Motion With Constant Acceleration
advertisement

Atomic Spectra
HISTORY AND THEORY
When atoms of a gas are excited (by high voltage, for instance) they will give off light.
Each element (in fact, each isotope) gives off a characteristic atomic spectrum, a set of particular
wavelengths. One of the great early triumphs of quantum theory was in explaining these spectra.
Quantum theory tells us that electrons can only occupy particular energy levels. The energy
levels are quantized, in other words. To conserve energy when an electron makes a transition
from a higher-energy state to a lower-energy state, a photon, with an energy corresponding to the
energy difference between the electron energies, is emitted.
The Danish physicist Neils Bohr presented a solar-system model of the atom, in which
the electrons occupy particular circular orbits centered on the nucleus. It is important to
understand that the Bohr model is not a true picture of how the electrons behave in an atom, but
we use the Bohr model because it is a good starting point in understanding how to determine the
energies of the energy levels.
Using his model Bohr predicted that the energy levels of the electron in a hydrogen atom
are given by:
En = −
µe 4
8ε h n
2
o
2
2
=−
13.6eV
n2
Equation 1
where n is any positive integer, and µ is known as the reduced mass. If me is the mass of an
electron and mH is the mass of the hydrogen nucleus, the reduced mass is given by:
µ=
m H me
m H + me
Equation 2
ABOUT THIS EXPERIMENT
This is a challenging experiment that requires you to work hard at your experimental
techniques. Note that the room should be dark when you do your measurements, so the room
lights do not overwhelm the light from your light source.
1
Atomic Spectra
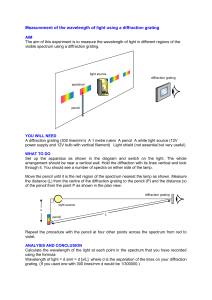
THE DIFFRACTION GRATING
In the laboratory you will use a diffraction grating to split a particular atomic spectrum into
its constituent colors. For a grating with a grating spacing of d, constructive interference occurs
at angles given by:
d sin θ = nλ
Equation 3
where n is an integer representing the order and λ is the wavelength. The figure below shows the
ray diagram for a particular first-order line.
The resolution of the diffraction grating is the smallest wavelength difference ∆λ necessary
to produce separate (resolved) images. For a grating with N lines the resolution is given by:
∆λ =
λ
Equation 4
nN
The dispersion of the grating is:
dθ
= nd cosθ
dλ
Equation 5
2
Atomic Spectra
OBJECTIVES AND APPARATUS
In this experiment your objective is to measure the wavelengths of light emitted by
hydrogen and other sources. You will then compare your results with the wavelengths predicted
by the Bohr Model. To do this you will make use of a spectrometer. The spectrometer uses a
diffraction grating to split the light into its constituent wavelengths, and also has a sensitive light
sensor to record the intensity of light observed at particular angles. To help you analyze the data
the readings from the light sensor, as well as readings of the angle, are passed to a computer so
you can easily obtain graphs of light intensity as a function of angle.
The basic components of the spectrometer are shown below.
•
The rotary motion sensor is used to measure the angle. The pinion connecting the
rotary motion sensor and the degree plate is designed to rotate 60 times for each
full revolution of the degree plate, and the calculations in the computer take that
into account.
•
The diffraction grating is blazed. The implication of this is that the spectral lines
on one side of the diffraction pattern will be brighter than the spectral lines on the
other, as you will notice when you take measurements. You should not touch the
diffraction grating with your fingers, nor try to clean it because it scratches
easily. If you need to move the grating touch the glass slide only by the edges.
•
The light sources you will use include hydrogen, helium, and deuterium discharge
tubes, as well as a sodium arc lamp. In each case the gas will be excited by a high
voltage, and will emit photons of particular wavelengths.
3
Atomic Spectra
EQUIPMENT SET-UP
It is important that the light reaching the diffraction grating is made up of parallel beams.
The apparatus includes collimating slits and a collimating lens near the light source to ensure this
(see figures 3 and 4). The slits are mounted on a movable slide. If you loosen the thumbscrew in
the slits plate you can move the slide back and forth to choose which slit the light passes
through.
Positioning the collimating slits and collimating lens:
1. If the diffraction grating is magnetically
mounted on the degree plate, remove it
carefully. Hold the glass slide at the edges.
2. Position the collimating lens about 10 cm
from the slits. Arrange a source so light
passes through a slit and then through the
collimating lens. Rotate the light sensor out of
the way so the light travels for a long
distance.
3. Adjust the distance between the slits and the
collimating lens so that, after passing through
the slit and lens, the beam of light is neither
converging nor diverging (i.e., the beam has a
constant width). You can check the beam
width by holding a piece of paper in the beam
at various distances from the collimating lens.
4. If the light beam is not perfectly vertical,
either loosen the thumbscrew that holds the
slits slide and adjust the slide, or loosen the brass thumbscrews that hold the slits plate
onto the holder and adjust the plate, until the collimated beam is vertical. Tighten the
screws again once the orientation is correct.
4
Atomic Spectra
Mounting the diffraction grating:
The diffraction grating is mounted on one side of a rectangular glass plate, on the same
side of the plate as the magnetic strips that hold the grating to the grating mount. Attach the
grating to the grating mount so the glass side of the grating faces the light source. Once again,
remember that the diffraction grating is very delicate, and it should not be touched or
cleaned for any reason.
Positioning the Focusing Lens
The focusing lens has magnets to hold it in place on the light sensor arm. Position the
focusing lens between the grating and the light sensor, about 10 cm in front of the aperture disk.
Markings on the degree plate indicate the approximate position in which to place the lens.
Arrange a light source and the collimating slits and lens so a beam of light shines through
the grating and focusing lens. The room should be fairly dark at this point. Position the light
sensor arm so the central ray of light (the “zeroth order”) is centered on the slit at the bottom of
the aperture disk. Adjust the position of the focusing lens until the spectral lines, which should
be visible on the aperture screen on either side of the zeroth order are sharply focused.
5
Atomic Spectra
6
Atomic Spectra
GENERAL INFORMATION
The Light Sensor
The High-Sensitivity Light Sensor has a gain (amplification) switch on the top with three
settings. When you measure a spectrum start with the lowest gain setting (1) to measure the
brightest lines. Then switch to the next setting (10) and re-scan the spectrum to measure the
dimmer lines. Then re-scan again at the highest setting (100) to measure the dimmest lines.
It is also possible to amplify the signal from the light sensor using the Data Studio
software. The normal sensitivity setting is “Low(1x)”. The other settings are “Medium(10x)” and
“High(100x)”. In general, your data will be better if you increase the gain setting on the sensor
before you increase the sensitivity setting in the software.
Note that using the switch on the light sensor, and amplifying the signal in the software,
is crucial to the success of the experiment. With the spectrum tubes in particular you are trying to
measure very low light levels. Blocking out as much stray light as possible (in particular, turning
the computer monitors away from the detector), and making extensive use of the amplification
you have available, will give you the best chance to see the low-intensity lines that are present.
You can even change the gain switch on the light sensor in the middle of taking data. For
instance, you might want to keep it at 100 when you’re scanning through the spectral lines but
move it to 1 when you move the sensor through the zeroth order, then switch back to 100 again.
Slit Widths
There are five slits on the collimating slits slide and six slits on the aperture disk. Using
wider slits allows more light to pass through to the light sensor but decreases the accuracy of the
measurements. If you don’t pick up any spectral lines, or you’re trying to find additional lines,
use wider slits on the collimating slide and/or the aperture disk. If you have plenty of spectral
lines try using narrower slits.
Angular Resolution
For every full rotation of the degree plate, the shaft of the Rotary Motion Sensor should
rotate approximately 60 times. Thus, a single rotation of the Rotary Motion Sensor shaft
represents approximately 6û of angular displacement for the degree plate. The maximum
resolution of the Rotary Motion Sensor is 1440 divisions per rotation, so the best-case angular
resolution of the spectrometer is 6û/1440, which is one quarter of a minute of arc, or 15 seconds
of arc. This corresponds to a wavelength resolution of about 2 nm, given the grating line spacing
of about 1667 nm.
7
Atomic Spectra
Scanning a Spectrum
To scan a spectrum:
•
Use the threaded post under the light sensor to move the light sensor arm so the
light sensor is beyond the far end of the first-order spectral lines on one side (but
not within the range of the second-order lines).
•
Start collecting data in the Data Studio program.
•
Scan the spectrum continuously but slowly in one direction by pushing on the
threaded post to rotate the light sensor arm. Scan through the first-order spectral
lines on one side of the central ray and continue all the way through the first-order
spectrum on the other side.
•
The angle θ of a particular line is half the difference between the angle of that line
on one side of the zeroth order and the angle of the corresponding line on the
other side of the zeroth order. If a dim line appears on only one side you can find
its angle by taking the difference between its angle and the angle at which the
zeroth order appears.
8
Atomic Spectra
THE EXPERIMENT
PART I
On the computer double-click on the “Spectroscopy” program in the “Modern” folder.
This will start the Data Studio program with appropriate settings and a graph window.
The number of lines on the diffraction grating is roughly 600 per millimeter. This
corresponds to a line spacing of about d = 1667 nm.
To find a more precise value for the line spacing, use the sodium lamp to calibrate the
grating. Note that the sodium lamp takes a few minutes to warm up. The average wavelength of
the sodium doublet (the two closely spaced yellow lines) is 589.3 nm. Measure the angle
between the two first-order yellow lines, one on one side of the zeroth order and one on the
other, and then divide by two to get the diffraction angle for the 589.3 nm wavelength. Use this
information to determine the grating spacing for your diffraction grating.
To do the measurement, scan through the spectrum following the procedure outlined on
the previous page. Position the light sensor beyond the last first-order spectral line on one side,
and then hit the “Start” button in the Data Studio program. Scan slowly through the spectrum
(see figure 6) and then hit the “Stop” button in the Data Studio program when you are finished.
The Smart Tool is useful for taking readings off the graph on the computer – you can find the
Smart Tool by clicking the appropriate button above the graph.
Note that you can delete data runs from Data Studio by using the Experiment menu.
Question 1: What is your value of d? Is the above method a reasonable way of calibrating
the grating? Comment on whether you think it would be better to use the secondorder yellow lines for sodium, in particular.
PART II
Now you are ready to scan through the spectra from the different elements you have
available. You should set up a reasonable table before each step to help you keep track of your
data and streamline your calculations.
For each trial, the Data Studio program takes the initial position of the Rotary Motion
Sensor as the zero position. For some measurements it may be helpful to start each trial at the
same position. If you take one of the small thumbscrews that are stored on the light sensor arm
and screw it into one of the holes near the outer edge of the degree plate, it will act as a stop so
you can start the degree plate from the same angle each time.
1. Hydrogen: carefully insert a hydrogen discharge tube into the power supply and scan
through the second-order and first-order spectra on both sides of the zeroth order.
Determine the angles and wavelengths of the lines in the hydrogen spectrum. You will
probably need to scan through the spectrum a few times to get good data. Use the gain
settings on the light sensor, and adjust the slit widths, as discussed in the “General
9
Atomic Spectra
Information” section of this write-up. Also, remember to try to block as much stray light
as possible from entering the light sensor while you are recording data.
The “Scale-to-fit” button, the left-most button above the graph, is rather useful. Press it to
see what happens, and then click-and-drag on the graph to select a region you want to
zoom in on and hit the “Scale-to-fit” button again.
2. Deuterium: the object here is to see if you can tell the difference between the deuterium
spectrum and the hydrogen spectrum. With the hydrogen spectrum still on the screen,
carefully replace the hydrogen tube with the deuterium tube without moving the positions
of any other part of the system. Then scan through the second-order and first-order lines
for deuterium. Keep the slit widths as narrow as possible to maximize your resolution.
3. Sodium: see if you can measure the separation between the sodium D lines (the two
yellow-orange lines). Measure the angular displacement between these two lines for as
many orders as you can.
4. Helium: carefully insert a helium discharge tube into the power supply and scan through
the first-order spectrum on both sides of the zeroth order. Determine the angles and
wavelengths of the lines in the helium spectrum.
QUESTIONS AND ANALYSIS
Question 2: For hydrogen, compare your measured wavelengths to the predicted
wavelengths for hydrogen. Assuming the lower level is the same for all the lines you
observed in the hydrogen spectrum, make an energy level diagram for hydrogen.
Label the energy axis in electron volts.
Question 3: A deuterium nucleus has a mass twice that of hydrogen – while the standard
hydrogen nucleus consists of just a proton, the deuterium has a proton and a neutron.
Calculate the difference in wavelength produced by the difference in reduced mass
µ for two or three of the visible lines. Predict the angle between these hydrogen and
deuterium lines. Can you resolve them with your spectrometer?
Question 4: Sodium “D” lines are at wavelengths of 5889.95Å and 5895.92Å. Based on this,
what angle do you expect to observe between them in the first order spectrum? What
angle did you actually measure?
Question 5: Based on your observations of deuterium and sodium, was the resolution of the
spectrometer limited only by diffraction? What else, if anything, limits the resolution
of the spectrometer?
10
Atomic Spectra
Question 6: Because helium has two electrons we expect its spectrum to differ from that of
hydrogen. However, for one electron in the ground state and the other in an excited
state, the effective charge seen by the excited electron is still just e+. Therefore, you
should observe transitions between states with similar energies as those seen in
hydrogen. If the two electrons have their spins aligned opposite to one another we call
that the singlet state, or parahelium; if the spins are parallel to one another that is the
triplet state, or orthohelium.
The difference in binding energy for the singlet and triplet states causes each energy
level to split into two states, leading to spectral line pairs (doublets). Examine your
helium data and identify the hydrogen-like transitions that you observed. Because the
lower level differs for different transitions, an energy level diagram (see Figure 7) is
very difficult to produce. You may simply want to compare your wavelengths to those
in Figure 8.
excitation
energy, eV
24.6
singlet states
(parahelium)
n
1
S
1
1
P
D
triplet states
(orthohelium)
3
1
F
S
3
3
P
3
D
F
4
3{
20
2{
Red
Yellow
Green
Blue
4026
4471
4388
4713
5015
4922
5876
6678
7065
Figure 7. Excitation Energies.
Violet
Figure 8. Spectrum of Helium.
Try to make an energy level diagram for helium. Compare it with Figure 7; note that
you probably observed transitions between the higher levels and any one of the four n=2
levels. Only transitions indicated by arrows are allowed (by the selection rules under spinorbit or LS coupling, namely ∆L = ± 1).
11
Atomic Spectra