1 Synthesis and Characterization of N(C2H5)4 TiF5 , N(CH3)4
advertisement

CJBAS Vol. (01) - August - Issue 01 (2013) 1-9
Synthesis and Characterization of N(C2H5)4+TiF5−, N(CH3)4+TiF4Cl− , N(C4H9)4+TiF4Br− and
Density functional theory calculations of TiF5−,TiF4Cl− and TiF4Br –anions
Shahriar Ghammamy
a
Department of Chemistry, Islamic Azad University, Takestan Branch, Takestan, Iran
Keywords:
Abstract
Titanium (IV)
The reaction of TiF4 with salts (C2H5)4NF, (CH3)4NCl and (C4H9)4NBr, in anhydrous
CH3CN produced complexes [TiF4X]- (X=F-,Cl-,Br-). These were characterized by
elemental analysis, IR, UV/Visible and 81Br NMR spectroscopy. In this paper, the
optimized geometries and frequencies of the stationary point and the minimum-energy
paths are calculated at the B3LYP/6-311G (d,p) level of theory too. Theoretical results
showed that the Ti-X (X= F, Cl, Br) bond length values for the [TiF4X]- in compounds
1-3 are 1.863, 2.453 and 2.610 Å respectively. On the other hand the Ti-F bond length
values in [TiF4X]- are 1.863, 1.838 and 1.837 Å, respectively. These results reveal that
the bond order for Ti-X bonds decrease from compounds 1 to 3, while for Ti-F bonds,
the bond orders increase. These results reveal that the bond order for Ti-X bonds
decrease from compounds 1 to 3, while for Ti-F bonds, the bond orders increase. It can
be concluded that the decrease of Ti-X bonds lengths and the increase of Ti-F5 bond
lengths in compounds 1-3 result from the increase of the hyperconjugation from
compounds 1 to 3. Harmonic vibrational frequencies and infrared intensities forTiF5−,
TiF4Cl− and TiF4Br− are studied by means of theoretical and experimental methods.
The calculated frequencies are in reasonable agreement with the experimental values.
fluorides, Synthesis
ab initio calculations
density functional
calculations
Vibrational analysis
B3LYP level
1.Introduction
Properties and applications of technologically important inorganic compounds of titanium are surveyed.
Raw materials and pigment production processes are reviewed. Mixed oxides, e.g., barium titanate, and
halides of titanium are important raw materials for electroceramics. Titanium tetafluoride and its derivatives
have applications in fluorohydrins; addition of carbanions to aldehydes and imines, chemoselective
synthesis and deprotection of geminaldiacetates of aldehydes. Titanium tetrafluoride and Lewis bases L
normally form 1:2 adducts TiF4L2 [1]. TiF4 is stable in water and even in basic
Corresponding author (E-mail: shghamami@yahoo.com , Fax: (+98) 281-3780040).
1
Shahriar Ghammamy CJBAS Vol. (01)-August – Issue 01 (2013) 1-9
solution and is not easily hydrolyzed by air moisture,
unlike the chlorides [2]. Titanium fluoride complexes
were found as a very efficient and enantioselective
bifunctional asymmetric catalysts [3-12].
a)
In this work, we report on the synthesis and
characterization of complexes of the type [TiF4X](X=F-,Cl-,Br-), compounds 1-3, obtained directly
from TiF4 and tetra alkyl ammonium salts.
b)
c)
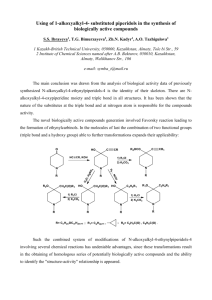
Figure 1. Optimized geometries of (a) [TiF5]-, (b) [TiF4Cl]- and (c) [TiF4Br]at B3LYP/6-311G(d,p) level of theory.
During this study we report synthesis,
characterization, optimized geometries and
infrared spectral measurements, assignments and
electronic structure calculations for compounds 1-3
(see Figure 1).
The structures of compounds have been optimized
by the density functional theory (DFT) based
method at B3LYP/6-311G levels of theory, using
the Gaussian 98 package of programs [3,4]. The
comparison between theory and experiment is
made (Tables 1-2).
Table 1. Geometrical parameters optimized of compounds1-3 bond length (Å) and angle (◦)
B3LYP/6-311G(d,p)
-
[TiF5]
[TiF4Cl]-
[TiF4Br]-
1.8265
1.8271
1.8263
1.8637
1.8637
1.8092
1.8072
1.8087
1.8389
2.4536
1.8077
1.8073
1.8085
1.8374
2.6107
F2-Ti1-F3
F2-Ti1-F4
F2-Ti1-F5
119.9
120
90
120.1
119.9
90.78
119.7
120
91
F2-Ti1-X6
90
89.1
89
Bond lengths (Å)
Ti1-F2
Ti1-F3
Ti1-F4
Ti1-F5
Ti1-X6
Bond angles (◦)
2
Shahriar Ghammamy CJBAS (01)-August – Issue 01 (2013) 1-9
spectrophotometer. The UV/Visible measurements
were made on an Uvicon model 922 spectrometer.
81
Br-NMR was recorded on a Bruker AVANCE
DRX 500 spectrometer at 500 MHz. The percent
composition of elements was obtained from the
Microanalytical Laboratories, Department of
Chemistry, OIRC, Tehran. The density functional
and ab initio calculation have been performed with
the Gaussian 98 program and the basis sets
implemented therein [4,13-15].
2. Materials and Methods
2.1. General
Acetonitrile (Fluka, P.A.) was distilled several
times from phosphorus pentaoxide before Use,
thereby reducing its water content to <4 ppm. TiF4
(Merck, p.a.) were used without further
purification. Anhydrous Et4N+F- were obtained by
a drying procedure of the tetrahydrate in high
vacuum (d, 130 0C). Infrared spectra were recorded
as KBr disks on a Shimadzu model 420
Table 2. Calculated and experimental frequencies of compounds 1-3 (cm-1)
B3LYP/6-311G(d,p)
[TiF5][TiF4Cl][TiF4Br]-
96, 99, 251, 253, 284, 285, 288,
525, 628, 687, 715, 716
Exptl
571, 649,793
94, 97, 215, 218, 254, 274, 275,
292, 607, 659, 742, 747
86, 91, 179, 201, 206, 271,
273,280,606, 659, 743, 745
566, 625
551, 637
mixture was filtered, washed ether, and dried at
room temperature. Mp 334–336˚C. Anal. Calc. for
C8H20NTiF5: C, 35.19; H, 7.33; N, 5.13. Found: C,
35.27; H, 7.41; N, 5.21%. IR (KBr) (cm-1): 3387,
3277, 3028, 3010, 2995, 2968, 2780, 2655, 2448,
1850, 1492, 1182, 1065, 783, 649, 571. UV-Vis in
CH3CN, λ/cm-1:45454, 40485, 35335, 26525.
(Figures 2-4, Tables 3,4).
2.2. Synthesis of
Tetraethylammoniumpantafluorotitanate
(IV),[(C2H5)4N][TiF5] (1)
To a solution of TiF4 (0.22 g, 1.77 mmol) in
MeCN, the solid powder tetraethtylammonium
fluoride (0.30 g, 2.01mmol) was added under
stirring at room temperature until a white solid
precipitate was formed. After 2 hours stirring, the
Figure 2. The FT-IR spectrum of (C4H9)4N[TiF4Br] (KBr Disk)
3
Shahriar Ghammamy CJBAS (01)-August – Issue 01 (2013) 1-9
filtered, washed ether, and dried at room
temperature. Mp 335-336˚C. Anal. Calc. for
C4H12NTiF4Cl: C, 20.56; H, 5.14; N, 5.99. Found:
C, 20.64; H, 5.22; N, 6.07%. IR (KBr) (cm-1):3381,
3225, 3017, 2980, 2962, 2780, 2655, 2466, 1850,
1489, 1405, 1293, 952, 625, 566. UV–Vis in
CH3CN, λ /cm-1:40650, 35460, 26246. (Figures 56, Tables 5-6).
2.3.
Synthesis
of
Tetrametyl
ammoniumhalotetrafluorotitanate
(IV),
[(CH3)4N][TiF4Cl] (2)
The preparation is like as pervious method: A
solution of TiF4 (0.26 g,2.09 mmol) in MeCN the
solid powder tetrametylammonium chloride (0.25
g, 2.28 mmol) was added under stirring at room
temperature until white off solid precipitate was
formed. After 2 hours stirring, the mixture was
1.6
1.4
1.2
1
0.8
0.6
0.4
0.2
0
200 250 300 350 400 450 500 550 600 650 700 750 800
Figure 4. The 81Br NMR spectrum of
[C4H9)4N[TiF4Br] in the CDCl3
Figure 3. The UV/Vis spectrum of
(C4H9)4N[TiF4Br]
Table 3. FT-IR data obtained for (C4H9)4N[TiF4Br]
υ( cm )
-1
Vibration
(C4H9)4N
Intensity
+
υ( cm-1)
Vibration
Intensity
1473
υ15,CH2,asym.def
(ms)
3383
υCH2+υ19
(m, br)
1384
υ16,CH2, sym.str
(s)
3315
υCH2+υ8
(w, br)
1168
υrock,CH2,roking υ14
(m)
3225
υCH2, asym.str
(sh)
1062
υ18, υ C4asym.str
(ms)
3010
υ13,υCH2, asym.str
(w,br)
463
υ19, υ C4,dif.
(w,br)
2962
υ14,asym.str
(w,br)
453
υ19, υ C4,dif.
(w,br)
2877
υ14,υCH2, asym.str
(w,br)
2765
υ7+ υ16
(w)
637
Ti -F
(vs)
2396
υ3+ υ8+ υ16
(w)
551
Ti-F
(vs)
2085
υ8+ υ15
(w,br)
393
Ti- Br
(m)
[TiF4Br]-
ammonium
The preparation is like as pervious method: A
halotetrafluorotitanate (IV), [(CH3)4N][TiF4Cl]
solution of TiF4 (0.26 g,2.09 mmol) in MeCN the
(2)
solid powder tetrametylammonium chloride (0.25
2.3.
Synthesis
of
Tetrametyl
4
Shahriar Ghammamy CJBAS (01)-August – Issue 01 (2013) 1-9
g, 2.28 mmol) was added under stirring at room
After 2 hours stirring, the mixture was filtered,
temperature until white off solid precipitate was
washed ether, and dried at room temperature.
formed.
Table 4. The electron transitions data of compound (C4H9)4N[TiF4Br]
λ1
(ε,M-1cm-1)
λ1
(ε,M-1cm-1)
λ2
(ε,M-1cm-1)
279(429)
382(55)
682(18)
Mp 335-336˚C. Anal. Calc. for C4H12NTiF4Cl:
1850, 1489, 1405, 1293, 952, 625, 566. UV–
C, 20.56; H, 5.14; N, 5.99. Found: C, 20.64;
Vis in CH3CN, λ /cm-1:40650, 35460, 26246.
H, 5.22; N, 6.07%. IR (KBr) (cm-1):3381,
(Figures 5-6, Tables 5-6)
3225, 3017, 2980, 2962, 2780, 2655, 2466,
Figure 5. The FT-IR spectrum of (CH3)4N[TiF4Cl] (KBr Disk)
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
200
250
300
350
400
450
500
Figure 6. The UV/Vis spectrum of (CH3)4N[TiF4Cl] (2.5×10-3 in acetonitrile)
5
Shahriar Ghammamy CJBAS (01)-August – Issue 01 (2013) 1-9
Table 5. FT-IR data obtained for (CH3)4N[TiF4Cl]
υ( cm )
-1
Vibration
Intensity
(CH3)4N+
υ( cm-1)
Vibration
Intensity
1489
υ15,CH2,asym.def
(ms)
3381
υCH2+υ19
(m, br)
1405
υ16,CH2, sym.str
(s)
3225
υCH2+υ8
(w, br)
1293
υrock,CH2,roking υ14
(m)
3017
υCH2, asym.str
(sh)
952
υ18, υ C4asym.str
(ms)
2980
υ13,υCH2, asym.str
(w,br)
499
υ19, υ C4,dif.
(w,br)
2962
υ14,asym.str
(w,br)
428
υ19, υ C4,dif.
(w,br)
2780
υ14,υCH2, asym.str
(w,br)
2655
υ7+ υ16
(w)
637
Ti -F
(vs)
2466
υ3+ υ8+ υ16
(w)
551
Ti-F
(vs)
1850
υ8+ υ15
(w,br)
393
Ti- Br
(m)
[TiF4Cl]
(0.397 g,1.23 mmol) was added under stirring at
room temperature until gold solid precipitate was
formed.
2.4. Synthesis of Tetrabuthyl ammonium
bromotetrafluorotitanate
(IV), [(C4H9)4N][TiF4Br](3)
A solution of TiF4 (0.139 g, 1.12 mmol) in MeCN
the solid powder tetrabuthtylammonium bromide
Table 6.The electron transitions data of (CH3)4N[TiF4Cl]
λ1
λ1
λ2
(ε,M-1cm-1)
246)237(
(ε,M-1cm-1)
282)190(
(ε,M-1cm-1)
381)108(
After 2 hours stirring, the mixture was filtered,
washed ether, and dried at room temperature.Mp.
63.4-65.1˚C. Anal. Calc. for C16H36NTiF4Br: C,
43.06.; H, 8.07; N, 3.14. Found: C, 43.14; H, 8.15;
N, 3.23 %. IR(KBr) (cm-1): 3383, 3315, 3225,
3010, 2962, 2877, 2765, 2396, 2085, 1473, 1384,
1164, 1062, 887, 803, 637, 551. 81Br NMR
(CDCl3) δ: 56.71. UV–Vis in CH3CN, λ /cm1
:35842, 26178,14662. (Figures 7-8, Tables 7,8).
2.5. Computational methods
structural parameters were used in the vibrational
frequency calculations at the HF and DFT levels to
characterize all stationary points as minima [6].
Harmonic vibrational frequencies (ν) in cm-1 and
infrared intensities (int) in Kilometer per mole of
all compounds were performed at the same level
on the respective fully optimized geometries.
3. Discussion and Conclusions
The complex (C2H5)4N[TiF5] was obtained by the
reaction of (C2H5)4NF with TiF4 in the
acetonitrile solvent (reaction (1)). The reaction of
TiF4 with (CH3)4NCl in acetonitrile solvent gave
(CH3)4N[TiF4Cl] (reaction (2)).also the reaction
of TiF4 with (C4H5)4NBr in acetonitrile solvent
gave (C4H9)4N[TiF4Br] (reaction (3)).
Density functional theory (DFT) calculations were
carried out at B3LYP/6-311G(d,p) levels of theory
with the Gaussian 98 package of programs [4,5]
which combines the exact Hartree-Fock exchange
with Becke,s and uses the Lee-Yang-Parr
correlation function in order to include the most
important correlation effects. The optimized
6
Shahriar Ghammamy CJBAS (01)-August – Issue 01 (2013) 1-9
Table 7. FT-IR data obtained for (C2H5)4N[TiF5]
-1
υ( cm )
Vibration
(C2H5)4N
Intensity
+
υ( cm-1)
Vibration
Intensity
1492
υ15,CH2,asym.def
(ms)
3387
υCH2+υ19
(m, br)
1449
υ16,CH2, sym.str
(s)
3277
υCH2+υ8
(w, br)
1182
υrock,CH2,roking υ14
(m)
3028
υCH2, asym.str
(sh)
1065
υ18, υ C4asym.str
(ms)
2995
υ13,υCH2, asym.str
(w,br)
499
υ19, υ C4,dif.
(w,br)
2968
υ14,asym.str
(w,br)
428
υ19, υ C4,dif.
(w,br)
2780
υ14,υCH2, asym.str
(w,br)
2655
υ7+ υ16
(w)
783
Ti -F
(vs)
2448
υ3+ υ8+ υ16
(w)
649
Ti-F
(vs)
1850
υ8+ υ15
(w,br)
571
Ti- Br
(m)
[TiF5]
-
(C2H5)4NF + TiF4 → (C2H5)4N[TiF5] (1)
(CH3)4NCl + TiF4 → (CH3)4N[TiF4Cl] (2)
(C4H9)4NF + TiF4 → (C4H9)4N[TiF4Br] (3)
Halotfluorotitanates were synthesized through
a one-step reaction. Our procedure for
producing these compounds has some
advantages. For example, there is no side
product in preparing these halotitanate
compounds in our method, the reaction is quite
fast and does not require any severe conditions
such as high pressure or high temperature, and
it is not sensitive to air.
In the present study, all compounds under
consideration are carried out with the Gaussian
98 program [4,7]. Geometry optimization, Fig.
1, show that symmetry for compounds 2 and 3
is C1 while compound 1 has D3h. Selected
bond distances and angles are reported in
Table1.We could not compare the calculation
results given in Table 1with the experimental
data. Because the crystal structure of the title
compound is not available till now. B3LYP/6311G results showed that the Ti-X (X= F, Cl,
Br) bond length values for the [TiF4X]- in
compounds 1-3 are 1.863, 2.453 and 2.610 Å,
respectively.
1.4
1.2
1
0.8
0.6
0.4
0.2
0
200
Figure 7. The FT-IR spectrum of (C2H5)4N[TiF5]
250
300
350
400
450
500
Figure 8. The UV/Vis spectrum of (C2H5)4N[TiF5])
(KBr Disk)
(1.2× 10-3 in acetonitrile)
7
Shahriar Ghammamy CJBAS (01)-August – Issue 01 (2013) 1-9
λ1 (ε,M-1cm-1)
220 (672)
Table 8. The electron transitions data of (C2H5)4N[TiF5]
λ2 (ε,M-1cm-1)
λ3 (ε,M-1cm-1)
247)461(
283)327(
Also, the Ti-F5 bond length values in [TiF4X]- are
1.863, 1.838 and 1.837 Å, respectively. These
results reveal that the bond order for Ti-X bonds
decrease from compounds 1 to 3, while for Ti-F5
bonds, the bond orders increase. It can be
concluded that the decrease of Ti-X bonds lengths
and the increase of Ti-F5 bond lengths in
compounds 1-3 result from the increase of the
hyperconjugation from compounds 1 to 3. Besides,
the θF2-Ti1-L6 bond angle values in compounds
1-3 are 90.0, 89.1 and 89.0, respectively (see Table
1). The decrease of θF2-Ti1-X6 bond angle values
from compounds 1 to 3, could again, be explained
by the increase of the hyperconjugation from
compounds 1 to 3.
The harmonic vibrational frequencies of all the
stationary points at the B3LYP/6-311G(d,p) level
along with the available experimental data [1, 8,9]
presented in Table 2.
The compounds structure shows the presence of
Ti-F stretching vibrations in the region 800–500
cm−1 which is the characteristic region for the
ready identification of the Ti–F stretching
vibrations. Hence, the FT-IR bands at 675-521
cm−1 in compounds 1-3 have been designated to
Ti–F stretching vibration. In general the
compounds Ti–F vibrations calculated theoretically
are in good agreement with the experimentally
reported values [10,11].Three tetraalkylammonium
salts of TiF4 were synthesized simply. These
compounds were characterized by elemental
analysis, IR, UV/Visible, and 81Br-NMR
techniques. Production of these compounds shows
the ability of tetraalkylammonium salts in halide
addition to transition metal and main group
elements compounds and the optimized geometry
parameters calculated at B3LYP/6-311+G(d,p)
level. The optimized structures are in good
agreement with the available experimental results.
In the present article, the infrared spectrum of the
Titanium halide complexes was studied using the
theoretical and experimental methods. Our
theoretical infrared spectrum of Compounds 1-3
λ4 (ε,M-1cm-1)
377)128(
are in very good agreement compared to our
experimental spectrum.
Acknowledgements
The authors would like to thank Dr. Gh. Rezaei
Behbahani and Dr. Mahjoub for valuable
discussions.
References
[1] Nikiforov G. B., Knapp C., Passmore J. Decken
A.: Interaction of TiF4 with the donor solvents
SO2, PhCN, and MeCN. Isolation and
structural characterization of the first trimeric
fluorine bridged donor acceptor adduct {TiF4
(PhCN)}3, Journal of Fluorine Chemistry, 127,
1398–1404 (2006).
DOI: 10.1016/j.jfluchem.2006.05.022
[2]
Yang X.; Paillaud J.-L.; van Breukelen
H.F.W.J.; Kessler H.: Duprey E.: Synthesis of
microporous titanosilicate ETS-10 with TiF4 or
TiO2, Microporous and Mesoporous Materials,
46, 1-11 (2001).
DOI:10.1016/S1387-1811(01)00267-0
[3] Nori-Shargh D., Roohi F., Deyhimi F., NaeemAbyaneh R.: DFT study and NBO analysis of
the metallotropic shifts in cyclopentadienyl
(trimethyl)
silane,-germane
andstannane. Journal of Molecular Structure:
THEOCHEM, 763, 21-28 (2006).
DOI: 10.1016/j.theochem.2006.01.011
[4] Frisch M J, Trucks G W, Schlegel H B, et al.
GASSIAN 98 (Revision A. 3) Gaussian Inc.,
Pittsburgh, PA, USA, 1998.
[5]
8
Becke
A.
D.:
Density-functional
thermochemistry. III. The role of exact
exchange, The Journal of Chemical Physics, 98,
5648-5652 (1993).
DOI: 10.1063/1.464913
Shahriar Ghammamy CJBAS (01)-August – Issue 01 (2013) 1-9
[6] Sundaraganesan N., Ilakiamani S., Dominic
Joshua
B.:
Vibrational
spectroscopy
investigation using ab initio and density
functional theory analysis on the structure of 3,
4-dimethylbenzaldehyde. Spectrochimica Acta
Part A: Molecular and Biomolecular
Spectroscopy, 68, 680-687 (2007).
DOI: 10.1016/j.saa.2006.12.046
[11] Fox J J and Martin A E. Infra-red spectra in
the 3µ region of naphthalene, α- and βmethylnaphthalenes,
crinoline,
and
isoquinoline: an aid to analysis. Journal of the
Chemical Society (Resumed), 72, 318-322
(1939).
DOI: 10.1039/JR9390000318
[7] He H. -Q., Liu J. –Y., Li Z. –S., Sun C. –C.:
Theoretical study for the reaction of
C2H5Cl/C2D5Cl with Cl atom. Journal of
Molecular Structure: THEOCHEM, 763, 59-66
(2006).
DOI: 10.1016/j.theochem.2005.12.038
[12] Alcolea Palafox M.: Scaling factors for the
prediction of vibrational spectra. I.Benzene
molecule, International Journal of Quantum
Chemistry , 77, 661-684 (2000).
DOI: 10.1002/(SICI)1097461X(2000)77:3<661::AID-QUA7>3.0.CO;2-J
[8] Nakamoto K.: Infrared and Raman spectra of
Inorganic and Coordination Compound. John
Wiley, New York (1978).
[13] Politzer P., Seminario, J. M. (Eds.).: Modern
density functional theory: a tool for chemistry:
a tool for chemistry. Access Online via
Elsevier, (1995).
[9] Levason W., Patel B., Reid G.: Titanium,
Zirconium and Hafnium Halide complexes of
Trithioether and Triselenoether Ligands.
Inorganica Chimica Acta, 357, 2115–2120
(2004).
DOI: 10.1016/j.ica.2003.11.042
[14] Lee C, Yang W., Parr R. G.: Development of
the Colle-Salvetti correlation-energy formula
into a functional of the electron density,
Physical Review B, 37, 785-789 (1988).
DOI: 10.1103/PhysRevB.37.785
[10] Colthup N. B., Daly L. H., Wiberley S. E.:
Introduction
to
Infrared
and
Raman
Spectroscopy, Academic Press, New York
(1964).
[15] Radom L., Schleyer P. V. R., Pople, J. A.: Ab
initio molecular orbital theory , Wiley, New
York (1986)
9