Section 4. Overview of Fuel oxidation, ATP generation: Glycolysis is
advertisement

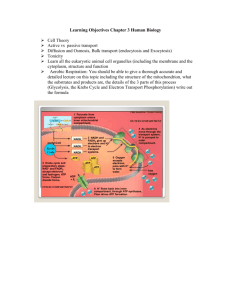
Section 4. Fuel oxidation, generation of ATP Fuel oxidation overview - respiration Phase 1: energy (e-) from fuel transfer to NAD+ and FAD; Acetyl CoA, TCA intermediates are central compounds Phase 2: electron transport chain convert e- to ATP; membrane proton gradient drives ATP synthase Section 4. Overview of Fuel oxidation, ATP generation: Physiological processes require energy transfer from chemical bonds in food: • • • Phase 3: ATP powers processes Electrochemical gradient Movement of muscle Biosynthesis of complex molecules 3 phases: • Oxidation of fuels (carbs, fats, protein) Fig.iv.1 • Conversion of energy to ~PO4 of ATP • Utilization of ATP to drive energy-requiring reactions Respiration occurs in mitochondria Glucose is universal fuel for every cell Respiration occurs in mitochondria: Glycolysis is universal fuel: 1 glucose -> 2 pyruvate + 2 NADH + 2 ATP • Most enzymes in matrix • Aerobic path: • Inner surface has • e- transport chain • ATP synthase • • • • • ATP transported through inner membrane, diffuses through outer • Some enzymes encoded by mitochondrion genome, • most by nuclear genes Fig. iv.2 Continued oxidation Acetyl CoA -> TCA, NADH, FAD(2H) -> e- transport chain Lots of ATP • Anaerobic: fermentation: Fig. iv.3 • ‘anaerobic glycolysis’ • Oxidation of NADH to NAD+ • Wasteful reduction of pyruvate • to lactate in muscles • to ethanol, CO2 by yeast Fig. iv.4 1 Chapt. 19 Cellular bioenergetics of ATP, O2 Ch. 19 Cellular bioenergetics Student Learning Outcomes: • Explain the ATP-ADP cycle • Describe how chemical bond energy of fuels can do cellular work through ~PO4 bond of ATP • Explain how NADH, FAD(2H) coenzymes carry electrons to electron transport chain • Describe how ATP synthesis is endergonic (requires energy) • Describe how ATP hydrolysis (exergonic) powers biosynthesis, movement, transport ATP Fuel oxidation makes ATP Cellular Bioenergetics of ATP and O2: • Chemical bond energy of fuels transforms to physiological responses necessary for life • Fuel oxidation generates ATP • ATP hydrolysis provides energy for most work • High energy bonds of ATP: • Energy currency of cell Fig. 19.1 Thermodynamics brief High energy phosphate bond of ATP: • Strained phosphoanhydride bond • ∆G0’ -7.3 kcal/mol standard conditions • Hydrolysis of ATP to ADP + Pi transfers PO4 to metabolic intermediate or protein, for next step Thermodynamics states what is possible: ∆G = change in Gibbs free energy of reaction: ∆G = ∆G0 + RT ln [P]/[S] (R = gas const; T = temp oK) ∆G0 = ∆G ∆ at standard conditions of1 M substrate & product and proceeding to equilibrium) ∆G0’ = ∆G0 under standard conditions of [H2O] = 55.5 M, pH 7.0, and 25oC [37oC not much different] Concentrations of substrate(s) and products(s): At equilibrium, ∆G = 0, therefore Fig. 19.2 ∆G0’ = -RT lnKeq’ = -RT ln[P]/[S] 2 Thermodynamics brief C. Exogonic, endogonic reactions Thermodynamics states what is possible: • Exergonic reactions give off energy (∆G0’ < 0) • typically catabolic • Endergonic reactions require energy (∆G0’ > 0) • typically anabolic • Unfavorable reactions are coupled to favorable reactions • Hydrolysis of ATP is very favorable • Additive ∆G0’ values determine overall direction Phosphoglucomutase converts G6P to/from G1P: • G6P to glycolysis • G1P to glycogen synthesis • Equilibrium favors G6P Exergonic reactions give off energy (DG0’ < 0) Endergonic reactions require energy (DG0’ > 0) Fig. 19.3 III. Energy transformation for mechanical work ATP powers transport ATP hydrolysis can power muscle movement: • Myosin ATPase hydrolyzes ATP, changes shape • ADP form changes shape back, moves along • Actin was activated by Ca2+ Active transport: ATP hydrolysis moves molecules: • Na+, K+ ATPase sets up ion gradient; bring in items • Vesicle ATPases pump protons into lysosome • Ca2+-ATPases pump Ca2+ into ER, out of cell Fig. 19.4 Fig. 10.6 3 III. ATP powers biochemical work Activated intermediates in glycogen synthesis ATP powers biochemical work, synthesis: Glycogen synthesis needs 3 ~P: Anabolic paths require energy: ∆Go’ additive • Couple synthesis to ATP hydrolysis: • Phosphoryl transfer to G6P Phosphoryl transfer reactions • Activated intermediate • • Activated intermediate with UDP covalently linked Fig. 19.5 Ex. Table 19.3: glucose + Pi -> glucose 6-P + H2O + 3.3 kcal/mol ATP + H2O -> ADP + Pi - 7.3 kcal/mol Sum: glucose + ATP -> glucose 6-P + ADP -4.0 Also Glucose -> G-1-P will be -2.35 kcal/mol overall: hydrolysis of ATP, through G-6-P to G-1-P ∆G depends on substrate, product concentrations ∆G depends on substrate, product concentrations ∆G = ∆G0 + RT ln [P]/[S] • Cells do not have 1M concentrations • High substrate can drive reactions with positive ∆G0’ • Low product (removal) can drive reactions with positive ∆G0’ Fig. 19.6 Activated intermediates with ~bonds Other compounds have high-energy bonds to aid biochemical work: (equivalent to ATP) • UTP, CTP and GTP also (made from ATP + NDP): • • • • Ex., even though equilibrium (∆G0’= +1.6 kcal/mol) favors G6P: G1P in a ratio 94/6, • If G1P is being removed (as glycogen synthesis), then equilibrium shifts ex. If ratio 94/3, then ∆G = -0.41 favorable UTP for sugar biosyn, GTP for protein, CTP for lipids • Some other compounds: • Creatine PO4 energy reserve muscle, nerve, sperm Glycolysis Ac CoA TCA cycle Fig. 19.7 4 V. Energy from fuel oxidation Oxidation/reduction Energy transfer from fuels through oxidative phosphorylation in mitochondrion: • NADH, FAD(2H) transfer e- to O2 • Stepwise process through protein carriers • Proton gradient created • e- to O2 -> H2O • ATP synthase makes ATP • lets in H+ Oxidation: reduction reactions: • Electron donor gets oxidized; recipient is reduced • LEO GER: •Loss Electrons = oxidation; gain electrons is reduction • use coenzyme e- carriers Fig. 19.9 NADH Fig. 19.10 FAD(2H) Fig. 19.8 Redox potentials Calorie content of fuels reflects oxidation state Redox potentials indicate energetic possibility: Calorie content of fuels reflects oxidation state: Energy tower; combine half reactions for overall: • C-H and C-C bonds will be oxidized: Ex. Table 19.4: • Glucose has many C-OH already: • 4 kcal/g ½ O2 + 2H+ + 2e- -> H2O NAD+ + 2H+ + 2e- -> NADH + H+ E0’ 0.816 -0.320 Combine both reactions (turn NADH -> NAD+) = 0.320 • Fatty acids very reduced: 9 kcal/g Total 1.136 (very big) = -53 kcal/mol • Cholesterol no calories: FAD(2H) gives less, since its only +0.20 (FAD(2H) -> FAD not oxidized in reactions giving NADH 5 Anaerobic glycolysis” = fermentation ‘Anaerobic glycolysis’ = fermentation In absence of O2, cell does wasteful recycling: • NADH oxidized to NAD+ (lose potential ATP) • pyruvate reduced to lactate • glycolysis can continue with new NAD+ • yeast makes ethanol, CO2 from pyruvate • bacteria make diverse acids, other products Oxidation not for ATP generation Most O2 used in electron transport chain. Some enzymes use O2 for substrate oxidation, not for ATP generation: • Oxidases transfer e- to O2 • • Oxygenases transfer eand O2 to substrate • • Fig. 19.11 VII Energy balance Energy expenditure reflects oxygen consumption: • Most O2 is used by ATPases [Cytochrome oxidase in electron transport chain] Peroxidases in peroxisome Form H2O and S-OH Hydroxylases • (eg. Phe -> Tyr) Fig. 19.12 Energy balance Portion of food metabolized is related to energy use: • Basal metabolic rate • Thermogenesis • Physical activity • Storage of excess Fig. 19.14 “If you eat to much and don’t exercise, you will get fat” (summarizes ATP-ADP cycle) 6