4 ULCER CARE - MAGNETIC DRESSING APCO March 06 Black
advertisement
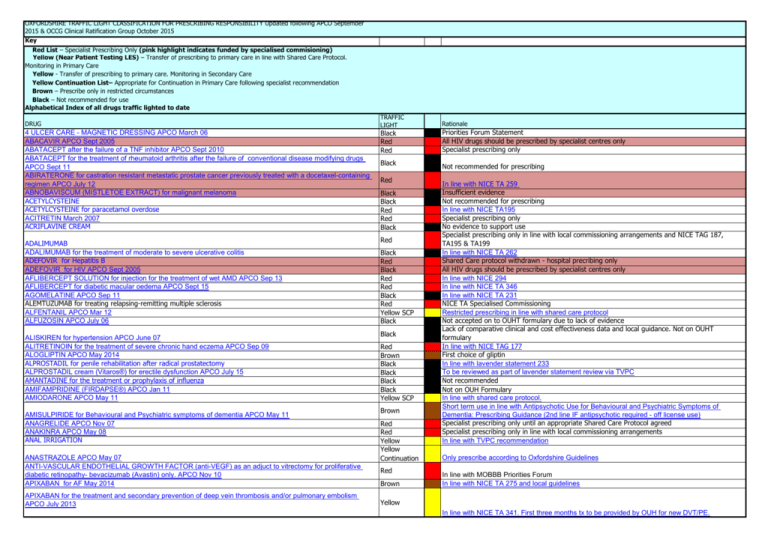
OXFORDSHIRE TRAFFIC LIGHT CLASSIFICATION FOR PRESCRIBING RESPONSIBILITY Updated following APCO September 2015 & OCCG Clinical Ratification Group October 2015 Key Red List – Specialist Prescribing Only (pink highlight indicates funded by specialised commisioning) Yellow (Near Patient Testing LES) – Transfer of prescribing to primary care in line with Shared Care Protocol. Monitoring in Primary Care Yellow - Transfer of prescribing to primary care. Monitoring in Secondary Care Yellow Continuation List– Appropriate for Continuation in Primary Care following specialist recommendation Brown – Prescribe only in restricted circumstances Black – Not recommended for use Alphabetical Index of all drugs traffic lighted to date DRUG 4 ULCER CARE - MAGNETIC DRESSING APCO March 06 ABACAVIR APCO Sept 2005 ABATACEPT after the failure of a TNF inhibitor APCO Sept 2010 ABATACEPT for the treatment of rheumatoid arthritis after the failure of conventional disease modifying drugs APCO Sept 11 ABIRATERONE for castration resistant metastatic prostate cancer previously treated with a docetaxel-containing regimen APCO July 12 ABNOBAVISCUM (MISTLETOE EXTRACT) for malignant melanoma ACETYLCYSTEINE ACETYLCYSTEINE for paracetamol overdose ACITRETIN March 2007 ACRIFLAVINE CREAM ADALIMUMAB ADALIMUMAB for the treatment of moderate to severe ulcerative colitis ADEFOVIR for Hepatitis B ADEFOVIR for HIV APCO Sept 2005 AFLIBERCEPT SOLUTION for injection for the treatment of wet AMD APCO Sep 13 AFLIBERCEPT for diabetic macular oedema APCO Sept 15 AGOMELATINE APCO Sep 11 ALEMTUZUMAB for treating relapsing-remitting multiple sclerosis ALFENTANIL APCO Mar 12 ALFUZOSIN APCO July 06 ALISKIREN for hypertension APCO June 07 ALITRETINOIN for the treatment of severe chronic hand eczema APCO Sep 09 ALOGLIPTIN APCO May 2014 ALPROSTADIL for penile rehabilitation after radical prostatectomy ALPROSTADIL cream (Vitaros®) for erectile dysfunction APCO July 15 AMANTADINE for the treatment or prophylaxis of influenza AMIFAMPRIDINE (FIRDAPSE®) APCO Jan 11 AMIODARONE APCO May 11 AMISULPIRIDE for Behavioural and Psychiatric symptoms of dementia APCO May 11 ANAGRELIDE APCO Nov 07 ANAKINRA APCO May 08 ANAL IRRIGATION ANASTRAZOLE APCO May 07 ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR (anti-VEGF) as an adjuct to vitrectomy for proliferative diabetic retinopathy- bevacizumab (Avastin) only. APCO Nov 10 APIXABAN for AF May 2014 APIXABAN for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism APCO July 2013 TRAFFIC LIGHT Black Red Red Black Red Black Black Red Red Black Red Black Red Black Red Red Black Red Yellow SCP Black Black Red Brown Black Black Black Black Yellow SCP Brown Red Red Yellow Yellow Continuation Red Brown Rationale Priorities Forum Statement All HIV drugs should be prescribed by specialist centres only Specialist prescribing only Not recommended for prescribing In line with NICE TA 259 Insufficient evidence Not recommended for prescribing In line with NICE TA195 Specialist prescribing only No evidence to support use Specialist prescribing only in line with local commissioning arrangements and NICE TAG 187, TA195 & TA199 In line with NICE TA 262 Shared Care protocol withdrawn - hospital precribing only All HIV drugs should be prescribed by specialist centres only In line with NICE 294 In line with NICE TA 346 In line with NICE TA 231 NICE TA Specialised Commissioning Restricted prescribing in line with shared care protocol Not accepted on to OUHT formulary due to lack of evidence Lack of comparative clinical and cost effectiveness data and local guidance. Not on OUHT formulary In line with NICE TAG 177 First choice of gliptin In line with lavender statement 233 To be reviewed as part of lavender statement review via TVPC Not recommended Not on OUH Formulary In line with shared care protocol. Short term use in line with Antipsychotic Use for Behavioural and Psychiatric Symptoms of Dementia: Prescribing Guidance (2nd line IF antipsychotic required - off license use) Specialist prescribing only until an appropriate Shared Care Protocol agreed Specialist prescribing only in line with local commissioning arrangements In line with TVPC recommendation Only prescribe according to Oxfordshire Guidelines In line with MOBBB Priorities Forum In line with NICE TA 275 and local guidelines Yellow In line with NICE TA 341. First three months tx to be provided by OUH for new DVT/PE. APIXABAN for preventing VTE after total hip or knee replacement in adults APCO Mar 13 APOMORPHINE APCO May 10 AQUADEKS APCO Mar 12 ARIPIPRAZOLE APCO Sep 04 ARIPIPRAZOLE for Behavioural and Psychiatric symptoms of dementia APCO Sep 11 ARIPIPRAZOLE for the treatment of schizophrenia in people aged 15 to 17 years APCO May 11 ARIPIPRAZOLE for treating moderate to severe manic episodes in adolescents with bipolar 1 disorder APCO Sep 13 Red Yellow Brown Yellow Continuation Brown Yellow Continuation In line with NICE TA 245 In line with the APCO approved shared care protocol Restricted prescribing for children with cystic fibrosis. Oxford Health (Mental Health) consultants only Short term use in line with Antipsychotic Use for Behavioural and Psychiatric Symptoms of Dementia: Prescribing Guidance (2nd line IF antipsychotic required - off license use) In line with TA 213 Red Yellow Continuation Black Red Yellow SCP Yellow In line with TA292 In line with TA 218 Shared Care Protocol Shared Care Protocol Shared Care Protocol Shared Care Protocol Shared Care Protocol Shared care protocol Shared Care Protocol No evidence to support its place in therapy over other cheaper sartans. AZITHROMYCIN for cystic fibrosis APCO March 2003 BACLOFEN INTRATHECAL for spasticity APCO Nov 2007 BALSALAZIDE APCO June 07 BARBITURATES APCO July 11 BARBITURATES for addiction APCO July 11 BARBITURATES for epilepsy APCO July 11 Bard Catheter Trays APCO Jan 14 BASILIXIMAB APCO Sept 05 BD Autoshield needles APCO Nov 14 BENDAMUSTINE for relapsed or refractory non-Hodgkin’s lymphoma APCO Sept 2010 BENDAMUSTINE for the treatment of chronic lymphocytic leukaemia APCO May 2011 Red Yellow (NPT) Yellow (NPT) Yellow (NPT) Yellow (NPT) Yellow (NPT) Yellow (NPT) Yellow (NPT) Black Yellow Continuation Red Black Red Black Yellow Black Red Black Red Red BENZODIAZEPINES - should not be used for GAD except as a short-term measure during crises APCO May 11 BETA-INTERFERON BETAMETHASONE 2.250mg medicated plaster (BETESIL®) APCO Mar 10 Brown Red Black In line with CG 113 Treatment from OUHT providing patient fits certain criteria Lack of comparative clinical and cost effectiveness data ARIPIPRAZOLE ORO-DISPERSIBLE APCO Nov 06 ARTHROTEC APCO Jan 14 ATAZANAVIR APCO Sept 2005 ATOMOXETINE APCO Nov 10 AURANOFIN AZACITIDINE for the treatment of myelodysplastic syndromes, chronic myelomonocytic leukaemia and acute myeloid leukaemia APCO May 2011 AZATHIOPRINE for inflammatory arthritis APCO Nov 2010 AZATHIOPRINE for inflammatory bowel disease APCO May 11 AZATHIOPRINE in idiopathic pulmonary fibrosis APCO Nov 10 AZATHIOPRINE for liver transplant and autoimmune liver disease APCO Sep 11 AZATHIOPRINE in children and adolescents (Rheumatology) APCO July 11 AZATHIOPRINE in dermatology APCO May 12 AZATHIOPRINE in sarcoidosis APCO Nov 11 AZILSARTAN APCO Sep 12 BEVACIZUMAB (first-line) for the treatment of advanced and/or metastatic renal cell carcinoma APCO Sept 2009 BEVACIZUMAB for diabetic macular oedema APCO Jan 12 BEVACIZUMAB for macular oedema caused by retinal vein occlusion APCO Jan 12 BEVACIZUMAB for metastatic colorectal cancer after first line chemotherapy APCO Jan 12 BEVACIZUMAB for neo-vascularisation in non wet-AMD conditions and glaucoma prior to laser treatment. APCO Jan 10 Black Black Black Black Red BEVACIZUMAB in combination with a taxane for the first-line treatment of metastatic breast cancer APCO May 11 Black BEVACIZUMAB in combination with capecitabine for the first line treatment of metastatic breast cancer APCO Black Sept 2012 BEVACIZUMAB in combination with gemcitabine and carboplatin for treating people with the first recurrence of Black platinum-sensitive advanced ovarian cancer Following recommendation Not in line with policy All HIV drugs should be prescribed by specialist centres only Oxford Health - Mental Health consultants only. Shared Care Protocol Not currently used in Oxfordshire Detailed information will be provided by the consultant Specialist prescribing only Not currently recommended for prescribing as not on the OUHT formulary Secondary care prescribing only for the treatment of addiction Phenobarbital for epilepsy More expensive than components Specialist initiation and prescribing only No GP prescribing, to be supplied by OH In line with MOBBB Priorities Forum and with NICE TA206 In line with TA 216 In line with NICE TAG 178 In line with MOBBB Commissioning Policy Statement 211 In line with MOBBB Commissioning Policy Statement 212 In line with NICE TA 242 In line with Priorities Forum In line with TA 214 In line with NICE TA 263 In line with NICE TA 285 BEVACIZUMAB in combination with oxaliplatin and either fluorouracil plus folinic acid or capecitabine for the treatment of metastatic colorectal cancer APCO Jan 11 BEVACIZUMAB in combination with paclitaxel and carboplatin for first-line treatment of advanced ovarian cancer APCO July 13 BEXAROTENE APCO Nov 10 BIOLOGICAL DISEASE MODIFYING DRUGS in rheumatoid arthritis (anti-TNF drugs) APCO July 11 BIOLOGICAL DISEASE MODIFYING DRUGS in rheumatoid arthritis (anti-TNF drugs) APCO July 11 BIOTIN for Paediatric Neurologists APCO Sep 13 BLEOMYCIN APCO July 08 BOCEPREVIR APCO May 12 BORTEZOMIB APCO July 2008 BORTEZOMIB and thalidomide for the first-line treatment of multiple myeloma APCO Sept 11 BORTEZOMIB for dialysis prevention in untreated myeloma BOSENTAN BOTULINUM TOXIN for anal fissure/bladder over activity BOTULINUM TOXIN for axillary hyperhidrosis BOTULINUM TOXIN Type A for chronic headache(excluding chronic migraine) APCO July 12 BOTULINUM TOXIN Type A for chronic migraine APCO July 12 BRIGHT EYES APCO Sep 12 BRIMONIDINE Gel for rosacea APCO July 14 BROMFENAC EYE DROPS APCO Nov 13 BUPRENORPHINE and NALOXONE (SUBOXONE®) APCO July 10 BUPRENORPHINE PATCHES APCO July 15 BUPRENORPHINE s/l tablets for the treatment of addiction APCO July 11 BUPROPION for smoking cessation APCO Nov 07 BUSERELIN for Central precocious puberty (CPP) APCO May 11 BUSULFAN APCO July 2008 CABAZITAXEL for hormone refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen APCO July 12 CALC FOLINATE APCO July 08 CALC LEVOFOLINATE APCO Jul 08 CALCITONIN for men with primary and scondary prevention of fractures APCO Nov 12 CANAGLIFLOZIN APCO July 14 CANAKINUMAB for the treatment of Gout APCO May 13 CANGRELOR for reducing atherothrombotic events in people undergoing percutaneous coronary intervention or awaiting surgery requiring interruption of anti‑platelet therapy APCO Sept 15 CANNABINOIDS (MEDICAL) CAPECITABINE APCO July 08 CAPREOMYCIN APCO July 2012 CAPSAICIN patch (QUETENZA®) APCO Sep 10 CARBOPLATIN APCO July 08 CARMUSTINE APCO July 08 CARMUSTINE IMPLANTS APCO Sept 10 CARNITINE APCO Jan 04 CAVILON APCO Mar 14 CEFUROXIME APCO Sep 12 CERAZETTE® APCO Jan 11 CERTOLIZUMAB PEGOL for rheumatoid arthritis APCO Mar 10 CETUXIMAB (for squamous cell carcinoma) APCO July 09 CETUXIMAB for metastatic colorectal cancer APCO Sept 11 CETUXIMAB for metastatic colorectal cancer after first line chemotherapy APCO Mar 12 CETUXIMAB for the first-line treatment of metastatic colorectal cancer APCO sept 09 Black In line with TA 212 Black Black Red Black Red Red Red Red Red Black Red Red Black Black Red Black Black Red Black In line with NICE TA 284 In line with MOBBB Priorities Forum indications within NICE in line with MOBB priorities statement 201 indications outside NICE in line with MOBB priorities statement 201 unlicensed Specialist prescribing only In line with NICE TA 253 Specialist prescribing only. Low priority for prescribing in Oxfordshire In line with TA 228 In line with MOBBB Priorities Forum Should only be prescribed by one of four specialist centres Prescribed by specialists only for anal fissure/bladder over activity Low priority for prescribing for axillary hyperhidrosis In line with MOBBB Commissioning Policy Statement 224. In line with NICE TA 260 No evidence to support its place in therapy. Lack of evidence, costly, awaiting TVPC Full supply to be provided by OUHT In line with APCO approved Guidance for Substance Misuse Yellow On recommendation by pain specialist only. For patients with stable pain who have swallowing difficulties and cannot tolerate other options. Not to be used for any other patient group. Under shared care drug misuse LES only For consideration second line after NRT in order to be consistent with guidance for varenicline Shared Care Protocol In line with NPSA Rapid Alert on Oral Anticancer Medicines Brown Brown Yellow Red black Red Red black Brown black Black Black Red Red Black Red Red Red Red Black Brown Brown Red Black Black Black Red In line with NICE TA 255 In line with NPSA Rapid Alert on Oral Anticancer Medicines Specialist prescribing only in Line with lavender statement 232 In line with NICE TA 315 In line with NICE terminated appraisal In line with NICE TA 351 In line with Priorities Forum In line with NPSA Rapid Alert on Oral Anticancer Medicines and NICE TA 191 Specialist prescribing only lack of clinical and cost effectiveness data Specialist prescribing only Secondary care prescribing only. Secondary care prescribing only. In line with lavender statement 83a, NPSA Rapid Alert on Oral Anticancer Medicines and TA 191 Renal physicians only Replaced by Medi Derma S as less expensive and similar efficacy In line with Oxfordshire paediatric antimicrobial guidelines- only for cat or dog bites if child is allergic to penicillin. Restricted use for use in women in whom a POP is indicated but who find the strict 3 hour regimen of standard POPs difficult to adhere to. In line with NICE TA186 Not recommended as per NICE guidance. In line with Policy Statement 208/MOBBB Statement 52 In line with NICE TA 242 In line with NICE TAG 176 CHLORAMBUCIL APCO July 08 CHLORMETHINE HYDROCHLORIDE APCO July 08 CHOLESTAGEL APCO Sept 08 CICLOSPORIN for inflammatory arthritis APCO Nov 10 CICLOSPORIN for inflammatory bowel APCO May 11 CICLOSPORIN for psoriasis APCO May 11 CICLOSPORIN for severe inflammatory skin disease APCO May 11 CILOSTAZOL APCO July 11 CINACALCET APCO Jan 05 CISPLATIN APCO July 08 CLADRIBINE APCO July 08 CLINDAMYCIN - oral APCO July 2010 CLINDAMYCIN for infective endocarditis APCO Nov 09 CLOFARABINE APCO July 08 CLOPIDOGREL with DYPYRIDAMOLE MR for the prevention of occlusive vascular events APCO Jan 11 CLOZAPINE APCO Jan 11 COLECALCIFEROL 800IU (Fultium-D3®) APCO Jan 12 COLECALCIFEROL CAPSULES (10,000IU and 50,000IU) APCO Sep 11 COLECALCIFEROL TABLETS (10,000IU and 50,000IU) APCO Sep 11 COLESEVELAM COLESEVELAM for bile acid malabsorption APCO Nov 13 COLISTIMETHATE SODIUM (Nebulised COLOMYCIN) APCO Nov 13 COLISTIMETHATE SODIUM (PROMIXIN®) APCO Mar 10 COMPRESSION HOSIERY for DVT and early use in oedema to prevent lymphodema APCO Sept 15 CO-PROXAMOL APCO May 05 CRISANTASPASE APCO July 08 CRIZOTINIB for previously treated non-small-cell lung cancer associated with an anaplastic lymphoma kinase fusion gene APCO Nov 13 CUTIMED SORBACT APCO Sept 14 CYCLOPHOSPHAMIDE APCO July 08 CYSTISTAT® (PURIFIED HYALURONIC ACID) APCO Jan 11 CYTARABINE APCO July 08 DABIGATRAN APCO May 2014 DACARBAZINE APCO July 08 DACTINOMYCIN APCO July 08 DALTEPARIN for DVT & long haul flight prophylaxis APCO Jan 15 DALTEPARIN for perioperative anticoagulation, extended thromboprophylaxis & post-partum, intermediate risk pregnancy and Trauma APCO Jan 15 DALTEPARIN for sub-therapeutic INRs, DVT in patients with cancer, IV drug users & first doses in high risk pregnancy APCO Jan 15 DAPAGLIFLOZIN July 13 DAPOXETINE APCO Mar 2014 DAPTOMYCIN APCO Jan 11 DARBEPOETIN APCO Nov 05 DARIFENACIN for overactive bladder syndrome APCO May 11 DASATINIB APCO July 2008 DASATINIB for CML resistant to standard dose imatinib APCO Mar 2012 DASATINIB for the first-line treatment of chronic myeloid leukaemia (part review of technology appraisal guidance 70) APCO May 2012 DAUNORUBICIN APCO july 2008 DEFERASIROX APCO Nov 06 Red Red Black Yellow (NPT) Yellow (NPT) Yellow (NPT) Yellow (NPT) Black Red Red Red Yellow continuation Brown Red Yellow Yellow (NPT) Brown Red Black Yellow Black Red Black Black Black Red Black Black Red Black Red Brown Red Red Brown Red Yellow In line with NPSA Rapid Alert on Oral Anticancer Medicines Specialist prescribing only Not appropriate for prescribing Shared Care Protocol Shared Care Protocol Shared Care Protocol Shared Care Protocol in line with NICE TA223 In line with NICE TA 117 Secondary care prescribing only. Specialist prescribing only Following recommendation Second line prophylaxis (penicillin allergy) for infective endocarditis. Specialist prescribing only In line with TA 210 Shared Care Protocol For osteoporosis patients with a high calcium intake. Similar costs to standard calcium and vitamin D preparations Hospital Prescribing only Not on OUH formulary. No prescribing in primary care In line with SCP NICE evidence summary Funding by NHS England Specialist Commissioning Not on OUH formulary. Rejected by MAC. No evidence of benefit. Can be used for prevention of recurrence of leg ulcers and for 3 months for varicose veins No longer suitable for prescribing Specialist prescribing only In line with NICE TA 296 Insufficient evidence In line with NPSA Rapid Alert on Oral Anticancer Medicines Limited clinical benefit, and any benefit demonstrated in published studies appears to be shortterm Specialist prescribing only In line with NICE TA 249 and local guidelines (to be updated) Specialist prescribing only Specialist prescribing only In line with DVT LES: One off, initial dose only, if outside of DVT clinic hours. Flight prophylaxis rarely required, see Oxfordshire dalteparin guidelines Specialist prescribing only. In line with Dalteparin – Guidelines for Prescribing in Primary Care Brown In line with Dalteparin – Guidelines for Prescribing in Primary Care In line with NICE TA288 Black Commissioning pathway under review Red Red Black Red Black Black Red Red For use at the OUH (NOC) only on advice of ID or Microbiology Consultants in specific situations All prescribing (and funding) transferred to Renal Unit in September 2005. For secondary care prescribing only Not recommended In line with NPSA Rapid Alert on Oral Anticancer Medicines In line with NICE TA 241 In line with NICE TA 251 Specialist prescribing only Specialist prescribing only DEFERIPRONE APCO Nov 06 DEGARELIX for tumour flare prevention in prostate cancer APCO May 11 DEHYDROEPIANDOSTERONE SULPHATE (DHEAS) APCO July 04 DENOSUMAB for the primary and secondary prevention of osteoporotic fractures APCO Aug 14 DENOSUMAB for the prevention of skeletal-related events in adults with bone metastases DENOSUMAB for the prevention of skeletal-related events in adults with bone metastases - prostate cancer related DENOSUMAB for therapy-induced bone loss in non-metastatic prostate cancer APCO Sep 10 DERMASILK GARMENTS APCO July 09 DESFERRIOXAMINE APCO Nov 06 DESMOPRESSIN NASAL SPRAY (OCTIM®) APCO Sep 05 DESSICATED THYROID PRODUCTS (eg ARMOUR THYROID) APCO Nov 12 DEXAMETHASONE intravitreal implant for treating diabetic macular oedema APCO Sept 15 DEXAMFETAMINE APCO jul 2011 DEXRAZOXANE APCO jul 08 DEXTROMORAMIDE for the treatment of addiction APCO July 11 DIAMORPHINE - 500mg APCO July 11 DIAMORPHINE in Substance Misuse APCO July 11 DIAMORPHINE reefers APCO July 11 DIDANOSINE APCO Sept 2005 DILTIAZEM 2% OINTMENT APCO Nov 06 DIPIPANONE (DICONAL®) APCO July 11 DISODIUM FOLINATE APCO jul 2008 DOCETAXEL APCO Jul 2008 DOMPERIDONE APCO jul 2014 DORNASE ALPA in children with cistic fibrosis DOSULEPIN APCO May 05 DOXAZOSIN for BPH APCO Mar 04 DOXAZOSIN for hypertension APCO Mar 04 DOXAZOSIN MR APCO Mar 04 DOXORUBICIN HYDROCHLORIDE APCO Jul 08 DRONEDARONE APCO Jan 11 DROTRECOGIN ALFA (ACTIVATED) DULOXETINE for depression APCO Mar 05 DULOXETINE for neuropathic pain APCO May 13 DULOXETINE for stress incontinence APCO Jul 06 DUODOPA INTESTINAL GEL DuoResp Spiromax DURAPHAT® HIGH FLOURIDE TOOTHPASTE APCO Jan 11 DUTASTERIDE APCO Sep 11 DYMISTA ® APCO May 13 EFALIZUMAB APCO Mar 09 EFAVIRENZ APCO sept 2005 EFLORNITHINE sept 2005 ELMIRON® (PENTOSAN POLYSULFATE SODIUM) APCO july 2010 ELTROMBOPAG for chronic immune idiopathic thrombocytopenic purpura APCO Nov 2010 EMPAGLIFLOZIN ELTROMBOPAG for the treatment of chronic immune or idiopathic thrombocytopenic purpura APCO Nov 2010 EMTRICITABINE APCO sept 2005 ENALAPRIL APCO Nov 05 ENFUVIRTIDE APCO sept 05 ENSURE SHAKE APCO NOV 14 Red Black Black Brown Red Black Black Black Red Red Black Red Yellow Red Black Red Red Black Red Black Black Red Red Brown Red Brown Black Brown Black Red Yellow (NPT) Red Yellow Continuation Brown Yellow Continuation Black Black Black Black Black Black Red Black Black Red Yellow Black Red Brown Red Brown Specialist prescribing only Not recommended In line with NICE TA204 and local guidelines In line with NICE TA 265 In line with NICE TA 265 In line with NICE TA194 Lack of evidence or cost-effectiveness data available. Specialist prescribing only Specialist prescribing only Not recommended for rxing as no eveidence to support use over licensed alternatives In line with NICE TA 349 In line with ADHD shared care protocol Specialist prescribing only In line with National Clinical Assessment Service (NCAS guidance) Specialist prescribing only Specialist prescribing only In line with National Clinical Assessment Service (NCAS guidance) All HIV drugs should be prescribed by specialist centres only Not accepted on to OUHT formulary In line with National Clinical Assessment Service (NCAS guidance) Specialist prescribing only Specialist prescribing only Short term use for nausea and vomiting only in line with MHRA Specialist prescribing only New patients should not be initiated in general practice Not recommended In line with NICE guidance for hypertension Not recommended - use standard release doxazosin Specialist prescribing only In line with APCO Shared Care Protocol To only be prescribed within an intensive care setting Prescribe following recommendation from Oxford Health (Mental Health) only Pain guidelines currently under review Prescribe following recommendation from specialists only In line with priorities statement Non Formulary in line with COPD guidelines Included on dental formulary therefore dentists should prescribe where appropriate rather than refer to the GP. No evidence of advantage over finasteride or that patients failing on finasteride will benefit from dutasteride No evdience of cost efficacy and not in line with current guidelines (use of intranasal corticosteroid with oral antihistamine recommended) Marketing authorisation suspended All HIV drugs should be prescribed by specialist centres only Low priority Not on OUH formulary , unlicensed & no cost- efficacy data In line with NICE TA 293 In line with NICE 336 In line with NICE TA205 All HIV drugs should be prescribed by specialist centres only No longer recommended for initiation in new patients All HIV drugs should be prescribed by specialist centres only Aymes first line ENOXAPARIN APCO Sep 08 ENTACAPONE ENTECAVIR APCO Sep 08 EPIPEN for home IV iron haemodialysis patients APCO Jan 14 EPIRUBICIN HYDROCHLORIDE EPLERENONE EPLERENONE APCO Nov 13 ERDOSTEINE ERECTILE DYSFUNCTION DRUGS ERIBULIN for the treatment of locally advanced or metastatic breast cancer APCO May 12 ERLOTINIB ERLOTINIB for 2nd line treatment of non-small cell lung cancer APCO Sep 12 ERLOTINIB for the first-line treatment of locally advanced or metastatic EGFR-TK mutation_positive none-small cell lung APCO Sep 12 ERLOTINIB monotherapy for the maintenance treatment of non-small cell lung cancer APCO Sep 11 Black Yellow Continuation Red Red Red Brown Yellow Continuation Brown Brown Black Red Black Black Black Red ERYTHROPOIETIN for renal disease APCO Nov 05 ESCITALOPRAM APCO Sep 02 ESOMEPRAZOLE 20mg ESOMEPRAZOLE 40mg ESOMEPRAZOLE GRANULES APCO July 13 ESTRAMUSTINE PHOSPHATE ETANERCEPT APCO Sep 10 ETOGLUCID ETOPOSIDE EVEROLIMUS (with an aromatase inhibitor) for HER2 negative, oestrogen receptor positive, locally advanced or metastatic breast cancer EVEROLIMUS for second-line treatment of advanced renal cell carcinoma APCO May 11 EVEROLIMUS for preventing organ rejection in liver transplantation APCO Sept 15 EXEMESTANE APCO May 07 EXENATIDE APCO July 13 EXENATIDE prolonged release suspension APCO May 12 EZETIMIBE APCO Jan 15 FEBUXOSTAT APCO Sep 08 link FENTANYL BUCCAL TABLETS (EFFENTORA®) APCO Nov 08 FENTANYL IONTOPHORETIC TRANSDERMAL SYSTEM (IONYSIS®) APCO May 08 FENTANYL LOZENGES (ACTIQ®) APCO Nov 08 FESOTERODINE APCO May 11 Fexofenadine Mar 14 FIDAXOMICIN for use in C Diff APCO Sep 13 FILGRASTIM (RECOMBINANT HUMAN GRANULOCYRE-COLONY STIMULATING FACTOR) APCO Sep 07 FINGOLIMOD for the treatment of highly active relapsing-remitting multiple sclerosis APCO May 12 FIRDAPSE APCO Sep 12 FLAMINAL APCO Mar 08 FLOXURIDINE FLUDARABINE PHOSPHATE FLUNARIZINE for migraine prophylaxis APCO July 15 Black Black Brown Brown Red Red Red Red Black Black Black Yellow Continuation Brown Brown Brown Brown Black Red Black Yellow Continuation Brown Red Red Red Black Black Red Red Red Not appropriate for prescribing Must be initiated and monitored by specialists in secondary care with expertise in management of patients with Parkinson's disease In line with NICE TA 153 Hospital supply iron with epipen Specialist prescribing only Prescribe in restricted circumstances Second line for patients that cannot tolerate spironolactone APCO March 2007 In line with DH and local guidance In line with NICE TA 250 In line with NPSA Rapid Alert on Oral Anticancer Medicines and NICE TA 258 Until erlotinib is equal in cost to docetaxal in line with NICE TA 162 In line with NICE TA 258 In line with NICE TA 227 All prescribing (and funding) transferred to Renal Unit in September 2005. For secondary care prescribing only. Shared Care protocol available for monitoring requirements IF these cannot be undertaken within secondary care. No significant advantage over citalopram Not on OUHT formulary. Omeprazole and lansoprazole capsules are current first line choices. Omeprazole 40mg is equivalent to Esomeprazole 20mg in terms of bioavailability. Restricted for patients with significant symptoms requiring very high dose PPI treatment. Restricted for use in enteral tube patients where a small tube is being used or where blockage problems have occurred with lansop fast tabs/Omep MUPs In line with NPSA Rapid Alert on Oral Anticancer Medicines Specialist prescribing only in line with local commissioning arrangements & NICE TA195 & TA199 Specialist prescribing only In line with NPSA Rapid Alert on Oral Anticancer Medicines In line with NICE TA 295 In line with TA 219 In line with NICE TA 348 In line with local guidelines In line with local GLP1 guidelines In line with agreed Exenatide Prescribing Guidelines In line with local guidelines Restricted prescribing in line with NICE TAG 164. Limited information available re place in therapy. Currently no request to use by Palliative Care Team. Specialist prescribing only Limited information available re place in therapy. Currently no request to use by Palliative Care Team. In line with PCT Prescribing guidance No advantage over other antihistamines, disadvantage of not available OTC Specialist prescribing within secondary care Specialist prescribing only Specialist prescribing only in line with NICE TA 254 In line with MOBB statement. Not recommended Specialist prescribing only In line with NPSA Rapid Alert on Oral Anticancer Medicines To be prescribed by specialist, unlicensed and named patient only FLUOCINOLONE ACETONIDE for diabetic macular oedema Fluocinolone acetonide intravitreal implant for the treatment of chronic diabetic macular oedema after an inadequate response to other therapy APCO Mar 13 FLUOROURACIL (ORAL) Black Black Red Brown FLUTICASONE FUROATE nasal spray (Avamys®) FLUTICASONE NASULES FOSAMPRENAVIR FOSFOMYCIN APCO Sep 13 FULVESTRANT APCO Nov 07 FULVESTRANT for the treatment of locally advanced or metastatic breast cancer APCO Jan 12 FUMADERM APCO Mar 04 GALANTAMINE for the treatment of Alzheimers Disease APCO Sep 11 GARMENTS INCLUDING SKINNIES AND DREAMSILK apco March 2015 GALANTAMINE for the treatment of dementia associated with Parkinson’s disease or Lewy Bodies GAMOLENIC ACID GARDASIL Human papillomavirus (HPV) vaccine GEFITINIB for the second-line treatment of locally advanced or metastatic non-small-cell lung cancer GEMCITABINE GLICLAZIDE MR APCO Sep 11 GLUCOSAMINE GLUTEN FREE FOODS APCO Sep 12 GLUTEN FREE FOODS for treatment of autism APCO Jan 03 GLYCOPYRONIUM BROMIDE APCO July 12 GLYCOPYRRONIUM BROMIDE (Seebri Breezhaler®) APCO Jan 13 GLYCOPYRRONIUM for hypersalivation for mental health patients APCO Sep 13 GLYCOPYRRONIUM INHALER for COPD APCO Nov 13 GLYCOPYRRONUIM for hypersalivation for neuro patients APCO Sep 13 GLYCOPYRRONUIUM for hyperhydrosis GOLD (SODIUM AUROTHIOMALATE) for inflammatory arthritis APCO Nov 10 GOLIMUMAB APCO Sep 11 GOSERELIN for Central precocious puberty (CPP) APCO May 11 GRANISETRON APCO May 10 GRAZAX® - ORAL GRASS POLLEN VACCINE APCO May 07 GROWTH HORMONE for “small for gestational age” (SGA) children APCO July 10 GROWTH HORMONE IN ADULTS APCO May 11 GROWTH HORMONE IN CHILDREN APCO July 10 GTN 0.4% ointment APCO June 07 HAELAN TAPE for cracked heels APCO May 13 HAELAN TAPE for eczema APCO May 13 HAELAN TAPE for keloid scars APCO July 13 HEAD LICE DEVICES as listed in Drug Tariff IXA APCO July 08 Brown Red Red Black Black Red Yellow Black Yellow Continuation Black Black Red Red Black Black Brown Black Brown Black Red Black Yellow Black Yellow (NPT) Red Black Black Black Yellow Yellow (NPT) Yellow Brown Black Black Black Black Black HEEL BALMS (DERMATONICS® and FLEXITOL®) APCO Sep 11 HOME PARENTERAL NUTRITION APCO Sep 03 HYALURONIC ACID APCO Mar 09 HYDROCOTISONE MR (Plenadren®) APCO Jan 13 HYDROXYCARBAMIDE (HYDROXYUREA) APCO July 11 HYDROXYCHLOROQUINE for inflammatory arthritis APCO Nov 10 HYDROXYCHLOROQUINE in children and adolescents (Rheumatology) APCO Jul 11 Yellow Red Black Yellow (NPT) Yellow Yellow Specialised commissioning decision Not recommended in line with NICE TA 271 In line with NPSA Rapid Alert on Oral Anticancer Medicines Restricted prescribing 3rd line if fluticasone is being considered. No comparative studies with beclomethasone (1st line) or momentasone (2nd line) which are more cost effective preparations Restricted prescribing for patients with hypo osmia, Aspirin Sensitive Asthma & after 6 week trial of steroid nasal spray for nasal polyps. All HIV drugs should be prescribed by specialist centres only Unlicensed Not recommended In line with NICE TA 239 For prescribing by Dermatologists only In line with PCMAS or specialist recommendation In line with other garments In line with PCMAS or specialist recommendation Priorities Forum Lavender Statement In line with Priorities Forum guidance In line with NICE TAG 175 and TA 192 Specialist prescribing only No clear evidence for using the MR formulation over the immediate release preparation Priorities Forum Lavender Statement 43b In line with agreed MOBB policy, products other than bread and flour are not recommended for prescribing. LS 42c Not prescribed on the NHS as defined by DoH Hypersalivation - limited to children and young adults with neurodevelopmental disabilities who have failed on standard treatment. Insufficient evidence Limited evidence No clinical advantage Moderate evidence No good evidence to support use Shared Care Protocol Specialist prescribing only in line with local commissioning arrangements and TA 220, TA 225 & TA 233 Shared Care Protocol In line with OUH guidance: Surgical antiemetic policy, Cancer antiemetic policy Low priority Shared Care Protocol NICE TA188 Shared Care Protocol Shared Care Protocol For anal fissure. Consider after lifestyle advice/use of topical anaesthetic prior to specialist referral lack of evidence ofcost effectiveness lack of evidence ofcost effectiveness In line with lavender statement 6e Not recommended Lack of good quality evidence comparing emollients. Policy statement 88 recommends that medicines that can be bought over the counter should be considered a low priority for prescribing Shared Care Protocol For limited use within OUH (NOC) as per agreed internal guidelines Not included on OUH formulary. No evidence of improved compliance Shared Care Protocol Shared Care Protocol Shared Care Protocol iAluRil May 2014 IBANDRONATE APCO May 07 IBANDRONIC ACID (oral) for the prevention of skeletal events in metastatic and advanced breast cancer APCO Sept 14 ICAPS® for age related muscular degeneration APCO July 04 IDARUBICIN HYDROCHLORIDE APCO july 2008 IFOSFAMIDE APCO Jul 08 IMATINIB APCO jul 04 IMATINIB HIGH DOSE for CML resistant to standard dose imatinib APCO Mar 12 IMIQUIMOD 3.75% CREAM for actinic keratosis APCO May 07 IMIQUIMOD 5% CREAM for recalcitrant anogenital warts APCO Aug 07 Immediate release FENTANYL intranasal spray for break through cancer pain APCO Mar 11 IMMUNOGLOBULINS: intravenous and subcutaneous APCO Mar 06 INDINAVIR APCO Sept 05 INFERTILITY TREATMENT INFLIXIMAB APCO May 08 INOSINE PRABONEX INOSITOL NICOTINATE APCO July 11 INSULIN DEGLUDEC APCO July 13 INSULIN DETEMIR APCO May 06 INSULIN GLARGINE INTANZA® APCO July 11 IRINOTECAN HYDROCHLORIDE APCO Jul 08 ISONIAZID ISOTRETINOIN – ORAL IVABRADINE – arrhythmia APCO Jan 08 IVABRADINE - Heart Failure APCO Jan 13 IVABRADINE- Chronic stable angina APCO Mar 13 KALCIPOS-D® APCO Sep 11 KETAMINE APCO Mar 12 LACOSAMIDE APCO May 12 LACTASE DROPS (COLIEF ®) APCO Mar 10 LACTOSE FREE MILK APCO Mar 12 LACTOSE FREE MILK for colic LAMIVUDINE for Hepatitis B APCO July 11 LAMIVUDINE for HIV LANREOTIDE for acromegaly APCO Nov 11 LANREOTIDE for neuroendocrine tumours APCO Jan 12 LANTHANUM APCO May 07 Lapatinib in combination with an aromatase inhibitor for the first-line treatment of metastatic hormone receptorpositive breast cancer that over expresses HER2 APCO July 12 LEFLUNOMIDE for inflammatory arthritis APCO May 11 LEFLUNOMIDE in children and adolescents (Rhematology) APCO July 11 LENALIDOMIDE APCO jul 2008 LETROZOLE APCO May 2007 LEUPRORELIN for Central Precocious puberty (CPP) APCO May 11 LIDOCAINE PLASTER for neuropathic pain LINACLOTIDE for IBS (constipation predominent) APCO Mar 13 Black Black Yellow Black Red Red Red Black Black Red Black Red Red Red Red Black Black Yellow cont Brown Brown Black Red Red Red Yellow Yellow SCP Yellow SCP Black Yellow Yellow Black Black Black Red Red Yellow SCP Yellow SCP Yellow Continuation Black Yellow (NPT) Yellow (NPT) Red Yellow Continuation Yellow Yellow Continuation Black specialist treatment - not on OUHT formulary Not recommended For patients that cannot tolerate injections NICE Technology Appraisal 155 (May 2012) Macular degeneration (age-related) – ranibizumab and pegaptanib In line with NPSA Rapid Alert on Oral Anticancer Medicines Specialist prescribing only For prescribing by secondary care oncologists/haematologists only. In line with NICE TA196 TA 209 In line with TA 241 Insufficient evidence of advantage over other treatment options or cost efficacy For specialist GUM consultant prescribing only unclear as to where this product should be used within the pathway, prescribing responsibilities and which patient groups it would be appropriate for use in. All should be provided through the OUHT All HIV drugs should be prescribed by specialist centres only In line with Priorities Forum Statement Specialist prescribing only in line with local commissioning arrangements NICE TAG 187, TA195 & TA199 Not recommended. Not on OUH formulary in line with NICE TA223 In line with local guidelines, for intractable hypos & repeated DKA admissions only Prescribe in line with national and local guidance Restricted prescribing in accordance with Lavender Statement Lack of evidence of improved efficacy compared to standard therapy Specialist prescribing only Specialist prescribing only In line with BNF advice Usually specialist prescribing only but may be appropriate for shared care where an individual, patient specific protocol is agreed. In line with SCP and NICE TA267 In line with SCP The amount of calcium is less than the usual recommended intake. More cost effective preparations available Restricted prescribing for palliative care only, in line with the agreed shared care protocol In line with the APCO approved shared care protocol In line with lactose free milk policy and colic treatment pathway. Lack of evidence for effectiveness. Not recommended for babies without proven intolerance Not recommended in line with lactose free milk policy Shared Care protocol withdrawn - hospital prescribing only All HIV drugs should be prescribed by specialist centres only Shared Care Protocol Only in line with Shared Care Protocol Second line a Shared Care Protocol is available In line with NICE TA 257 Shared Care Protocol Shared Care Protocol In line with NPSA Rapid Alert on Oral Anticancer Medicines In line with local guidance. Shared Care Protocol Prescribe following recommendation from pain specialists only Lack of evidence of efficacy compared with current treatments LINAGLIPTAN APCO Mar 13 LINEZOLID APCO Jan 05 LIRAGLUTIDE 1.2mg (VICTOZA®) for the treatment of Type 2 diabetes mellitus APCO Jan 11 LIRAGLUTIDE 1.8mg (VICTOZA®) for the treatment of Type 2 diabetes mellitus APCO Jan 11 LISDEXAMFETAMINE DIMESYLATE (ELVANSE®) APCO July 13 LITHIUM LIXISENATIDE APCO July 13 LOMITAPIDE for homozygous, familial hypercholesterolaemia May 2014 LOMUSTINE APCO Jul 2008 LOPINAVIR APCO sept 2005 LOXAPINE inhalation for treating acute agitation and disturbed behaviours associated with schizophrenia and bipolar disorder APCO July 13 LUBIPROSTONE APCO Sept 2014 LURASIDONE APCO March 2015 MACUSHIELD® (vitamin prep) APCO Jan 11 MAGNESIUM ASPARTATE APCO March 2015 MANNITOL DRY POWDER for inhalation for the treatment of cystic fibrosis APCO Jan 13 MARAVIROC MELATONIN (CIRCADIN®) for jet lag or primary insomnia APCO July 08 MELATONIN (Oxford Health-Mental Health) APCO Jan 12 MELATONIN for circadian rhythm sleep disorder APCO Jan 12 MELATONIN for sleep EEG APCO Jan 12 MELATONIN paediatric neurology use APCO May 05 MELPHALAN APCO July 2008 MEMANTINE for the treatement of Alzheimers discease APCO Sep 11 MEPITEL, MEPILEX & MEPITAC APCO Jul 06 MERCAPTOPURINE for inflammatory bowel disease APCO May 11 MESNA APCO Jul 2008 METHADONE ampoules APCO July 11 METHADONE CONCENTRATE (10mg, 20mg, 50mg/ml) APCO July 11 METHADONE solution 1mg/ml APCO July 11 METHADONE tablets 5mg APCO July 11 METHOTREXATE (oral) in children and adolescents (Rheumatology) APCO July 11 METHOTREXATE 10MG TABLETS METHOTREXATE for dermatology APCO May 11 METHOTREXATE for inflammatory bowel disease APCO May 11 METHOTREXATE for inflammatory eye conditions APCO May 11 METHOTREXATE for neurology APCO May 11 METHOTREXATE for sarcoidosis APCO July 11 METHOTREXATE ORAL for paediatric gastroenterology APCO Mar 09 METHOTREXATE SUBCUTANEOUS (EBETREX®) APCO Sep 11 METHOTREXATE SUBCUTANEOUS for inflammatory arthritis APCO May 11 METHOTREXATE SUBCUTANEOUS For paediatric rheumatology APCO Sep 11 METHOTREXATE TABLETS for inflammatory arthritis APCO May 11 METHYLNALTREXONE APCO Sep 08 METHYLPHENIDATE APCO July 11 MIDAZOLAM BUCCAL (BUCCOLAM and EPISTATUS® - Unlicensed) APCO Sep 11 Brown Red Yellow Black Red Yellow Brown Black Red Red Black In line with NICE TA 286 In line with NICE TA 318 Psychiatrists use only Low priority for prescribing Continuation after initiation by gastroenterologists In line with NICE TA 266. Unclear whether this will be the responsibility of the National Commissioning Board or CCGs from April 2013. Specialist prescribing only Not recommended Brown Red Black Yellow Red Red Black Red Black Red Yellow Red Yellow Red Yellow Red Black Black Brown Black Yellow Red Yellow Yellow Yellow Yellow Yellow Red (NPT) (NPT) (NPT) (NPT) (NPT) (NPT) (NPT) Black Yellow (NPT) Yellow (NPT) Yellow (NPT) Black Yellow Yellow Continuation Red MIDAZOLAM INTRA-NASAL APCO Mar 11 MIDODRINE APCO May 05 Mirabegron APCO jul 13 For use in patients with renal impairment in line with local Guidelines and NICE CG 87 Secondary care prescribing only In line with APCO Liraglutide Guidance In line with NICE TA203 Secondary care prescribing only In line with Shared Care Protocol In line with local GLP1 guidelines specialised commissioning/not routinely commissioned In line with NPSA Rapid Alert on Oral Anticancer Medicines All HIV drugs should be prescribed by specialist centres only Red Yellow Specialist prescribing within Oxford Health Mental Health - for child and adolescent prescribing only Not recommended Secondary care prescribing only An individual Shared Care Protocol will be issued each time melatonin is recommended for children under the care of paediatric neurology depending on the indication In line with NPSA Rapid Alert on Oral Anticancer Medicines In line with PCMAS or specialist recommendation In line with local Wound Management Guidance Shared Care Protocol Secondary care prescribing only In line with National Clinical Assessment Service (NCAS guidance) In line with APCO approved Guidance for Substance Misuse Under shared care drug misuse LES only In line with APCO approved Guidance for Substance Misuse Shared Care Protocol Secondary care prescribing only Shared Care Protocol Shared Care Protocol Shared Care Protocol Shared Care Protocol Shared Care Protocol Secondary care prescribing only Metoject® is the brand of choice for Oxfordshire – restricted to one brand across the health economy Shared Care Protocol Shared Care Protocol Shared Care Protocol Not appropriate for prescribing To be used following recommendations from Oxford Health (Mental Health) consultants only. A Shared Care Protocol is available Shared Care Protocol For use by the Oxfordshire Salaried Primary Care Dental Service only for special care adults and adults with needle phobia - subject to the medico-legal aspects being agreed with Oxford Health. Secondary care prescribing only In line with OAB guidelines MITOBRONITOL APCO jul 08 MITOMYCIN APCO jul 08 MITOTANE APCO sept 05 MITOXANTRONE APCO jul 08 MODAFINIL MODAFINIL for excessive daytime sleepiness in myotopic dystrophy May 2014 MOLLUDAB APCO Sept 14 MOVICOL APCO Jan 05 MYCOPHENOLATE for rheumatology APCO Nov 10 MYCOPHENOLATE in Autoimmune Liver Disease and Liver Transplant APCO Jan 12 MYCOPHENOLATE in dermatology APCO Sep 11 MYCOPHENOLATE in Inflammatory Bowel Disease APCO Jan 12 MYCOPHENOLATE in Interstitial Lung Disease APCO Nov 11 MYCOPHENOLATE in renal transplant APCO Sept 05 NALMEFENE APCO Nov 14 NALOXEGOL for treating opioid induced constipation APCO Sept 15 NALTREXONE APCO Nov 04 NALTREXONE low dose for multiple sclerosis APCO Mar 05 NANO-PARTICLE BOUND PACLITAXEL for metastatic breast cancer APCO sept 2010 NAPROXEN & ESOMEPRAZOLE (VIMOVO®) APCO Jan 12 NATALIZUMAB for treatment of MS APCO sept 2012 NEGATIVE PRESSURE WOUND THERAPY NELARABINE APCO Jul 08 NELFINAVIR APCO Sept 2005 NEVIRAPINE APCO Sept 2005 NICOTINAMIDE for Bullous Pemphigoid APCO Jan 14 NICOTINE REPLACEMENT THERAPY NILOTINIB for CML resistant to standard dose imatinib APCO Jun 2007 NINTEDANIB for previously treated, locally advanced, metastatic or locally recurrent non-small-cell lung cancer APCO Sept 15 NUTRITIONAL SUPPLEMENTS (oral) for patients in care and nursing homes Sept 15 NUTRITIONAL SUPPLEMENTS – dessert style supplements (eg FORTICREME, FRESUBIN CREME) NUTRITIONAL SUPPLEMENTS 1kcal/ml (eg ENSURE®, FRESUBIN®) APCO Jan 11 NUTRITIONAL SUPPLEMENTS 2kcal/ml (eg TWOCAL®, ENSURE TWOCAL®) NUVARING APCO July 09 OBINUTUZUMAB in combination with chlorambucil for untreated chronic lymphocytic leukaemia APCO July 15 OCRIPLASMIN for treating vitreomacular traction APCO Nov 13 OCTREOTIDE for acromegaly APCO Nov 11 OCTREOTIDE for neuroendocrine tumours OCUVITE for age related muscular degeneration APCO july 04 OFATUMUMAB for the treatment of chronic lymphocytic leukaemia refractory to fludarabine and alemtuzumab APCO Nov 10 OFATUMUMAB in combination with chlorambucil or bendamustine for untreated chronic lymphocytic leukaemia APCO July 15 OLANZAPINE for Behavioural and Psychiatric symptoms of dementia APCO Sep 11 OLANZAPINE IM APCO May 04 OMALIZUMAB for previously treated chronic spontaneous urticaria APCO July 15 OMALIZUMAB in asthma for adults and children over 12 Red Red Red Red Yellow Continuation Red Black Brown Yellow (NPT) Yellow (NPT) Yellow (NPT) Yellow (NPT) Yellow (NPT) Red Red Brown Yellow Continuation Black Black Black Red Yellow Red Red Red Black Brown Red Red Black Brown Black Brown Brown Red Red Yellow Red Black Black Red Brown Red Red Red In line with NPSA Rapid Alert on Oral Anticancer Medicines Specialist prescribing only In line with NPSA Rapid Alert on Oral Anticancer Medicines Specialist prescribing only Prescribe only following recommendation from local specialist in sleep disorders In line with EMA safety guidance Limited evidence, can be purchased Should only be second/third line for patients who cannot tolerate lactulose/fybogel Shared Care Protocol Shared Care Protocol Shared Care Protocol Shared Care Protocol Shared Care Protocol Renal transplant drug for specialist initiation and prescribing only Refer patients to Public Health Drug and Alcohol Service for patients who have not responded to other treatments in line with NICE TA 345 May be initiated by a consultant hepatologist for cholestatic itching Unlicensed with little supporting evidence – should not be prescribed Specialised commissioning decision No studies comparing with standard treatment. Esomeprazole 20mg is also classified locally as black. In line with NICE TA 127 Guidelines Specialist prescribing only All HIV drugs should be prescribed by specialist centres only All HIV drugs should be prescribed by specialist centres only Unlicesnsed - lack of evidence Priorities Forum Lavender Statement In line with TA 241 In line with NICE TA 347 NOT PEG TUBE PATIENTS. Food fortification by care home staff should be used in most cases. Exceptions are patients with head and neck cancer or motor neurone disease Restricted for use only where recommended by a dietitan / Speech & Language therapist in dysphagia. In line with local Guidelines for the Management of Undernutrition. Use 1.5kcal/ml supplements (such as Complan Shake) where a nutritional supplement is appropriate to prescribe. In line with local Guidelines for the Management of Undernutrition. Restricted for use only where a patient is under a specialist/dietitian. 2kcal/ml supplements can be used to reduce the volume in bolus feeding via PEG tube or in fluid restricted patients, such as ascites in liver disease. In line with local Guidelines f 2nd or 3rd line option in line with specific patient criteria. in line with NICE TA 343 In line with NICE guidance Only in line with Shared Care Protocol Specialised Commissioning only Low priority for prescribing In line with NICE TA202 In line with NICE TA 344 Short term use in line with Antipsychotic Use for Behavioural and Psychiatric Symptoms of Dementia: Prescribing Guidance (2nd line IF antipsychotic required - off license use) Oxford Health (Mental Health) specialists only in line with NICE TA 339 Specialist prescribing only in line with NICE TA133 OMALIZUMAB for the treatment of severe persistant allergic asthma in children aged 6-11 APCO Nov 2010 OMEGA 3 SUPPLEMENTS for hypertriglyceridaemia APCO Mar 11 ONDANSETRON APCO May 10 ORLISTAT OSELTAMIVIR – for the treatment or prophylaxis of influenza OXALIPLATIN APCO Jul 08 OXCODONE 50mg/5mL APCO May 11 OXYBUTYNIN PATCH APCO May 11 OXYCODONE APCO may 11 OXYCODONE and NALOXONE (TARGINACT®) APCO Jul 09 PABRINEX® APCO Nov 11 PACLITAXEL APCO Jul 08 PALIFERMIN APCO July 08 PALIPERIDONE APCO sept 07 PALIVIZUMAB APCO Nov 10 PANITUMUMAB APCO July 08 PANITUMUMAB for metastatic colorectal cancer after first line chemotherapy APCO Mar 12 PANITUMUMAB in combination with chemotherapy for treatment of metastatic colorectal cancer APCO Jan 12 PARACETAMOL INJECTION APCO Jan 06 PARACHLOROPHEYLOLANINE APCO Jul 08 PARATHYROID hormone for men with primary and secondary prevention of fractures APCO Nov 12 PAZOPANIB for the first line treatment of metastatic renal cell carcinoma APCO May 11 PEG INTERFERON APCO July 05 PEGAPTANIB PEGAPTANIB for diabetic macular oedema APCO Jan 12 PEGINTERFron alfa and ribavirin (VIRAFERONPEG®) for the treatment of chronic hepatitis C (part review of TAGs 75 and 106) APCO Nov 10 PEGLOTICASE for treating severe debilitating chronic tophaceous gout APCO July 13 PEGVISOMANT APCO Sep 05 PEMETREXED APCO jul 2008 PENICILLAMINE APCO Nov 10 PENTOSTATIN APCO jul 08 PENTOXIFYLLINE APCO July 11 PENTOXIFYLLINE for gastric/liver indications APCO Jan 14 PENTOXIFYLLINE for venous leg ulcer APCO Nov 13 PENTOXIFYLLINE in osteoradionecrosis (ORN) of the jaws APCO Nov 12 PERAMPANEL APCO Nov 13 PERHEXILINE APCO Nov 13 PERINDOPRIL ARGININE PLUS INDAPAMIDE (COVERSYL®) APCO July 09 PHARMALGEN APCO Mar 12 PIMECROLIMUS cream APCO Jul 11 PIRFENIDONE for treating idiopathic pulmonay fibrosis APCO May 13 PLERIXAFOR for Mutiple myeloma PLERIXAFOR for non-Hodgkin lymphoma PORFIMER SODIUM POSACONAZOLE APCO Nov 06 PRAMIPEXOLE for Idiopathic Restless Leg Syndrome APCO Sep 11 PRASUGREL APCO May 10 PREDNISOLONE 0.01%,0.03% & 0.1% EYE DROPS APCO Jan 04 PREDNISOLONE 0.03% & 0.3% PRESERVATIVE FREE EYE DROPS APCO Jan 04 PREDNISOLONE EC tabs APCO Jan 11 PREGABALIN for neuropathic pain PRISTINAMYCIN APCO Nov 06 Black Black Yellow Brown Brown Red Brown Brown Brown Black Yellow Red Red Black Red Red Black Black Red Red Black Red Red Black Black Red Black Black Red Yellow (NPT) Red Black Red Yellow Yellow Yellow Shared Care Red Black Red Yellow Red Black Red Red Red Yellow Yellow Red Red Black Yellow Continuation Red In line with NICE TA201 In line with NICE CG172 In line with OUH guidance: Surgical antiemetic policy, Cancer antiemetic policy Only prescribe following NICE CG43 Only prescribe according to guidelines Specialist prescribing only Restricted use – within syringe drivers where volume is an issue In line with Prescribing guidance, for patients with swallowing difficulties In line with Opoid Guidelines Lack of evidence of cost-effectiveness In line with Guidance for Primary Care Alcohol Detoxification Specialist prescribing only Specialist prescribing only APCO September 2007 Specialist prescribing only Specialist prescribing only In line with NICE TA 242 In line with NICE TA 240 Used selectively in OUHT Specialist prescribing only in Line with lavender statement 232 In line with TA 215 Secondary care prescribing only Not recommended in line with NICE TA155 NICE TA 274 In line with NICE TA200 In line with NICE TA 291 Considered low priority for prescribing In line with NPSA Rapid Alert on Oral Anticancer Medicines NICE TAG 190 Shared Care Protocol Specialist prescribing only in line with NICE TA223 Specialist use only In line with Shared Care Protocol In line with SCP In line with Shared Care Protocol Unlicensed In line with OUH MAC decision In line with NICE TA 246 For moderate atopic eczema on the face and neck of children aged 2-16yrs in line with NICE TA 82 In line with NICE TA 282 Specialised commissioning decision Specialist prescribing only Specialist prescribing only Specialist prescribing only Shared Care Protocol In line with NICE TA 182 – patient should be stabilised by OUH and length of treatment should be advised when primary care is requested to take over prescribing. All specially prepared eye drops to be prescribed and dispensed by the OUHT All specially prepared eye drops to be prescribed and dispensed by the OUHT Lack of evidence of cost-efficacy Prescribe following recommendation from pain specialists only Specialist prescribing only PRO-BIOTICS PROCARBAZINE HYDROCHLORIDE PROFLAVINE CREAM APCO Sep 13 PROPIVERINE APCO May 11 PRUCALOPRIDE for chronic constipation in women APCO May 12 PYRAZINAMIDE QLAIRA® phased combined hormonal contraceptive APCO July 11 QUETIAPINE for Behavioural and Psychiatric symptoms of dementia APCO July 13 QUETIAPINE for GAD APCO July 13 QUETIAPINE XL APCO Sep 09 RACECODATRIL for children and adults APCO Mar 13 RALTEGRAVIR RALTITREXED RAMELTEON APCO May 08 RANIBIZUMAB for choroidal neovascularisation associated with a pathological myopia RANIBIZUMAB for diabetic macular oedema APCO Mar 13 RANIBIZUMAB for macular oedema caused by retinal vein occlusion APCO Jan 12 RANIBIZUMAB for wet AMD RANOLAZINE APCO July 12 RASAGILINE APCO Nov 13 RAZOXANE RECOMBINANT HUMAN GRANULOCYTE-COLONY STIMULATING FACTOR RELVAR ELLIPTA for Asthma March 2014 RELVAR ELLIPTA for COPD May 2015 RELVAR ELLIPTA for non-compliant, poorly controlled teenage asthmatics at stage 5 of BTS May 2014 RESPERATE® APCO Mar 12 RETAPAMULIN OINTMENT APCO May 08 RETIGABINE for the adjunctive treatment of adults with partial onset seizures in epilepsy with and without secondary generalisation APCO Sep 11 RETIN-A for stretch marks RIFAMPICIN APCO May 12 RIFAMPICIN for TB APCO July 09 RIFAXIMIN for hepatic encephalopathy APCO May 15 RILUZOLE APCO May 13 RISPERIDONE for Behavioural and Psychiatric symptoms of dementia APCO Sep 11 RISPERIDONE IM APCO July 05 RITONAVIR RITUXIMAB RITUXIMAB for mantle cell lymphoma RIVAROXABAN for the prevention of stroke and systemic embolism in people with atrial fibrillation. APCO Sep 12 RIVAROXABAN for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary embolism(TA261) APCO May 13 RIVAROXABAN for preventing adverse outcomes of acute coronary system RIVASTIGMINE (EXELON®) PATCH APCO Sep 09 RIVASTIGMINE for the treatment of Alzheimers Disease APCO Sep 11 RIVASTIGMINE for the treatment of dementia associated with Parkinson’s disease or Lewy Bodies ROFECOXIB INJECTION ROFLUMILAST APCO Sep 12 Black Red Black Black Brown Red Black Brown Black Red Black Red Red Black Red Red Red Red Yellow Yellow Red Red Black Yellow Yellow continuation Black Black Yellow Continuation Black Brown Red Red Yellow Brown Red Red Red Black Brown Yellow Yellow Yellow Continuation Yellow Yellow Continuation Black Black In line with Priorities Forum Policy Statement 125 In line with NPSA Rapid Alert on Oral Anticancer Medicines No evidence to support use In line with local prescribing guidance In line with TA 211 Specialist prescribing only No evidence of clinical benefit over alternatives and more expensive, no long term safety data. Short term use in line with Antipsychotic Use for Behavioural and Psychiatric Symptoms of Dementia: Prescribing Guidance (2nd line IF antipsychotic required - off license use) Limited evidence suggests no greater efficacy than antidepressants No clinical evidence of increased tolerability compared to immediate release therapy. Only anecdotal evidence of benefit in titration. Limited evidence In line with Priorities Forum Lavender Statement Specialist prescribing only Currently unlicensed - No Prescribing In line with NICE ta 298 In line with NICE TA 274 In line with NICE TA 283 In line with NICE TA 155 Shared Care Protocol Parkinson's treatment for specialist initiation, second line after selegiline. Specialist prescribing only Specialist prescribing only Safety concerns and lack of robust evidence 2nd line option if steroid inhaler indicated In line with local COPD guidelines for non-compliant, poorly controlled teenage asthmatics at stage 5 of BTS on recommendation from paediatric respiratory only Routine provision of relaxation therapies by primary care teams is not currently recommended within NICE CG 127 Hypertension Not recommended In line with NICE TA 232 Not recommended in line with Priorities Forum statement 6 Restricted prescribing in line with antimicrobial guidance Specialist prescribing only In line with NICE TA 337 In line with NICE TA20 and Shared Care Protocol Short term use in line with Antipsychotic Use for Behavioural and Psychiatric Symptoms of Dementia: Prescribing Guidance (1st line IF antipsychotic required) Secondary care prescribing only All HIV drugs should be prescribed by specialist centres only Specialist prescribing only in line with local commissioning arrangements and TA 193, TA195, TA226 & TA243 In line with Policy Statement 207/MOBBB Statement 54 In line with NICE TA 256 and local guidance In line with NICE TA 261. First three months tx to be provided by OUH for new DVT/PE. In line with NICE TA 335 Only if patient unable to tolerate oral therapy, clinical evidence not sufficient to recommend first line. Shared Care Protocol and in-line with TA 217 In line with Priorities Forum Statement 134 Not on OUHT formulary for acute pain due to lack of evidence In line with NICE TA 244 - recommended only as part of a clinical trial ROMIPLOSTIM for the treatment of chronic immune or idiopathic thrombocytopenic purpura APCO May 11 ROPINIROLE for restless legs - mild to moderate ROPINIROLE for restless legs – severe & persistent Red Black Red In line with TA 221 In line with Priorities Forum July 2008 Specialist prescribing only in line with Priorities forum July 2008 Brown For use only where other first and second line statins have not been tolerated, or for very high risk secondary prevention patients where first and second line agent have not been effective. Following specialist diagnosis in line with the recommendations for the management of Parkinson disease in NICE Clinical Guideline 35 and • In patients unable to tolerate ropinirole or • In patient with an insufficient response to ropinerole or • In patient unable to swallow oral medicines Specialist only in Lennox Gastaut Syndrome In line with NICE TA 289 All HIV drugs should be prescribed by specialist centres only Effect size of reducing spasticity is small, with very little translation into patient benefit. No cost effectiveness studies. Few long term cannabinoids studies. For use in line with In line with NICE Type 2 Diabetes CG87. Alogliptin is first choice DPP4 APCO May 14 In line with NICE TA 350 Shared Care Protocol Jan 12 Specialist prescribing only In line with lavender statement 233 For use in line with In line with NICE Type 2 Diabetes CG87. Alogliptin is first choice DPP4 APCO May 14 Only for treatment of hypercalcaemia anociated skeletal metastases in patients with breast cancer or multiple myeloma following recommendation from specialists ROSUVASTATIN APCO March 2011 Brown ROTIGOTINE PATCH for parkinsons disease or restless legs APCO Mar 11 RUFINAMIDE APCO Sep 08 RUXOLITINIB for disease-related splenomegaly or symptoms in adults with myelofibrosis APCO Jul 13 SAQUINAVIR APCO Nov 06 appendix B SATIVEX® APCO Nov 10 SAXAGLIPTIN APCO Nov 10 SECUKINUMAB for treating moderate to severe plaque psoriasis APCO Sept 15 SEVELAMER APCO Aug 07 SILDENAFIL for pulmonary artery hypertension APCO Mar 11 SILDENAFILfor penile rehabilitation after radical prostatectomy Priorities Committee Sep 12 SITAGLIPTIN (JANUVIA®) APCO Jul 11 SODIUM CLODRONATE APCO Nov 06 SODIUM OXYBATE SOLIFENACIN 5mg SOLIFENACIN 10MG SOLIFENACIN and TAMSULOSIN combination APCO Sept 2014 SOMATROPIN for the treatment of growth failure in children APCO Jul 10 SORAFENIB (first- and second-line) for the treatment of advanced and/or metastatic renal cell carcinoma APCO Sep 09 SORAFENIB for the treatment of advanced hepatocellular carcinoma APCO Jul 10 SOUVENAID ® APCO Jan 13 STAVUDINE APCO Nov 06 appendix B STIRIPENTOL APCO Nov 14 Red Black Red Black Brown Red Yellow Red Black Brown Yellow Continuation Red Yellow continuation Yellow continuation Black Yellow Black Black Black Red Red Black STOMA products APCO July 11 STRIVERDI RESPIMAT Nov 14 STRONTIUM STRONTIUM for men with primary and scondary prevention of fractures APCO Nov 12 SULPHAMETHOXYPYRIDAZINE for pemphigoid May 2014 SULPHASALAZINE for inflammatory arthritis APCO Nov 10 SULPIRIDE for Behavioural and Psychiatric symptoms of dementia APCO May 11 SUMATRIPTAN RADIS SUNITINIB SUNITINIB for the treatment of gastrointestinal stromal tumours APCO Nov 09 SYLK® Paraben free personal lubricant APCO Jan11 TACROLIMUS for liver transplantation APCO Jul 11 TACROLIMUS for renal transplantation TADALAFIL (for pulmonary artery hypertension) Black Brown Black Black Yellow (NPT) Brown Black Red Red Black Yellow Red Red In line with OAB guidelines In line with OAB guidelines Shared Care Protocol, Growth Hormone Paediatric In line with NICE TA178 In line with NICE TA189 Some evidence of improved memory but limited to very early disease only and no improvement in cognition demonstrated. Can be purchased OTC. All HIV drugs should be prescribed by specialist centres only Specialist prescribing only certain Suportx, Cuiwear, Comfizz, Simplicity products considered to be cosmetic rather than clinical. Refer to Stoma Prescribing Guidance and 'Stoma products' hyperlink on left for more detail. LABA only licensed in COPD, no advantage over existing treatment options See MHRA safety alert in Line with lavender statement 232 Hospital only drug - not on OUHT formulary Shared Care Protocol Short term use in line with Antipsychotic Use for Behavioural and Psychiatric Symptoms of Dementia: Prescribing Guidance (2nd line IF antipsychotic required - off license use) Not recommended Secondary care prescribing only for the first line treatment of advanced and/or metastatic renal cell carcinoma In line with NICE TA179 Not recommended for prescribing, available for OTC purchase Shared Care Protocol Specialist initiation and prescribing only Specialist prescribing only TADALAFIL for penile rehabilitation pre- and post- radical prostatectomy May 2014 Tadalafil for the treatment of symptoms associated with benign prostatic hyperplasia APCO Mar 13 TADALAFIL ONCE A DAY (CIALIS®) APCO Mar 09 TALAPREVIR for the treatment of genotype 1 chronic hepatitis C APCO May 12 TAMSULOSIN MR (FLOMAXTRA-XL®) APCO Sep 11 TAPENTADOL APCO July 11 TARGINACT apco Jul 09 TEGAFUR APCO Jul 08 TEICOPLANIN APCO Jan 05 TELBIVUDINE TEMOPORFIN TEMOZOLOMIDE - dose-dense in recurrent glioblastoma multiforme TEMOZOLOMIDE for malignant glioma TEMSIROLIMUS (first-line) for the treatment of advanced and/or metastatic renal cell carcinoma TEMSIROLIMUS for the treatment of relapsed or refractory mantle cell lymphoma (terminated appraisal) APCO Nov10 TENOFOVIR APCO Sept 05 TENOFOVIR DISOPROXIL for chronic hepatitis B APCO Sept 09 TERBINAFINE TERIPARATIDE APCO May 12 TERIPARATIDE for men with primary and scondary prevention of fractures APCO Nov 12 TESTOSTERONE (ANDROPATCH®) PATCH APCO Aug 07 TESTOSTERONE (INTRINSA®) PATCH for hypoactive sexual disorder APCO June 07 TESTOSTERONE (STRIANT®) BUCCAL TABLETS APCO Aug 07 TESTOSTERONE GEL (TESTIM® & TESTOGEL®) APCO Jan 10 TESTOSTERONE GEL (TOSTRAN®) APCO Jan 09 TESTOSTERONE UNDECANOATE (NEBIDO®) APCO Nov 11 THALIDOMIDE and Bortezomib for the first-line treatment of multiple myeloma THICKENERS for dysphasia APCO Sept 06 THIOTEPA APCO Jul 08 TICAGRELOR for patients with ACS APCO Nov 12 TINZAPARIN APCO Jan 14 TINZAPARIN APCO Sep 08 TIOGUANINE Jul 08 NPSA alert TIOTROPIUM for ASTHMA March 2015 TIOTROPIUM RESPIMAT APCO Jul 08 TOBRAMYCIN (INHALED) (TOBI PODHALER®) APCO Nov11 TOBRAMYCIN (INHALED) (TOBI® & BRAMITOP®) APCO Jan 11 TOCILIZUMAB for rheumatoid arthritis APCO Mar 12 TOCILIZUMAB for the treatment of juvenile idiopathic arthritis APCO Jan12 TOLCAPONE APCO Jul 06 TOLTERODINE APCO May 11 TOLTERODINE MR APCO May11 TOPICAL steroid for Wound Management APCO Mar 13 TOPOTECAN APCO Jan10 TRABECTEDIN APCO Mar10 TRABECTEDIN for the treatment of relapsed ovarian cancer APCO May 11 TRAMACET APCO Mar06 TRASTUZUMAB APCO Jan11 TRASTUZUMAB in combination with an aromatase inhibitor for the first-line treatment of metastatic hormone receptor- positive breast cancer that over expresses HER2 APCO Jul12 TREDAPTIVE for hypertryglyceridaemia APCO Mar 11 Black Black Black Red Black Black Black Red Red Black Red Red Red Black Black Red Red Brown Yellow Black Black Black Black Yellow (NPT) Yellow (NPT) Yellow (NPT) Red Yellow Continuation Red Yellow Continuation Yellow shared care Black Red Yellow Brown Black Yellow Red Red Black Brown Black Brown Red Red Black Black Red Black Black In line with lavender statement 233 NICE appraisal terminated Not recommended In line with NICE TA252 No evidence of clinical advantage over standard preparation Lack of evidence of clinical efficacy compared to standard treatment lack of evidence of cost effectiveness In line with NPSA Rapid Alert on Oral Anticancer Medicines Secondary care prescribing only In line with NICE TA154 Specialist prescribing only Specialist prescribing only Specialist prescribing only In line with NICE TA 178 In line with NICE TA207 All HIV drugs should be prescribed by specialist centres only In line with NICE TA 173 Follow recommendations from Priorities Forum Lavender Statement. Shared Care Protocol in Line with lavender statement 232 Not recommended for use as no more clinically effective than testogel, testim and sustanon but more expensive For hypoactive sexual disorder in the general female population (out of license use) Not recommended for use as no more clinically effective than testogel, testim and sustanon but more expensive Prescribe for males only within licensed indications according to shared care protocols Prescribe for males only within licensed indications according to shared care protocols Prescribe for males only within licensed indications according to shared care protocols In line with TA 228 Only on recommendation of Speech and Language Therapy Specialist prescribing only on recommendation from cardiologists only, in line with agreed guidelines For SE locality patients referred to RBH ONLY Not appropriate for prescribing In line with NPSA Rapid Alert on Oral Anticancer Medicines Specialist initiation add on to LABA and corticosteroids Restricted for patients unable to use Handihaler No request to include on OUH formulary Shared Care Protocol In line with TA 247 In line with NICE TA 238 Not recommended. Awaiting consideration by OUHT MAC See OAB guidelines see OAB guidelines In line with agreed local guidelines Specialist prescribing only. NICE TA 184 gives guidance for the treatment of relapsed small-cell lung cancer Specialist prescribing only in line with NICE TA185. In line with TA 222 Not recommended for prescribing due to sub-therapeutic doses Specialist prescribing only. TA 208 In line with NICE TA 257 Withdrawn from market TREOSULFAN APCO Jul 08 TRETINOIN IV TRIADCORTYL APCO Jan 14 TRICLOFLOS APCO May 04 TRIMOVATE for wound management APCO Nov 13 TRIPTORELIN (DECAPEPTYL SR®) APCO Sep 11 TRIPTORELIN for central precocious puberty APCO Sept 05 TROPISETRON APCO May 10 TROSPIUM APCO Jan 14 ULIPRISTAL ACETATE (ELLAONE®) APCO Nov 09 ULIPRISTAL ACETATE (ESMYA®) APCO July 15 USTEKINUMAB for the treatment of adults with moderate to severe psoriasis APCO Nov09 USTEKINUMAB for treating psoriatic arthritis APCO Jul14 VACUUM ERECTION DEVICES for penile rehabilitation after radical prostatectomy APCO Nov 12 VARENICLINE (CHAMPIX®) APCO Sept07 VEDOLIZUMAB for treating moderately to severely active ulcerative colitis APCO July 14 VENLAFAXINE for Hot Flushes APCO Nov 05 Brown Red Black Black VENLAFAXINE MR CAPSULES APCO Sep 11 VIAGRA® Black Brown VILDAGLIPTIN APCO July 11 VINBLASTINE SULPHATE APCO Jul08 VINCRISTINE SULPHATE APCO Jul08 VINDESINE SULPHATE APCO Jul 08 VINORELBINE APCO Jul 08 VIRULITE COLD SORE MACHINE APCO Mar 08 Red Red Red Red Black VITAMIN PREPARATIONS for age related macular degeneration APCO May 07 VORICONAZOLE APCO July 03 YASMIN® APCO Jan 11 YES APCO Jul14 ZALCITABINE APCO Sept 05 ZANAMIVIR - for the TREATMENT or PROPHYLAXIS of influenza APCO Mar09 ZIDOVUDINE APCO Sept 05 ZOELY ® APCO May 13 ZOLEDRONIC ACID for men with primary and scondary prevention of fractures APCO Nov 12 ZOLEDRONIC ACID for Pagets, Osteoporosis and home TPN patients APCO Jun 07 ZOLEDRONIC ACID for steroid induced osteoporosis APCO Mar 10 ZONISAMIDE APCO Nov 10 ZUCLOPENTHIXOL ACETATE APCO May 2014 Drug Jaydess 13.5 mg intrauterine delivery system Red Red Red Red Yellow Yellow Yellow Black Brown Brown Black Red Black Black Black Red Brown Black Red Brown Red Black Black Red Brown Yellow Red Traffic Light Holding List In line with NPSA Rapid Alert on Oral Anticancer Medicines Specialist prescribing only Prescribed and dispensed by the OUHT Specialist initiation for wound bed healing In line with other analogues, 6 monthly administration and is cost neutral Shared Care Protocol In line with OUH guidance: Surgical antiemetic policy, Cancer antiemetic policy In line with OAB guidelines For women who present between 72 -105 hours and an IUD is not felt to be appropriate For uterine fibroids - no request to use this from OUH gynae In line with NICE TA 180 NICE TA313 In line with lavender statement 233 To be used second line following NRT or where intolerant to NRT - APCO approved local guidance for patients intolerant of NRT or where NRT is unsuccessful. in line with NICE TA 342 Not licensed. Awaiting a review of the evidence No evidence for using MR capsules rather than tablets – the MR tablets have significant cost savings benefits Generic prescribing only APCO Sep 14 For use in line with In line with NICE Type 2 Diabetes Mellitus clinical guideline. Vildagliptan not first choice DPP4 in line with local Guidelines. Specialist prescribing only Specialist prescribing only Specialist prescribing only In line with NPSA Rapid Alert on Oral Anticancer Medicines Not recommended Not recommended. Including Ocuvite Presser Vision, VisiVite Original Formula, Viteyes AREDS Formula – Specialist prescribing only Restricted use for women who are unable to tolerate alternative COCs Can be purchased OTC policy lavender statement 88 All HIV drugs should be prescribed by specialist centres only Only prescribe according to guidelines NICE TA168 All HIV drugs should be prescribed by specialist centres only No evidence of clinical benefit over alternatives and more expensive, no long term safety data. in Line with lavender statement 232 Specialist prescribing only. In line with Secondary Prevention of Fragility Fractures Algorithm for Treatment In line with local steroid induced OP pathway In line with shared care protocol. to be used under the direction of consultant psychiatrist Rationale Awaiting Public Health review







