree. Low energy potential relative to other resources • Burning emits CO2 and other pollutants • Possible toxic byproducts from societal waste • Loss of habitat when trees harvested, unless sustainable tree farms Low economic cost, though high start up costs Destruction of farmlands, dislocation of people, loss of habitat, alteration of stream flows • Renewable • Clean resource with high efficiency • Influenced by climate and geography • Renewable • High economic cost particularly in terms of startup • Dependent on climate and geographical location • Need a storage system for the energy to ensure reliability • Not advanced enough for global use • Renewable • Central-thermal systems to convert solar energy directly to heat • More competitive economically than photovoltaics • Dependent on climate and geographical location • Extracts heat from underground masses of hot rock. • Technology is still undeveloped. • Can be geographically dependent Electromagnetic energy from the sun Solar Power (solar thermal) Electromagnetic energy from the sun Geothermal Gravitational pressure and nuclear reactions in the Earth's core Wind Power Gravitational & electromagnetic energy from the sun • Renewable • Unlimited resource that is a very clean process, no pollutants Strong nuclear forces in nuclear bonds • Non renewable resource U235 (uranium) • Highly technological infrastructure necessary for safe operation • Production of nuclear energy has a high cost due in part to regulations • High water usage for cooling Weak nuclear • Technology is not yet viable and requires research investment • Technology still not developed enough to make this a viable source Nuclear Fusion Environmental Impact Non renewable Solar Power (photovoltaics) Nuclear Fission Usage • Technology is already in use for remote applications and noncentralized uses where it is economically competitive with alternatives • Unlimited resource that is clean, efficient, safe, and renewable • Solar energy technology not advanced enough for global use • Many industrial plants use solar • Consumption is localized • Efficient • Economic cost comparable to current technologies • System must be designed to operate reliably at variable rotor speeds • Technology not advanced enough for global societal us Currently accounts for 10-12% of the world’s electricity Disrupts natural geyser activity Aesthetic issues, needs lots of land, and possible bird impacts • Byproduct is highly radioactive and highly toxic • Produces radioactive wastes that have a long lifetime • Disposal solution complex technically and politically • Safety issues in terms of operating a facility with the potential to release radiation to the atmosphere • Public perception problem in terms of radiation, etc. Possibility high for water pollution because of radioactive tritium Table 9: Energy Sources and related information. Energy System 34