approx. bond angle
advertisement

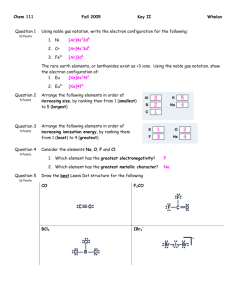
Los Angeles City College Chemistry 60 Bonding Practice Problems with ANSWERS 1. Consider each of the following ions/molecules shown below and write your final structures in the boxes provided: A. B. C. D. Beginning with the best Lewis dot structure, use VSEPR theory to draw each of the listed species, including resonance where appropriate. Don’t forget to include lone pairs! Describe the geometry about EACH central atom. Include both electronic and molecular geometries. Give the approximate bond angle(s) around each central atom. Determine whether the overall species is polar or nonpolar. i. ii. POCl3 ClO3+ Structure #1 iii. iv. SCNSbBr3 Structure #2 electronic geometry: electronic geometry: molecular geometry: molecular geometry: approx. bond angle: approx. bond angle: polar or nonpolar? polar or nonpolar? Structure #3 Structure #4 electronic geometry: electronic geometry: molecular geometry: molecular geometry: approx. bond angle: approx. bond angle: polar or nonpolar? polar or nonpolar? 2. Use VSEPR theory to draw the two structural isomers for C2H6S. Which isomers are polar/nonpolar? Make sure to clearly label your structures. 3. Consider the protein aspartame shown below, a common artificial sweetener derived from aspartic acid and phenylalanine: H C H O H B. C. D. CH NH2 CH C CH H2C C C C O A. O H HC C H CH O N C H O H C H H Use an arrow labeled “A” (A →) to identify ONE central atom with a bent geometry and an approximate bond angle of 109.5º. Use an arrow labeled “B” (B →) to identify ONE central atom with a trigonal pyramidal geometry and an approximate bond angle of 109.5º. Use an arrow labeled “C” (C →) to identify ONE central atom with a trigonal planar geometry and an approximate bond angle of 120º. Does this particular protein exhibit resonance? Briefly explain why or why not. 4. Consider each of the following ions/molecules shown below and write your final structures in the boxes provided: A. B. C. D. Beginning with the best Lewis dot structure, use VSEPR theory to draw each of the listed species, including resonance where appropriate. Don’t forget to include lone pairs! Describe the geometry about EACH central atom. Include both electronic and molecular geometries. Give the approximate bond angle(s) around each central atom. Determine whether the overall species is polar or nonpolar. i. ii. SOCl2 HCHO Structure #1 iii. iv. OCNHONO2 Structure #2 electronic geometry: electronic geometry: molecular geometry: molecular geometry: approx. bond angle: approx. bond angle: polar or nonpolar? polar or nonpolar? Structure #3 Structure #4 electronic geometry: electronic geometry: molecular geometry: molecular geometry: approx. bond angle: approx. bond angle: polar or nonpolar? polar or nonpolar? For Questions 5 – 7, reference the structure of Vitamin E below: H2 C H C HO C C HC C CH3 H2 C CH O C H 5. CH2 C H2 CH3 H2 C CH C H2 CH3 H2 C CH C H2 C H2 C H2 CH C H2 CH3 Based on your knowledge of VSEPR, which of the following geometries is NOT present in Vitamin E? A. B. C. D. E. trigonal planar tetrahedral bent trigonal pyramidal All of the above are present in Vitamin E. 6. Select “A” for True OR “B” for False regarding the following statement: Vitamin E does not possess any resonance. 7. Select “A” for True OR “B” for False regarding the following statement: Vitamin E is a slightly polar molecule although predominantly nonpolar. 8. Consider the INCOMPLETE Lewis structure for the naphthalene molecule (C10H8) shown below. All the observed C-C bond lengths in the molecule are known to be intermediate between C-C single and C=C double bonds. Explain, making sure to complete the resonance representation shown below. H H H H C C C H C C C H H C C C C H H H H H C C H C C C C C C C C H H H ANSWERS 1. Note that terminal atoms are assumed to have an octet of electrons (excluding hydrogen) – omitted for the sake of clarity! O P Cl Cl O O O Cl Cl Cl O O O O Cl Structure #1 O O Structure #2 electronic geometry: tetrahedral electronic geometry: trigonal planar molecular geometry: tetrahedral molecular geometry: trigonal planar approx. bond angle: 109.5 degrees approx. bond angle: 120 degrees polar or nonpolar? polar polar or nonpolar? ionic (nonpolar) S C N S S C C N Sb N Br Structure #3 Br Br Structure #4 electronic geometry: linear electronic geometry: tetrahedral molecular geometry: linear molecular geometry: trigonal pyramidal approx. bond angle: 180 degrees approx. bond angle: 109.5 degrees polar or nonpolar? polar polar or nonpolar? polar 2. Structural isomers are molecules with the same chemical formula but different connectivity of atoms. Consider the two structures below: H H H C C H S H Polar H H S H C and H C H H Polar H 3. Consider the protein aspartame shown below, a common artificial sweetener derived from aspartic acid and phenylalanine: H C all carbons in ring are "C" HC A C CH O H B B. C. D. H O H2C C C C C C O A. CH CH NH2 CH CH A C H O N C H O H C H H B Use an arrow labeled “A” (A →) to identify ONE central atom with a bent geometry and an approximate bond angle of 109.5º. see above Use an arrow labeled “B” (B →) to identify ONE central atom with a trigonal pyramidal geometry and an approximate bond angle of 109.5º. see above Use an arrow labeled “C” (C →) to identify ONE central atom with a trigonal planar geometry and an approximate bond angle of 120º. see above Does this particular protein exhibit resonance? Briefly explain why or why not. Yes, one example is circled above. Electrons can be delocalized throughout the protein via single/double bonds. 4. Note that terminal atoms are assumed to have an octet of electrons (excluding hydrogen) – omitted for the sake of clarity! O S O Cl H Cl Structure #1 C H Structure #2 electronic geometry: tetrahedral electronic geometry: trigonal planar molecular geometry: trigonal pyramidal molecular geometry: trigonal planar approx. bond angle: 109.5 degrees approx. bond angle: 120 degrees polar or nonpolar? polar polar or nonpolar? polar O C N O C N H O O C N N O Structure #3 O H O N O O Structure #4 electronic geometry: linear electronic geometry: (O) tetrahedral; (N) trigonal planar molecular geometry: (O) bent; (N) trigonal planar approx. bond angle: (O) 109.5 degrees; (N) 120 degrees polar or nonpolar? polar molecular geometry: linear approx. bond angle: 180 degrees polar or nonpolar? polar For Questions 5 – 7, reference the structure of Vitamin E below: C C HC C C H 5. H2 C H C HO CH3 CH2 H2 C CH O C H2 CH3 H2 C CH C H2 C H2 CH3 H2 C CH C H2 C H2 CH C H2 CH3 Based on your knowledge of VSEPR, which of the following geometries is NOT present in Vitamin E? A. B. trigonal planar tetrahedral C. D. E. bent trigonal pyramidal All of the above are present in Vitamin E. 6. Select “A” for True OR “B” for False regarding the following statement: Vitamin E does not possess any resonance. 7. Select “A” for True OR “B” for False regarding the following statement: Vitamin E is a slightly polar molecule although predominantly nonpolar. 8. Consider the INCOMPLETE Lewis structure for the naphthalene molecule (C10H8) shown below. All the observed C-C bond lengths in the molecule are known to be intermediate between C-C single and C=C double bonds. Explain, making sure to complete the resonance representation shown below. H H H H C C H H H H C C H C C C C C C C C C C C C C C H H H H C C H H H The resonance structures shown above depict the delocalization of the carbon double bonds, giving each carbon atom both single/double bond character. As a result, the observed C-C bond lengths in the molecule are known to be intermediate between C-C single and C=C double bonds.