Sample Lab Report in PDF Format
advertisement

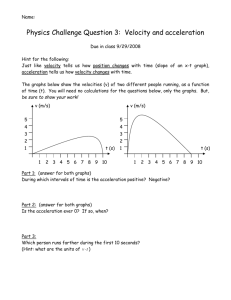
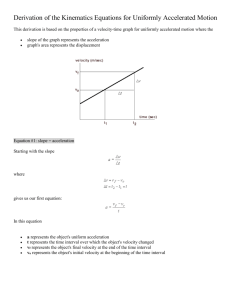
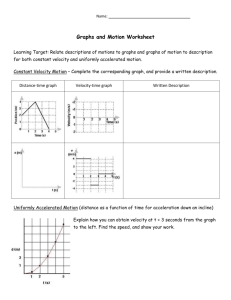
How to Write a Laboratory Report For each experiment you will submit a laboratory report. Laboratory reports are to be turned in at the beginning of the lab period, one week following the completion of the experiment. As a general rule, lab reports will not be accepted late. The laboratory report is to be used to express in a written form the purpose, methods employed, results obtained and conclusions reached in your experiment. It should be very neat, concise and complete. It should be prepared using a word processor for the text, spreadsheet software for tables, and graphing software for graphs. Sample Laboratory Report On the pages that follow a sample laboratory report is shown. The large numbers shown on the report correspond to numbers in parentheses in the discussion that follows. This sample report is to be used only as a general guide. Your laboratory instructor may have additional specific instructions and requirements for your laboratory reports. Each report should be clearly identified with (1) a title, (2) your name and the name(s) of your partners, (3) the date the experiment was performed, and (4) an abstract. The abstract should be a very brief overview of the goals and the main results of the experiment. If a known physical quantity was measured in the experiment then you should state the numerical value of the result that you obtained for that quantity and also state how close your result was to the expected result. It would be a good practice to write the abstract last, after you have written the remainder of the experiment. In a section entitled Description of Experiment (5) you should describe the purpose of the experiment, the physics principles that are studied, and the procedures that were employed to complete the experiment. It is not necessary (in fact it is undesirable) to give a step-by-step account of your activities in the experiment. Rather you should summarize the main techniques that you used to get to your final result. It may be helpful to include a sketch of the experimental geometry (6). This can be done by hand or can be cut and pasted into your report. You must have a section entitled Data and Analysis (7) in which you list and describe the raw experimental data collected during the experiment. Include any tables, graphs, results of best fits etc. as is appropriate. Describe how quantities were calculated from your raw data. If it is necessary to show a formula employed during the experiment then you may simply leave some space and write the equation in by hand as is shown by Equation 3 in the sample lab report. Finally, you should have a section entitled Results and Conclusions (8) in which you state the main result(s) of the experiment and compare your result(s) to the accepted or theoretical value(s) (if available) by computing a percent discrepancy. State what you consider to be the most likely causes of these discrepancies. If possible discuss these potential reasons for error quantitatively by calculating (or estimating) how much effect each source of uncertainty may have on the final result. An example of this type of reasoning is shown in the sample lab report at (9). Data Tables (10) may be incorporated inline into your lab report or may be placed at the end (as in the example) if it is more convenient. Data tables should be identified with a descriptive header. Each column should have a heading that describes the physical quantity that is recorded in the column. The column heading should also show the units of the physical quantity and the uncertainty in the quantity (if known). Numerical values recorded in the table should be rounded to the appropriate number of significant digits Graphs (11) may be incorporated inline into your lab report or may be placed at the end (as in the example) if it is more convenient. Graphs should be prepared on the computer. You should adhere to the following guidelines when preparing a graph. 1. Title – Every graph should have a title which identifies the graph by a number (i.e. Graph 1) along with some descriptive text that tells exactly what is plotted (i.e. Velocity vs. Time) and perhaps the purpose of the graph (i.e. Determination of Acceleration and Initial Velocity). 2. Axes and Axes Labels – Both axes should be labeled with descriptive text that tells the name of the physical quantity that is plotted on that axis and the units of that physical quantity (i.e. Velocity (cm/s)). Numerical values of the physical quantity should be printed at the major tick marks. 3. Size and Clarity – All graphs should be printed at a size that is sufficiently large so that the information can be easily read. Typically this means to make your graphs occupy most of the printed page. Choose WKU University Physics Laboratory I-3 How to Write a Lab Report axes limits so that the region of interest occupies most of the graph. Choose font sizes that are sufficiently large to be easily readable. 4. Graph Modes – You should adjust the graph mode so that individual data points are shown with some form of marker or with dots. Data values should never be connected with a jagged line. Show relationships that represent the best fit to data using lines with no markers. 5. Annotation – Whenever a best fit is performed on a graph, the results of the fit should be clearly displayed on the graph. In the case of a linear fit one should show the values of the slope and the yintercept. Be sure to include also the associated uncertainties and units. It is a good idea to include a measure of the goodness of fit (either the chi-squared value or the linear correlation coefficient – both discussed later) on the graph. Also, if possible, describe the physical significance of the slope and intercept as is done in the graph above. Finally, be sure to place your name (and your partner’s names if appropriate) and the date on the graph. 6. Uncertainty Bars – Whenever the uncertainties of individual data values are known they should be represented on the graph with uncertainty bars (often called error bars) as is shown in the above graph. At the end of the laboratory report staple your DATA Sheets (12) that you used during the laboratory period to record important data and results. Here you should show an example of each calculation that is used throughout the experiment. You will want to write the equation that was used and then show the use of the equation with numerical values substituted into it. If you make a mistake then simply cross out the suspected error and write the correct result as is shown near (13). WKU University Physics Laboratory I-4 How to Write a Lab Report Newton’s Second Law Joe Student Partner: Sue Student January 27, 2000 Abstract The position of a system of two masses was recorded as a function of time using a spark-timer apparatus. Using these data the instantaneous velocity was approximated by calculating the average velocity over 1/30 second time intervals. The acceleration of the system, which was calculated from the slope of a graph of velocity versus time, was 59.1 ± 1.5 cm/s2. The value of the acceleration predicted using Newton’s Second Law was 60.9 ± 0.1 cm/s2. The discrepancy between these two values for the acceleration of the mass was only 3%. WKU University Physics Laboratory I-5 Sample Lab Report Newton’s Second Law Description of Experiment In this experiment we verified Newton’s Second Law by measuring the acceleration of a system & subject to a net external force. Newton’s second law states that the acceleration a of an object is directly proportional to the net force acting on the object and inversely proportional to the object’s mass m. Newton’s second law can be expressed as an equation using & & Fnet = m a . (1) The unbalanced force was supplied by a mass falling in the earth’s gravitational field as is shown in Figure 1. Mass M rested on a smooth horizontal air track and was attached to mass m by a light tape passing over a pulley. When the system was released M was pulled along the track by the force supplied by the suspended mass m. The air track and pulley had small openings through which jets of air were ejected to create a nearly frictionless surface. The two masses were connected by a very light recording tape on which marks are made every 1/60 second by a spark timer. Figure 1 –Geometry for Newton’s Second Law Experiment We ignored the friction in the pulley as well as the friction between the mass and the air track. Also, the mass of the string was assumed to be negligible. With these assumptions Newton’s second law was WKU University Physics Laboratory I-6 Sample Lab Report applied to m and M. The resulting system of equations were solved to eliminate the tension in the string yielding a= m g m+M (2) for the predicted value of the acceleration of this system. We measured the velocity of M by measuring the distance between marks on the tape and dividing by the time interval that occurred between the creation of the marks. A graph of velocity versus time was made. The slope of this linear graph was the constant acceleration of the system. This result was compared to the value predicted from Newton’s second law given by Equation 2. Data and Analysis The masses of the system shown in Figure 1 were determined to be m = 10.23 ± 0.02 g and M = 154.35 ± 0.02 g using a mechanical balance. The position of the marks on the spark tape were measured using a meter stick and recorded to the nearest 0.02 cm in Data Table I. This table also shows the velocity at each twelfth dot which was estimated using the formula for average velocity (3) where ∆xi is the displacement between previous and successive dots and ∆ti is the corresponding time (2/60 s = 1/30 s). The total elapsed time (ti) required for the mass to move from the zeroth dot to the ith dot was determined knowing that the dots were separated in time by 1/60 second. Results and Conclusion Graph I shows a plot of the velocity versus time. The slope of this graph is the experimental value of the acceleration of the system. Using a least-squares fitting package, the acceleration was found to be aexp = slope = (59.1 ± 1.5) cm/s2. The value of the acceleration predicted by substituting measured values of the masses and g = 980 cm/s2 into equation (2) was (60.9 ± 0.1) cm/s2. The percent discrepancy between the two values is only -3.0%. WKU University Physics Laboratory I-7 Sample Lab Report The good agreement between the measured value of the acceleration of the system and the value predicted by Newton’s provides justification for the validity of Newton’s second law. Much of the difference between the measured and predicted values of acceleration can be attributed to uncertainties in the measurements in the distance between dots. This measurement uncertainty is on the order of ±0.02 cm. The displacement is then, using worse case propagation of error, uncertain by an amount ±0.04 cm. The uncertainty in the velocity calculated from equation (6) is then ±1.2 cm/s. For a typical velocity measurement this uncertainty is on the order of 2% accounting for most of the discrepancy. Additional sources of uncertainty include incorrect location of dots on the paper because the sparks may not be always perpendicular to the tape and friction along the air track and in the pulley. WKU University Physics Laboratory I-8 Sample Lab Report Data Table I Position and Velocity of Falling Mass as Function of Time Dot Number Total Time Total Time Position Displacement Time Change Velocity i ti ( ?/60 sec) ti (sec) xi (cm) ∆xi (cm) ∆ti (s) vi (cm/s) ± 0.02 cm ± 0.04 cm 11 11/60 0.183 15.78 12 12/60 0.200 16.39 13 13/60 0.217 17.02 23 23/60 0.383 24.33 24 24/60 0.400 25.16 25 25/60 0.417 26.00 35 35/60 0.583 35.26 36 36/60 0.600 36.28 37 37/60 0.617 37.30 47 47/60 0.783 48.52 48 48/60 0.800 49.76 49 49/60 0.817 51.03 59 59/60 0.983 64.25 60 60/60 1.000 65.61 61 61/60 1.017 67.04 71 71/60 1.183 82.22 72 72/60 1.200 83.82 73 73/60 1.217 85.45 WKU University Physics Laboratory I-9 ± 1.2 cm/s 1.24 2/60 37.2 1.67 2/60 50.1 2.04 2/60 61.2 2.51 2/60 75.3 2.79 2/60 83.7 3.23 2/60 96.9 Sample Lab Report WKU University Physics Laboratory I-10 Sample Lab Report WKU University Physics Laboratory I-11 Sample Lab Report WKU University Physics Laboratory I-12 Sample Lab Report