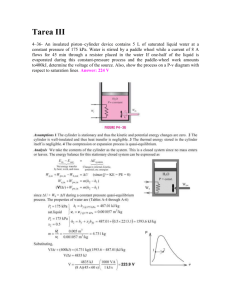
m 0.008 ( /kg m 0.4525 1 1 A
advertisement

Chapter 4 The First Law of Thermodynamics 4-61 Air is accelerated in a nozzle from 30 m/s to 180 m/s. The mass flow rate, the exit temperature, and the exit area of the nozzle are to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 Air is an ideal gas with constant specific heats. 3 Potential energy changes are negligible. 4 The device is adiabatic and thus heat transfer is negligible. 5 There are no work interactions. Properties The gas constant of air is 0.287 kPa.m3/kg.K (Table A-1). The specific heat of air at the anticipated average temperature of 450 K is Cp = 1.02 kJ/kg.°C (Table A-2). &1 = m &2 = m & . Using the ideal gas relation, the Analysis (a) There is only one inlet and one exit, and thus m specific volume and the mass flow rate of air are determined to be RT1 (0.287 kPa ⋅ m 3 /kg ⋅ K )(473 K ) = = 0.4525 m 3 /kg 300 kPa P1 1 1 m& = A1 V1 = (0.008m 2 )(30m/s) = 0.5304 kg/s 3 v1 0.4525m /kg v1 = (b) We take nozzle as the system, which is a control volume since mass crosses the boundary. The energy balance for this steady-flow system can be expressed in the rate form as ∆E& system Ê0 (steady) = =0 E& − E& out 1in 424 3 144 42444 3 Rate of net energy transfer by heat, work, and mass Rate of change in internal, kinetic, potential, etc. energies E& in = E& out & ≅ ∆pe ≅ 0) m& (h1 + V12 / 2) = m& (h2 + V22 /2) (since Q& ≅ W 0 = h2 − h1 + V22 − V12 V 2 − V12 → 0 = C p , ave (T2 − T1 ) + 2 2 2 Substituting, 0 = (1.02 kJ/kg ⋅ K )(T2 − 200 o C) + It yields (180 m/s) 2 − (30 m/s) 2 2 T2 = 184.6°°C 1 kJ/kg 2 2 1000 m /s (c) The specific volume of air at the nozzle exit is v2 = m& = RT2 (0.287 kPa ⋅ m 3 /kg ⋅ K )(184.6 + 273 K ) = = 1.313 m 3 /kg 100 kPa P2 1 1 A2 V2 → 0.5304kg/s = A2 (180m/s ) v2 1.313m 3 /kg P1 = 300 kPa T1 = 200°C V1 = 30 m/s A1 = 80 cm2 AIR A2 = 0.00387 m2 = 38.7 cm2 4-62 EES solution of this (and other comprehensive problems designated with the computer icon) is available to instructors at the Instructor Manual section of the Online Learning Center (OLC) at www.mhhe.com/cengel-boles. See the Preface for access information. 4-43 P2 = 100 kPa V2 = 180 m/s Chapter 4 The First Law of Thermodynamics 4-67 Air is decelerated in a diffuser from 230 m/s to 30 m/s. The exit temperature of air and the exit area of the diffuser are to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 Air is an ideal gas with variable specific heats. 3 Potential energy changes are negligible. 4 The device is adiabatic and thus heat transfer is negligible. 5 There are no work interactions. Properties The gas constant of air is 0.287 kPa.m3/kg.K (Table A-1). The enthalpy of air at the inlet temperature of 400 K is h1 = 400.98 kJ/kg (Table A-17). &1 = m &2 = m & . We take diffuser as the system, Analysis (a) There is only one inlet and one exit, and thus m which is a control volume since mass crosses the boundary. The energy balance for this steady-flow system can be expressed in the rate form as E& − E& out 1in 424 3 = Rate of net energy transfer by heat, work, and mass ∆E& system Ê0 (steady) 144 42444 3 =0 Rate of change in internal, kinetic, potential, etc. energies 1 E& in = E& out AIR & ≅ ∆pe ≅ 0) m& (h1 + V12 / 2) = m& (h2 + V22 /2) (since Q& ≅ W 0 = h2 − h1 + V22 − V12 2 , or, h2 = h1 − V22 − V12 (30m/s )2 − (230m/s )2 = 400.98kJ/kg − 2 2 1 kJ/kg 1000m 2 /s 2 = 426.98kJ/kg T2 = 425.6 K From Table A-17, (b) The specific volume of air at the diffuser exit is v2 = ( ) RT2 0.287kPa ⋅ m 3 /kg ⋅ K (425.6K ) = = 1.221m 3 /kg P2 (100kPa ) From conservation of mass, m& = m& v 2 (6000 3600 kg/s)(1.221 m 3 /kg ) 1 A2 V2 → A2 = = = 0.0678 m 2 V2 30 m/s v2 4-48 2 Chapter 4 The First Law of Thermodynamics Turbines and Compressors 4-75C Yes. 4-76C The volume flow rate at the compressor inlet will be greater than that at the compressor exit. 4-77C Yes. Because energy (in the form of shaft work) is being added to the air. 4-78C No. 4-79 Steam expands in a turbine. The change in kinetic energy, the power output, and the turbine inlet area are to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 Potential energy changes are negligible. 3 The device is adiabatic and thus heat transfer is negligible. Properties From the steam tables (Tables A-4 through 6) P1 = 10MPa v1 = 0.02975m 3 /kg T1 = 450 o C h1 = 3240.9kJ/kg P1 = 10 MPa T1 = 450°C V1 = 80 m/s and P2 = 10 kPa h2 = h f + x 2 h fg = 191.83 + 0.92 × 2392.8 = 2393.2kJ/kg x 2 = 0.92 Analysis (a) The change in kinetic energy is determined from ∆ke = V22 − 2 V12 (50m/s ) 2 = − (80m/s) 2 2 1kJ/kg 1000m 2 /s 2 = −1.95kJ/kg &1 = m &2 = m & . We take the (b) There is only one inlet and one exit, and thus m turbine as the system, which is a control volume since mass crosses the boundary. The energy balance for this steady-flow system can be expressed in the rate form as E& − E& out 1in 424 3 = Rate of net energy transfer by heat, work, and mass ∆E& system Ê0 (steady) 144 42444 3 =0 Rate of change in internal, kinetic, potential, etc. energies E& in = E& out & ≅ ∆pe ≅ 0) m& (h1 + V12 / 2) = W& out + m& (h2 + V22 /2) (since Q V 2 − V12 W& out = − m& h2 − h1 + 2 2 Then the power output of the turbine is determined by substitution to be W& out = −(12 kg/s)(2393.2 − 3240.9 − 1.95)kJ/kg = 10.2 MW (c) The inlet area of the turbine is determined from the mass flow rate relation, m& = m& v (12 kg/s)(0.02975 m 3 /kg ) 1 A1 V1 → A1 = 1 = = 0.00446 m 2 V1 v1 80 m/s 4-55 STEAM m· = 12 kg/s · W P2 = 10 kPa x2 = 0.92 V2 = 50 m/s Chapter 4 The First Law of Thermodynamics 4-86 Refrigerant-134a is compressed steadily by a compressor. The power input to the compressor and the volume flow rate of the refrigerant at the compressor inlet are to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 Kinetic and potential energy changes are negligible. 3 The device is adiabatic and thus heat transfer is negligible. Properties From the refrigerant tables (Tables A-11 through 13) T1 = −20 o C v1 = 0.1464m 3 /kg sat.vapor h1 = 235.31kJ/kg 2 P2 = 0.7MPa h2 = 307.01kJ/kg T2 = 70 o C &1 = m &2 = m & . We Analysis (a) There is only one inlet and one exit, and thus m take the compressor as the system, which is a control volume since mass crosses the boundary. The energy balance for this steady-flow system can be expressed in the rate form as E& − E& out 1in 424 3 = Rate of net energy transfer by heat, work, and mass ∆E& system Ê0 (steady) 144 42444 3 =0 Rate of change in internal, kinetic, potential, etc. energies E& in = E& out & 1 = mh & 2 (since Q& ≅ ∆ke ≅ ∆pe ≅ 0) W& in + mh W& in = m& (h2 − h1 ) Substituting, W& in = (1.2kg/s )(307.01 − 235.31) kJ/kg = 86.04kJ/s (b) The volume flow rate of the refrigerant at the compressor inlet is V&1 = m& v1 = (1.2kg/s )(0.1464m 3 /kg ) = 0.176m 3 /s 4-61 R-134a 1 Chapter 4 The First Law of Thermodynamics Throttling Valves 4-92C Because usually there is a large temperature drop associated with the throttling process. 4-93C Yes. 4-94C No. Because air is an ideal gas and h = h(T) for ideal gases. Thus if h remains constant, so does the temperature. 4-95C If it remains in the liquid phase, no. But if some of the liquid vaporizes during throttling, then yes. 4-96 Refrigerant-134a is throttled by a valve. The temperature drop of the refrigerant and specific volume after expansion are to be determined. √ Assumptions 1 This is a steady-flow process since there is no change with time. 2 Kinetic and potential energy changes are negligible. 3 Heat transfer to or from the fluid is negligible. 4 There are no work interactions involved. Properties The inlet enthalpy of R-134a is, from the refrigerant tables (Tables A-11 through 13), P1 = 0.8MPa T1 = Tsat = 31.33o C sat.liquid h1 = h f = 93.42kJ / kg &1 = m &2 = m & . We take Analysis There is only one inlet and one exit, and thus m the throttling valve as the system, which is a control volume since mass crosses the boundary. The energy balance for this steady-flow system can be expressed in the rate form as E& in − E& out = ∆E& system Ê0 (steady) =0 → E& in = E& out → & 1 = mh & 2 → mh h1 = h2 since Q& ≅ W& = ∆ke ≅ ∆pe ≅ 0 . Then, P2 = 0.14MPa h f = 25.77kJ/kg, Tsat = −18.8 o C (h2 = h1 ) h g = 236.04kJ/kg Obviously hf <h2 <hg, thus the refrigerant exists as a saturated mixture at the exit state and thus T2 = Tsat = -18.8°C. Then the temperature drop becomes P1 = 800 kPa Sat. liquid R-134a . = −50.13o C ∆T = T2 − T1 = −18.8 − 3133 The quality at this state is determined from x2 = h2 − h f h fg = P2 = 140 kPa 93.42 − 25.77 = 0.322 210.27 − 25.77 Thus, v 2 = v f + x 2 v fg = 0.0007381 + 0.322 × 0.13876 = 0.0454m 3 /kg 4-66 Chapter 4 The First Law of Thermodynamics 4-106 Feedwater is heated in a chamber by mixing it with superheated steam. If the mixture is saturated liquid, the ratio of the mass flow rates of the feedwater and the superheated vapor is to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 Kinetic and potential energy changes are negligible. 3 There are no work interactions. 4 The device is adiabatic and thus heat transfer is negligible. Properties Noting that T < Tsat @ 800 kPa = 170.43°C, the cold water stream and the mixture exist as a compressed liquid, which can be approximated as a saturated liquid at the given temperature. Thus, h1 ≅ hf @ 50°C = 209.33 kJ/kg h3 ≅ hf @ 800 kPa = 721.11 kJ/kg and P2 = 800kPa h2 = 2839.3kJ/kg T2 = 200 o C Analysis We take the mixing chamber as the system, which is a control volume since mass crosses the boundary. The mass and energy balances for this steady-flow system can be expressed in the rate form as & in − m & out = ∆m & system Ê0 m Mass balance: Energy balance: E& − E& out 1in 424 3 = Rate of net energy transfer by heat, work, and mass (steady) ∆E& system Ê0 (steady) 144 42444 3 =0 → & in = m & out m =0 &1 + m &2 = m &3 → m T1 = 50°C ·1 m Rate of change in internal, kinetic, potential, etc. energies H2O (P = 800 kPa) Sat. liquid E& in = E& out & 1h1 + m & 2 h2 = m & 3h3 m Combining the two, & 2 yields Dividing by m (since Q& ≅ W& ≅ ∆ke ≅ ∆pe ≅ 0) m& 1 h1 + m& 2 h 2 = (m& 1 + m& 2 ) h3 y h1 + h 2 = ( y + 1) h3 y= Solving for y: h3 − h2 h1 − h3 &1 / m & 2 is the desired mass flow rate ratio. Substituting, where y = m y= 721.11 − 2839.3 = 4.14 209.33 − 721.11 4-71 T2 = 200°C · m2 Chapter 4 The First Law of Thermodynamics 4-110 Refrigerant-134a is to be cooled by air in the condenser. For a specified volume flow rate of air, the mass flow rate of the refrigerant is to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 Kinetic and potential energy changes are negligible. 3 There are no work interactions. 4 Heat loss from the device to the surroundings is negligible and thus heat transfer from the hot fluid is equal to the heat transfer to the cold fluid. 5 Air is an ideal gas with constant specific heats at room temperature. Properties The gas constant of air is 0.287 kPa.m3/kg.K (Table A-1). The constant pressure specific heat of air is Cp = 1.005 kJ/kg· °C (Table A-2). The enthalpies of the R-134a at the inlet and the exit states are (Tables A-11 through A-13) P3 = 1MPa h3 = 313.20kJ/kg T3 = 80 o C P4 = 1MPa h4 ≅ h f @30oC = 91.49kJ/kg T4 = 30 o C Analysis The inlet specific volume and the mass flow rate of air are RT 0.287kPa ⋅ m 3 /kg ⋅ K (300K ) = 0.861m 3 /kg v1 = 1 = 100kPa P1 and V& 800m 3 /min = 929.2kg/min m& = 1 = v1 0.861m 3 /kg ( AIR 1 R-134a 3 ) 2 4 We take the entire heat exchanger as the system, which is a control volume. The mass and energy balances for this steady-flow system can be expressed in the rate form as Mass balance ( for each fluid stream): m& in − m& out = ∆m& system Ê0 (steady) = 0 → m& in = m& out → m& 1 = m& 2 = m& a and m& 3 = m& 4 = m& R Energy balance (for the entire heat exchanger): E& − E& out 1in 424 3 = Rate of net energy transfer by heat, work, and mass ∆E& system Ê0 (steady) 144 42444 3 =0 Rate of change in internal, kinetic, potential, etc. energies E& in = E& out & 1h1 + m & 3h3 = m & 2 h2 + m & 4 h4 (since Q& = W& = ∆ke ≅ ∆pe ≅ 0) m Combining the two, &R : Solving for m m& a (h2 − h1 ) = m& R (h3 − h 4 ) m& R = C p (T2 − T1 ) h2 − h1 m& a ≅ m& a − h3 h4 h3 − h4 Substituting, m& R = (1.005 kJ/kg⋅ o C)(60 − 27) o C (929.2 kg/min ) = 139.0 kg/min (313.20 − 91.49) kJ/kg 4-74 Chapter 4 The First Law of Thermodynamics 4-112 Refrigerant-134a is condensed in a water-cooled condenser. The mass flow rate of the cooling water required is to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 Kinetic and potential energy changes are negligible. 3 There are no work interactions. 4 Heat loss from the device to the surroundings is negligible and thus heat transfer from the hot fluid is equal to the heat transfer to the cold fluid. Properties The enthalpies of R-134a at the inlet and the exit states are (Tables A-5 and A-6) P3 = 800kPa h3 = 305.50kJ/kg T3 = 70 o C P4 = 800kPa h4 = h f @800kPa = 93.42kJ/kg sat.liquid Water 1 R-134a 3 Water exists as compressed liquid at both states, and thus 2 h1 ≅ hf @ 15°C = 62.99 kJ/kg h2 ≅ hf @ 30°C = 125.79 kJ/kg 4 Analysis We take the heat exchanger as the system, which is a control volume. The mass and energy balances for this steady-flow system can be expressed in the rate form as Mass balance (for each fluid stream): m& in − m& out = ∆m& system Ê0 (steady) = 0 → m& in = m& out → m& 1 = m& 2 = m& w and Energy balance (for the heat exchanger): E& − E& out 1in 424 3 = Rate of net energy transfer by heat, work, and mass ∆E& system Ê0 (steady) 144 42444 3 =0 Rate of change in internal, kinetic, potential, etc. energies E& in = E& out & 1h1 + m & 3h3 = m & 2 h2 + m & 4 h4 (since Q& = W& = ∆ke ≅ ∆pe ≅ 0) m Combining the two, m& w (h2 − h1 ) = m& R (h3 − h4 ) m& w = &w : Solving for m h3 − h4 m& R h2 − h1 Substituting, m& w = (305.50 − 93.42) kJ / kg (8 kg / min) = 27.0 kg / min (125.79 − 62.99) kJ / kg 4-76 m& 3 = m& 4 = m& R