Formal Charge, Electron Pushing
advertisement

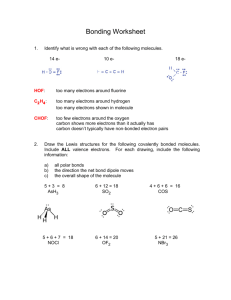
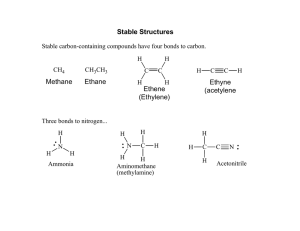
Formal charges, electron pushing, and mechanisms Oxygen. Oxygen can have a formal charge of -1, 0, or +1 The easiest way to see the charges on oxygen is to look at water: Water is said to be amphiphilic, meaning it can either gain or lose H+. Notice that in the sketch above, only H+ is being added or removed. The total number of electrons in each case is 8. Also, notice that the formal charge on the oxygen changes. These three depictions of oxygen are almost always how oxygen will ever appear: 1. negative oxygen: 1 bond, 3 lone pairs 2. neutral oxygen: 2 bonds, 2 lone pairs 3. positive oxygen: 3 bonds, 1 lone pairs. Nitrogen. Nitrogen is very similar to oxygen, except that it needs 3 electrons, and therefore forms three bonds under neutral conditions: 1. negative nitrogen: 2 bonds, 2 lone pairs 2. neutral nitrogen: 3 bonds, 1 lone pairs 3. positive nitrogen: 4 bonds, 0 lone pairs. Carbon attains charges differently than N or O. Since it normally has two bonds, it will attain a positive charge if a bond is broken and both electrons leave. It will attain a negative charge if a bond is broken and both electrons stay: Besides the halides and some occasional others, these are usually the 9 main components of any compound or intermediate. In order to succeed in organic chemistry, you should recognize these 9, and know their formal charges. Drawing mechanisms: Mechanisms allow us to track what’s really happening with a reaction…where the electrons are going, and what intermediates are being formed. With a good understanding of mechanisms, it’s easy to predict how reactions will occur. As a matter of bookkeeping, mechanisms will always use curved arrows to show the motion of electrons. A full arrow means two electrons are moving. A half-arrow means only one. The direction of the arrows should match the electron count before and after the change occurs. 1 Complete each of the following mechanisms by filling in the missing electrons and the formal charges. Show the transfer of electrons by drawing curved arrows.. 2 Draw Lewis Structures for each of the following molecules. Indicate whether each molecule has a dipole (and show direction), and indicate the geometry (T, Tr.P, L) of each central atom. H2CO H2NNH2 C2H4 CH3OCH3 C2H2 CH3NCF2 Draw Lewis structures for each of the following ionic compounds. Show all nonzero formal charges. C2H5ONa (CH3)4NCl CH3CO2K Draw Lewis structures and use curved arrows to show the mechanisms for the following acid-base reactions: NH2- CH3OH + NH3 + HCOOH CH3O- CHOO- 3 + + NH4+ NH3