AP Biology: Diffusion Mini-Lab
advertisement

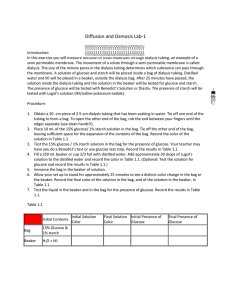
AP Biology Mini-Lab: Diffusion Introduction: In this exercise you will measure diffusion of small molecules through dialysis tubing, an example of a semi- permeable membrane. The movement of a solute through a semipermeable membrane is called dialysis (as well as diffusion). The size of the minute pores in the dialysis tubing determines which substance can pass through the membrane. A solution of glucose and starch will be placed inside a bag of dialysis tubing. Distilled water will be placed in a beaker, outside the dialysis bag. After 30 minutes have passed, the solution inside the dialysis tubing and the solution in the beaker will be tested for glucose and starch. The presence of glucose will be tested with glucose test strips. The presence of starch will be tested with Lugol's solution (iodine-potassium -iodide). Procedure: 1. Pour 160-170 mL of de-i water into a 250 mL beaker. Add approximately 4 mL of IKI solution to the water and mix well. Record the initial color of the solution in Table 1.1. 2. Dip a glucose test strip into the solution and record the initial glucose test results in Table 1.1. Use a “+” to denote a positive test for glucose and a “-” for a negative result. For best results, let the glucose test strip sit for about 60 seconds before determining results. 3. Dip a fresh glucose test strip into the glucose/starch solution. Record your results in Table 1.1. 4. Obtain a piece of dialysis tubing that has been soaking in water. The tubing should be soft and pliable. Roll the tubing between your thumb and forefinger to open it (see Figure 1). Close one end by rolling it and knotting it off. This will form a bag. 5. Using a small funnel, pour 15 mL of glucose/starch solution in the dialysis bag. Smooth out the top of the bag, running it between your thumb and index finger to expel air. Tie off the open end of the bag. Record the initial color of the glucose/starch solution. 6. Immerse the dialysis bag in the solution in your beaker. Make sure that the portion of the bag that contains the glucose/starch solution is completely covered by the solution in the cup at all times. Wait 30 minutes. Indicate the initial locations (inside or outside of the bag) of all the kinds of molecules that are available for diffusion through the dialysis membrane. For each of the molecules you list in a diagram below, predict their net overall diffusion: into the bag, out of the bag, both into and out of the bag equally, or none. Give a reason for each prediction. Molecule Net Movement Prediction & Explanation IKI Starch Glucose Water 7. After 30 minutes, remove the bag from the beaker, blot it on paper towel, and cut a slit in the bag with a razor large enough to insert a glucose test strip. Fill in the final columns of Table 1.1. 8. Finally, test for the presence of glucose in the liquid contained in the beaker with another glucose test strip. Table 1.1 : Dialysis Tubing Changes Initial Contents Bag 15% glucose/ 1% starch solution Beaker H 2O + IKI Initial Solution Color Final Solution Color Initial Presence of Glucose Final Presence of Glucose Analysis: 1. Which substance(s) are entering the bag and which are leaving the bag? What experimental evidence supports your answer? 2. Explain the results you obtained. Include the concentration differences and membrane pore size in your discussion. 3. Quantitative data uses numbers to measure observed changes. How could this experiment be modified so that quantitative data could be collected to show that water diffused into the dialysis bag? 4. Based on your observations, rank the following by relative size, beginning with the smallest: glucose molecules, water molecules, IKI molecules, membrane pores, starch molecules. 5. What results would you expect if the experiment started with glucose and IKI solution inside the bag and only starch and water outside? Why?