Hydrogen Atom in a Constant Electric Field
advertisement

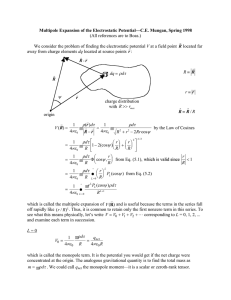
Hydrogen Atom in a Constant Electric Field Preliminaries The classical energy of this system is the sum of the kinetic energies of both particles, their Coulomb attraction and the energy due to the external field. E= 1 e2 r 1 r r r mN r&N2 + me r&e2 − r r + eφ (rN ) − eφ (re ) 2 2 4πε 0 re − rN r r r The external field is given by F = −∇φ . Lets choose F to be along the z axis, F = Fzˆ , r and since its constant we have φ ( r ) = φ ( 0 ) − Fz . We choose φ ( 0 ) = 0 with no loss of generality since it simply shifts the zero of energy. The energy due to the external field is then r r eφ ( rN ) − eφ ( re ) = −ez N F + eze F = eF ( ze − z N ) We can separate the center of mass motion from the internal motion in the usual way r r r r r r r = re − rn and R = (me re + mN rN ) / mt where mt = me + mn . From these we get r m r r r r m r rN = R − e r & re = R + N r mt mt and the classical energy becomes 1 r& 2 1 r& 2 e2 mt R + μ r − + eFz 2 2 4πε 0 r mm where μ = e N is the reduced mass. The center of mass energy is me + mN E= Ecm = 1 r& 2 mt R 2 while the internal or energy due to the relative motion is Eint = 1 r& 2 e2 μr − + eFz 2 4πε 0 r The Hamiltonian for the two particles is then J. F. Harrison Michigan State University 1/24/2008 1 rˆ rˆ Pcm2 Pint2 Ze 2 ˆ ˆ ˆ + − + eFz H = H cm + H int = 2mt 2 μ 4πε 0 r The Schrodinger equation is then Ĥ Ψ = E Ψ r r r r and if we write Ψ ( R, r ) = φ ( R )ψ ( r ) the eigenvalue E can be written as the sum of the center of mass energy and the internal energy, E = Ecm + Eint where r r Hˆ cmφcm ( R) = Ecmφcm ( R) r r & Hˆ intψ int (r ) = Eintψ int (r ) where h2 2 Hˆ cm = − ∇ cm 2mt and h2 2 e2 ∇ − + eFz Hˆ int = − 2μ 4πε 0 r Note that since the system is neutral the center of mass Hamiltonian is not affected by the external field. We will concentrate on the internal motion. Additionally we will use atomic units so the Hamiltonian becomes 1 1 1 1 Hˆ = − ∇ 2 − + Fz = − ∇ 2 − + Fr cos θ 2 r 2 r where we have approximated μ = me ≈ me which in atomic units is 1. Note that this me 1+ mp Hamiltonian commutes with L̂z and so its eigenfunctions must have the form Ψ( r,θ ,φ ; F ) = ψ ( r,θ ; F ) eimφ . Since we will be interested in the energy of the ground 2π state in the field and since the ground state in the absence of the filed has m=0 we will look for solutions of the form ψ ( r,θ ; F ) . J. F. Harrison Michigan State University 1/24/2008 2 Ground State Exact solution of the perturbation equations The first order correction to the wavefunction satisfies the equation ( ) ⎛ ˆ 0 1 ⎞ (1) (1) ( 0) ⎜ H + 2 ⎟ Ψ100 + Fz − E100 Ψ100 = 0 ⎝ ⎠ 0 = where Ψ100 1 π e−r and we have recognized that the ground state energy in au is − 1 2 . The first order correction to the energy is given by () 0 0 E100 = Ψ100 Fz Ψ100 =0 1 and is zero by symmetry. Because the perturbed wave function has the form ψ ( r,θ ; F ) it ∞ θ ) where P(cos θ ) is a Legendre can be written as ψ ( r,θ ; F ) = ∑ Rl ( r,F )P(cos l l l =0 polynomial which suggests that we look for a solution of the ∞ (1) = ∑ fl ( r,F )P(cos θ ) , where we have excluded l=0 so that the first order form Ψ100 l l =1 correction is orthogonal to the ground state. Recognizing that Ĥ 0 = − L̂2 1 1 ∂ 2 ∂ + − and L̂2 Pl = l( l + 1 )Pl r 2r 2 ∂r ∂r 2r 2 r (1) and inserting the ansatz for Ψ100 into the above equation results in ∞ ⎛ 1 ∂ 2 ∂f l l( l + 1 ) fl fl f l ⎞ 0 r =0 + − + ⎟ + FrP1Ψ100 2 2r 2 r 2⎠ ∂r ∂r ∑ P ⎜⎝ − 2r l =1 l from which we see that only the l=1 term contributes and so − 1 d ⎛ 2 df ⎞ f f f rFe− r r + − + + = 0 , where we have dropped the subscript on f. ⎜ ⎟ 2r 2 dr ⎝ dr ⎠ r 2 r 2 π This form also suggests that we make the substitution f ( r ) = g ( r ) e− r which results in J. F. Harrison Michigan State University 1/24/2008 3 d 2g ⎛ 1 ⎞ dg 2 g 2 Fr − 2 ⎜1 − ⎟ − 2 − =0 2 dr π ⎝ r ⎠ dr r If we try a power series solution ∞ g ( r ) = ∑ an r n we find g ( r ) = n =0 − Fr ⎛ r ⎞ ⎜1 + ⎟ π ⎝ 2⎠ we obtain ⎛ r2 ⎞ (1) 0 Ψ100 = − F ⎜ r + ⎟ P1 ( cos θ ) Ψ100 2 ⎝ ⎠ The second order correction to the energy is given by ( ) () 0 = Ψ100 =F E100 Fr cos θ Ψ100 2 1 4π (1) 0 . Ψ100 rY10 Ψ100 3 The angular integrations are immediate with the resulting radial integral ( 2) E100 ∞ 4 2 ⎛ 4 r 5 ⎞ −2 r 9 = − F ∫ ⎜ r + ⎟e dr = − F 2 and if we write this in terms of the 4 3 0⎝ 2⎠ 1 9 9 ( 2) polarizability α , we have E100 = − α F 2 = − F 2 and α = in atomic units. What is it 2 4 2 in the SI system? Energy and electric filed in the SI and au systems are related by ⎛ e2 ⎞ ⎛ e2 ⎞ 1 and = ESI = ⎜ E F F so if ESI = − α SI FSI2 then ⎟ au ⎜ SI 2 ⎟ au 2 ⎝ 4πε 0 a0 ⎠ ⎝ 4πε 0 a0 ⎠ 2 ⎛ e2 ⎞ 1 e ⎞ 2 ⎛ and so ESI ⎜ ⎟ Eau = − α SI Fau ⎜ 2 ⎟ a a πε πε 4 2 4 0 0 ⎠ 0 0 ⎠ ⎝ ⎝ α SI is usually reported and is called the polarizability 4πε 0 volume. Note α au a03 = 0.1481847 x10−30 α au m3 . The polarizability volume of the H atom in α the SI system is SI = 0.1481847 x10−30 9 m3 . 2 4πε 0 α SI = α au 4πε 0 a03 . The quantity ( ) We can determine the energy to higher orders and the second order correction to the wavefunction satisfies the differential equation J. F. Harrison Michigan State University 1/24/2008 4 ⎛ 0 1 ⎞ ( 2) (1) ( 2) 0 ⎜ Ĥ + ⎟ Ψ100 + Fz Ψ100 = E100 Ψ100 and using the same technique as above one can show 2⎠ ⎝ (Buckingham, Coulson & Lewis, Proc. Phys. Soc. 1956, A, 69, 639) that ( 2) 0 Ψ100 = F 2 ( b + cP2 ) Ψ100 where b = 1 4 1 r + 6r 3 + 18r 2 ) and c = ( 2r 4 + 10r 3 + 15r 2 ) . ( 24 24 This allows us to calculate the energy through 5th order or up to F5. ( 3) 0 The third order function is given by Ψ100 = F 3 ( dP1 + eP3 ) Ψ100 where 1 ( 6r 6 + 64r 5 + 344r 4 + 852r 3 + 1590r 2 + 3180r ) and 480 1 e= 2r 6 + 18r 5 + 63r 4 + 8r 3 ) and allows the energy to be calculated through 7th order ( 240 or F7. d= Exercise: Calculate the energy of the hydrogen atom in a constant electric field up to terms that depend on F4. ( 2) Series solution for E100 Usually one cannot solve the differential equation for the first order correction to the wavefunction and one calculates the second order change in the energy using the general formula 2 Eμ( ) = ∑ Vμν Vνμ which assumes that the unperturbed functions are complete with Eμ0 − Eν0 respect to the perturbed solutions. There are two classes of eigenfunctions for the H atom, Those that describe the bound states, E < 0 , and those that describe the continuum states, E > 0 . Since we have solved the differential equation exactly we know the exact second 9 ( 2) order energy, E100 = − F 2 , so lets see what the error is if we use only the bound 4 wavefunctions to calculate the second order correction. Accordingly ν ≠μ (2) 100 E =∑ nlm 100 Fz nlm E10 − En0 2 =F 2 ∑ nlm 100 z nlm E10 − En0 2 = 2F 2 ∑ nlm 100 z nlm 2 1 ⎞ ⎛ ⎜ −1 + 2 ⎟ n ⎠ ⎝ The required matrix element is J. F. Harrison Michigan State University 1/24/2008 5 100 z nlm = 2π π 0 0 ∞ Y00 cos θ Ylm ∫ dr R10 ( r ) r 3 Rnl ( r ) ∫ dφ ∫ sin θ dθ 0 and the angular factor can be easily evaluated by observing that cos θ = 2π π ∫ dφ ∫ sin θ dθ 0 0 Y00 cos θ 1 Yl = 3 m 2π π 0 0 0 m ∫ dφ ∫ sin θ dθ Y1 Yl = 4π 0 Y so 3 1 1 δ1lδ 0 m so 3 ∞ 100 z nlm = 1 1 δ1lδ 0 m ∫ dr R10 ( r ) r 3 Rn1 ( r ) = δ1lδ 0 m R10 r Rn1 3 3 0 2 (2) 100 E 2 2 ∞ R10 r Rn1 =− F ∑ . Note that only the npz states contribute to the summation. 1 ⎞ 3 n=2 ⎛ ⎜ +1 − 2 ⎟ n ⎠ ⎝ The radial integral can be evaluated and the series summed (W. Gordon, Annalen der Physik 1929, 394, 1031) resulting in ( 2) E1s 9 29 2 ∞ n ( n − 1) =− F ∑ = −1.8225 F 2 2n+6 3 n = 2 ( n + 1) 2 n −6 The bound state wavefunctions then account for ~81% of the exact result. A remarkable error! The need to include the continuum solutions in the expansion makes sense physically because in the electric field the electron feels the potential 1 − + Fr cos θ and if cos θ < 0 the perturbation will eventually overwhelm the Coulomb r term and instead of the potential going to zero at large r it will eventually become lower than the 1s energy and the electron will be able to tunnel into the continuum. J. F. Harrison Michigan State University 1/24/2008 6