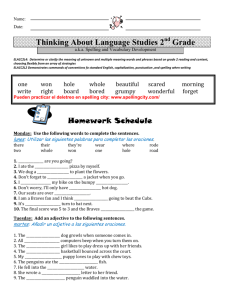
Template for Electronic Submission of Organic Letters
advertisement

a H1 H7 H6 Reações de superfície de compostos derivados de biomassa – C3 C2 H5 Surface reactions of biomass-derived oxygenates H2 C1 O4 Guo Shiou Foo,1 John R. Copeland,1 Jungseob So,1 Carsten Sievers1 O3 O2 H3 H4 Georgia Institute of Technology, School of Chemical & Biomolecular Engineering, 311 Ferst Dr. NW, Atlanta, GA 30332-0100, H8 E.U.A., carsten.sievers@chbe.gatech.edu 1 Abstract Al2 Al1 O1 ABSTRACT – Economically viable production of chemicals from biomass will require H7 catalytic processes with high selectivity. Heterogeneous catalysts will play a key role in b H5 such processes due to their facile separation. To reach the desired selectivity, it will be H1 H6 C2 H2 necessary to understand and control the interactions of biomass-derived compounds with the C1 C3 O2 active sites of catalysts. Since most biomass-derived compounds are oxygenates with multiple functional groups, their surface chemistry has an added layer of complexity. In this H4 H3 O4 O3 presentation, FTIR and NMR spectroscopy, adsorption isotherms, and DFT calculations will H8 be used to identify surface species formed from polyols and sugars and to deduce reaction pathways for their conversion. For example, glycerol can favourably compete with water for binding sites on metal oxides like -Al2O3, TiO2, and N2O5 [1-3]. The most stable surface species is formed when one of Al1 Al2 the primary alcohol groups forms a bridging alkoxy bond on a Lewis acid site (LAS), while the other primary alcohol group interacts non-dissociatively with the same site (Figure 1). In Figure 1: Most stable surface addition, the secondary hydroxyl group forms a hydrogen bond with the surface [1]. species formed from glycerol on Sufficient spatial separation between the alcohol groups is necessary for these multidentate Al2O3 based on DFT calculations surface species to form without excessive strain. and IR spectroscopy. Through the interaction with a LAS, the primary C-O bonds of this surface species are polarized making them more susceptible to cleavage. However, dehydration only occurs on metal oxide like Nb2O5 that also contain Brønsted acid sites (LAS) [3]. When LAS and BAS are involved, the primary alcohol group is removed preferentially, and acetol is formed. Without the involvement of a LAS, the secondary alcohol group is removed due to the higher stability of the secondary carbenium ion that is formed as the transition state. The intermediate 3-hydroxypropionaldehyde readily undergoes a second dehydration step to acrolein. Keywords: dehydration, acid-base reactions, alkoxy bond, polarization, adsorption. References [1] J.R. Copeland, X.-R. Shi, D.S. Sholl, C. Sievers, Langmuir 29 (2013) 581. [2] J.R. Copeland, I.A. Santillan, S.M. Schimming, J.L. Ewbank, C. Sievers, J. Phys. Chem. C 117 (2013) 21413. [3] G.S. Foo, D. Wei, D.S. Sholl, C. Sievers, ACS Catalysis 4 (2014) 3180.