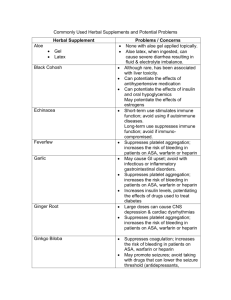
chapter 57 Drugs That Affect Blood Coagulation
advertisement

chapter 57 Drugs That Affect Blood Coagulation Objectives AFTER STUDYING THIS CHAPTER, THE STUDENT WILL BE ABLE TO: 1. Describe important elements in the physiology of hemostasis and thrombosis. 2. Discuss potential consequences of blood clotting disorders. 3. Discuss characteristics and uses of anticoagulant, antiplatelet, and thrombolytic agents. 4. Compare and contrast heparin and warfarin in terms of indications for use, onset and duration of action, route of administration, blood tests used to monitor effects, and nursing process implications. 5. Teach clients on long-term warfarin therapy protective measures to prevent abnormal bleeding. 6. Discuss antiplatelet agents in terms of indications for use and effects on blood coagulation. 7. With aspirin, contrast the dose and frequency of administration for antiplatelet effects with those for analgesic, antipyretic, and antiinflammatory effects. 8. Describe thrombolytic agents in terms of indications and contraindications for use, routes of administration, and major adverse effects. 9. Discuss the use of anticoagulant, antiplatelet, and thrombolytic drugs in special populations. 10. Describe systemic hemostatic agents for treating overdoses of anticoagulant and thrombolytic drugs. Critical Thinking Scenario Juan Sanchez, a 56-year-old migrant farmer without health insurance, is admitted to the hospital after an episode of syncope. He is diagnosed with atrial fibrillation and is started on a calcium channel blocker and Coumadin. Before his discharge, you are responsible for patient teaching. Reflect on: Assessment data that would be helpful to individualize your teaching plan. Discuss the rationale for use of Coumadin for clients with atrial fibrillation. Identify side effects of Coumadin therapy. Consider strategies that might help Mr. Sanchez comply with therapy and experience limited side effects. OVERVIEW A nticoagulant, antiplatelet, and thrombolytic drugs are used in the prevention and management of thrombotic and thromboembolic disorders. Thrombosis involves the formation (thrombogenesis) or presence of a blood clot (thrombus) in the vascular system. Blood clotting is a normal body defense mechanism to prevent blood loss. Thus, thrombogenesis may be lifesaving when it occurs as a response to hemorrhage; however, it may be life threatening when it occurs at other times, because the thrombus can obstruct a blood vessel and block blood flow to tissues beyond the clot. When part of a thrombus breaks off and travels to another part of the body, it is called an embolus. 832 Atherosclerosis is the basic disease process that often leads to pathologic thrombosis. It begins with accumulation of lipidfilled macrophages (ie, foam cells) on the inner lining of arteries. Foam cells develop in response to elevated blood lipid levels and eventually become fibrous plaques (ie, foam cells covered by smooth muscle cells and connective tissue). Advanced atherosclerotic lesions also contain hemorrhages, ulcerations, and scar tissue. Atherosclerosis can affect any organ or tissue, but often involves the arteries supplying the heart, brain, and legs. Over time, plaque lesions become larger and extend farther into the lumen of the artery. Eventually, a thrombus may develop at plaque sites and partially or completely occlude an artery. In coronary arteries, a thrombus may precipi- CHAPTER 57 DRUGS THAT AFFECT BLOOD COAGULATION tate myocardial ischemia (angina or infarction) (see Chapter 53); in carotid or cerebral arteries, a thrombus may precipitate a stroke; in peripheral arteries, a thrombus may cause intermittent claudication (pain in the legs with exercise) or acute occlusion. Thus, serious impairment of blood flow may occur with a large atherosclerotic plaque or a relatively small plaque with superimposed vasospasm and thrombosis. Consequences and clinical manifestations of thrombi and emboli depend primarily on their location and size. Normally, thrombi are constantly being formed and dissolved (thrombolysis), but the blood stays fluid and flow is not significantly obstructed. If the balance between thrombogenesis and thrombolysis is upset, thrombotic or bleeding disorders result. Thrombotic disorders occur much more often than bleeding disorders and are emphasized in this chapter; bleeding disorders may result from excessive amounts of drugs that inhibit clotting. To aid understanding of drug therapy for thrombotic disorders, normal hemostasis, endothelial functions in relation to blood clotting, platelet functions, blood coagulation, and characteristics of arterial and venous thrombosis are described. HEMOSTASIS Hemostasis is prevention or stoppage of blood loss from an injured blood vessel and is the process that maintains the integrity of the vascular compartment. It involves activation of several mechanisms, including vasoconstriction, formation of a platelet plug (a cluster of aggregated platelets), sequential activation of clotting factors in the blood (Table 57–1), and growth of fibrous tissue (fibrin) into the blood clot to make it more stable and to repair the tear (opening) in the damaged blood vessel. Overall, normal hemostasis is a complex process involving numerous interacting activators and inhibitors, including endothelial factors, platelets, and blood coagulation factors (Box 57–1). TABLE 57–1 833 CLOT LYSIS When a blood clot is being formed, plasminogen (an inactive protein found in many body tissues and fluids) is bound to fibrin and becomes a component of the clot. After the outward blood flow is stopped and the tear in the blood vessel repaired, plasminogen is activated by plasminogen activator (produced by endothelial cells or the coagulation cascade) to produce plasmin. Plasmin is an enzyme that breaks down the fibrin meshwork that stabilizes the clot; this fibrinolytic or thrombolytic action dissolves the clot. THROMBOTIC AND THROMBOEMBOLIC DISORDERS Thrombosis may occur in both arteries and veins. Arterial thrombosis is usually associated with atherosclerotic plaque, hypertension, and turbulent blood flow. These conditions damage arterial endothelium and activate platelets to initiate the coagulation process. Arterial thrombi cause disease by obstructing blood flow. If the obstruction is incomplete or temporary, local tissue ischemia (deficient blood supply) occurs. If the obstruction is complete or prolonged, local tissue death or infarction occurs. Venous thrombosis is usually associated with venous stasis. When blood flows slowly, thrombin and other procoagulant substances present in the blood become concentrated in local areas and initiate the clotting process. With a normal rate of blood flow, these substances are rapidly removed from the blood, primarily by Kupffer cells in the liver. A venous thrombus is less cohesive than an arterial thrombus, and an embolus can easily become detached and travel to other parts of the body. Venous thrombi cause disease by two mechanisms. First, thrombosis causes local congestion, edema, and perhaps inflammation by impairing normal outflow of venous blood (eg, thrombophlebitis, deep vein thrombosis [DVT]). Sec- Blood Coagulation Factors Number Name Functions I Fibrinogen II III IV V VII VIII IX X XI Prothrombin Thromboplastin Calcium Labile factor Proconvertin or stable factor Antihemophilic factor Christmas factor Stuart factor Plasma thromboplastin antecedent XII XIII Hageman factor Fibrin-stabilizing factor Forms fibrin, the insoluble protein strands that compose the supporting framework of a blood clot. Thrombin and calcium are required for the conversion. Forms thrombin, which catalyzes the conversion of fibrinogen to fibrin Converts prothrombin to thrombin Catalyzes the conversion of prothrombin to thrombin Required for formation of active thromboplastin Accelerates action of tissue thromboplastin Promotes breakdown of platelets and formation of active platelet thromboplastin Similar to factor VIII Promotes action of thromboplastin Promotes platelet aggregation and breakdown, with subsequent release of platelet thromboplastin Similar to factor XI Converts fibrin meshwork to the dense, tight mass of the completely formed clot 834 BOX 57–1 SECTION 9 DRUGS AFFECTING THE CARDIOVASCULAR SYSTEM HEMOSTASIS AND THROMBOSIS The blood vessels and blood normally maintain a balance between procoagulant and anticoagulant factors that favors anticoagulation and keeps the blood fluid. Injury to blood vessels and tissues causes complex reactions and interactions among vascular endothelial cells, platelets, and blood coagulation factors that shift the balance toward procoagulation and thrombosis. Endothelial Cells Endothelial cells play a role in all aspects of hemostasis and thrombosis. Normal endothelium helps to prevent thrombosis by producing anticoagulant factors, inhibiting platelet reactivity, and inhibiting activation of the coagulation cascade. However, endothelium promotes thrombosis when its continuity is lost (eg, the blood vessel wall is torn by rupture of atherosclerotic plaque, hypertension, trauma), its function is altered, or when blood flow is altered or becomes static. After a blood clot is formed, the endothelium also induces its dissolution and restoration of blood flow. Antithrombotic Functions • Synthesizes and releases prostacyclin (prostaglandin I2), which inhibits platelet aggregation • Releases endothelium-derived relaxing factor (nitric oxide), which inhibits platelet adhesion and aggregation • Blocks platelet exposure to subendothelial collagen and other stimuli for platelet aggregation • May inhibit platelet reactivity by inactivating adenosine diphosphate (ADP), a platelet product that promotes platelet aggregation • Produces plasminogen activators (eg, tissue-type or tPA) in re- • sponse to shear stress and such agonists as histamine and thrombin. These activators convert inactive plasminogen to plasmin, which then breaks down fibrin and dissolves blood clots (fibrinolytic effects). Produces thrombomodulin, a protein that helps prevent formation of intravascular thrombi by inhibiting thrombin-mediated platelet aggregation. Thrombomodulin also reacts with thrombin to activate proteins C and S, which inhibit the plasma cascade of clotting factors. Prothrombotic Functions • Produces antifibrinolytic factors. Normally, the balance be- • • tween profibrinolysis and antifibrinolysis favors fibrinolysis (clot dissolution). In pathologic conditions, including atherosclerosis, fibrinolysis may be limited and thrombosis enhanced. In pathologic conditions, may induce synthesis of prothrombotic factors such as von Willebrand factor. Von Willebrand factor serves as a site for subendothelial platelet adhesion and as a carrier for blood coagulation factor VIII in plasma. Several disease states are associated with increased or altered production of von Willebrand factor, including atherosclerosis. Produces tissue factor, which activates the extrinsic coagulation pathway after exposure to oxidized low-density lipoprotein cholesterol, homocysteine, and cytokines (eg, interleukin-1, tumor necrosis factor–alpha) Platelets Platelets (also called thrombocytes) are fragments of large cells called megakaryocytes. They are produced in the bone marrow and released into the bloodstream, where they circulate for approximately 7 to 10 days before they are removed by the spleen. They contain no nuclei and therefore cannot repair or replicate themselves. The cell membrane of a platelet contains a coat of glycoproteins that prevents the platelet from adhering to normal endothelium but allows it to adhere to damaged areas of endothelium and subendothelial collagen in the blood vessel wall. It also contains receptors for ADP, collagen, blood coagulation factors such as fibrinogen, and other substances. Breakdown of the cell membrane releases arachidonic acid (which can be metabolized to produce thromboxane A2) and allows leakage of platelet contents (eg, thromboplastin and other clotting factors), which function to stop bleeding. The cytoplasm of a platelet contains storage granules with ADP, fibrinogen, histamine, platelet-derived growth factor, serotonin, von Willebrand factor, enzymes that produce thromboxane A2, and other substances. The cytoplasm also contains contractile proteins that contract storage granules so they empty their contents and help a platelet plug to retract and plug a hole in a torn blood vessel. The only known function of platelets is hemostasis. When platelets come in contact with a damaged vascular surface, they become activated and undergo changes in structure and function. They enlarge, express receptors on their surfaces, release mediators from their storage granules, become sticky so that they adhere to endothelial and collagen cells, and form a platelet thrombus (ie, a cluster or aggregate of activated platelets) within seconds. The thrombus blocks the tear in the blood vessel and prevents further leakage of blood. Platelets usually disappear from a blood clot within 24 hours and are replaced by fibrin. Formation of a platelet thrombus proceeds through the phases of activation, adhesion, aggregation, and procoagulation. Activation Platelet activation occurs when agonists such as thrombin, collagen, ADP, or epinephrine bind to their specific receptors on the platelet cell membrane surface. Activated platelets release von Willebrand factor, which aids platelet adhesion to blood vessel walls. They also secrete ADP and thromboxane A2 into the blood. The ADP and thromboxane A2 activate and recruit nearby platelets. Adhesion Platelet adhesion involves changes in platelets that allow them to adhere to endothelial cells and subendothelial collagen exposed by damaged endothelium. Adhesion is mediated by interactions between platelets and substances in the subendothelial tissues. Platelets contain binding sites for several subendothelial tissue proteins, including collagen and von Willebrand factor. In capillaries, where blood shear rates are high, platelets also can bind indirectly to collagen through von Willebrand factor. Von Willebrand factor is synthesized by endothelial cells and megakaryocytes. Although it contains binding sites for platelets and collagen, it does not normally bind with platelets until they are activated. Aggregation Aggregation involves the accumulation of platelets at a site of injury to a blood vessel wall and is stimulated by ADP, collagen, thromboxane A2, thrombin, and other factors. It requires the binding of extracellular fibrinogen to platelet fibrinogen receptors. The fibrinogen receptor is located on a complex of two glycoproteins (GPIIb and IIIa) in the platelet cell membrane. Although many GP IIb/IIIa complexes are on the surface of each platelet, they do not function as fibrinogen receptors until the platelet is activated by an agonist. Each activated GP IIb/IIIa CHAPTER 57 DRUGS THAT AFFECT BLOOD COAGULATION BOX 57–1 835 HEMOSTASIS AND THROMBOSIS (Continued) complex is capable of binding a single fibrinogen molecule. However, a fibrinogen molecule may bind to receptors on adjacent activated platelets, thus acting as a bridge to connect the platelets. Activated GP IIb/IIIa complexes can also bind von Willebrand factor and promote platelet aggregation when fibrinogen is lacking. Aggregated platelets produce and release thromboxane A2, which acts with ADP from platelet storage granules to promote additional GP IIb/IIIa activation, platelet secretion, and aggregate formation. The exposure of functional GP IIb/IIIa complexes is also stimulated by thrombin, which can directly stimulate thromboxane A2 synthesis and granule secretion without initial aggregation. Collagen stimulates additional aggregation by increasing the production of thromboxane A2 and storage granule secretion. Overall, aggregated platelets release substances that recruit new platelets and stimulate additional aggregation. This activity helps the platelet plug become large enough to block blood flow out of a damaged blood vessel. If the opening is small, the platelet plug can stop blood loss. If the opening is large, a platelet plug and a blood clot are both required to stop the bleeding. Procoagulant Activity In addition to forming a platelet thrombus, platelets also activate and interact with circulating blood coagulation factors to form a larger and more stable blood clot. Activation of the previously inactive blood coagulation factors leads to formation of fibrin threads that attach to the platelets and form a tight meshwork of a fully developed blood clot. More specifically, the platelet plug provides a surface on which coagulation enzymes, substrates, and cofactors interact at high local concentrations. These interactions lead to activation of coagulation factor X and the conversion of prothrombin to thrombin. ond, embolization obstructs the blood supply when the embolus becomes lodged. The pulmonary arteries are common sites of embolization. DRUGS USED IN THROMBOTIC AND THROMBOEMBOLIC DISORDERS Drugs given to prevent or treat thrombosis alter some aspect of the blood coagulation process. Anticoagulants are widely used in thrombotic disorders. They are more effective in preventing venous thrombosis than arterial thrombosis. Antiplatelet drugs are used to prevent arterial thrombosis. Thrombolytic agents are used to dissolve thrombi and limit tissue damage in selected thromboembolic disorders. These drugs are described in the following sections and in Drugs at a Glance: Anticoagulant, Antiplatelet, and Thrombolytic Agents. Anticoagulants Anticoagulant drugs are given to prevent formation of new clots and extension of clots already present. They do not dis- Blood Coagulation The blood coagulation process causes hemostasis within 1 to 2 minutes. It involves sequential activation of clotting factors that are normally present in blood and tissues as inactive precursors and formation of a meshwork of fibrin strands that cements blood components together to form a stable, dense clot. Major phases include release of thromboplastin by disintegrating platelets and damaged tissue; conversion of prothrombin to thrombin, which requires thromboplastin and calcium ions; and conversion of fibrinogen to fibrin by thrombin. Blood coagulation results from activation of the intrinsic or extrinsic coagulation pathway. Both pathways, which are activated when blood passes out of a blood vessel, are needed for normal hemostasis. The intrinsic pathway occurs in the vascular system; the extrinsic pathway occurs in the tissues. Although the pathways are initially separate, the terminal steps (ie, activation of factor X and thrombin-induced formation of fibrin) are the same. The intrinsic pathway is activated when blood comes in contact with collagen in the injured vessel wall and coagulation factor XII interacts with biologic surfaces. The normal endothelium prevents factor XII from interacting with such surfaces. The activated form of factor XII is a protease that starts the interactions among factors involved in the intrinsic pathway (eg, prekallikrein, factor IX, factor VIII). The extrinsic pathway is activated when blood is exposed to tissue extracts and tissue factor interacts with circulating coagulation factor VII. Activated factors VII and IX both act on factor X to produce activated factor X, which then interacts with factor V, calcium, and platelet factor 3. Platelet factor 3, a component of the platelet cell membrane, becomes available on the platelet surface only during platelet activation. The interactions among these substances lead to formation of thrombin, which then activates fibrinogen to form fibrin, and the clot is complete. solve formed clots, improve blood flow in tissues around the clot, or prevent ischemic damage to tissues beyond the clot. Heparins and warfarin are commonly used anticoagulants; danaparoid and lepirudin are newer agents. Clinical indications include prevention or management of thromboembolic disorders, such as thrombophlebitis, DVT, and pulmonary embolism. The main adverse effect is bleeding. Heparin Heparin is a pharmaceutical preparation of the natural anticoagulant produced primarily by mast cells in pericapillary connective tissue. Endogenous heparin is found in various body tissues, most abundantly in the liver and lungs. Exogenous heparin is obtained from bovine lung or porcine intestinal mucosa and standardized in units of biologic activity. Heparin combines with antithrombin III (a natural anticoagulant in the blood) to inactivate clotting factors IX, X, XI, and XII, inhibit the conversion of prothrombin to thrombin, and prevent thrombus formation. After thrombosis has developed, heparin can inhibit additional coagulation by inactivating thrombin, preventing the conversion of fibrinogen to 836 SECTION 9 DRUGS AFFECTING THE CARDIOVASCULAR SYSTEM Drugs at a Glance: Anticoagulant, Antiplatelet, and Thrombolytic Agents Generic/Trade Name Anticoagulants Heparin Argatroban (Argatroban) Bivalirudin (Angiomax) Indications for Use Routes and Dosage Ranges Prevention and management of thromboembolic disorders (eg, deep vein thrombosis, pulmonary embolism, atrial fibrillation with embolization) Adults: IV injection, 5000 units initially, followed by 5000–10,000 units q4–6h, to a maximum dose of 25,000 units/d; IV infusion, 5000 units (loading dose), then 15–25 units/kg/h DIC, IV injection, 50–100 units/kg q4h; IV infusion, 20,000–40,000 units/d at initial rate of 0.25 units/kg/min, then adjusted according to aPTT; SC 10,000–12,000 units q8h, or 14,000–20,000 units q12h Low-dose prophylaxis, SC 5000 units 2 h before surgery, then q12h until discharged from hospital or fully ambulatory Children: DIC, IV injection, 25–50 units/kg q4h; IV infusion, 50 units/kg initially, followed by 100 units/kg q4h or 20,000 units/m2 over 24 h IV continuous infusion 2 mcg/kg/min Thrombosis prophylaxis or management in heparininduced thrombocytopenia Clients with unstable angina undergoing PTCA Dalteparin (Fragmin) Prophylaxis of DVT in clients having hip replacement surgery; also clients at high risk of thromboembolic disorders who are having abdominal surgery Danaparoid (Orgaran) Prophylaxis of DVT in clients having hip replacement surgery Prevention and management of DVT and pulmonary embolism Management of unstable angina, to prevent myocardial infarction Enoxaparin (Lovenox) Fondaparinux (Arixtra) Lepirudin (Refludan) Tinzaparin (Innohep) Warfarin (Coumadin) Antiplatelet Agents Aspirin Abciximab (ReoPro) Anagrelide (Agrylin) Prevention of DVT following hip fracture surgery or knee or hip replacement Heparin alternative for anticoagulation of clients with heparin-induced thrombocytopenia and associated thromboembolic disorders Management of DVT, with or without PE; may be given in conjunction with warfarin Long-term prevention or management of venous thromboembolic disorders, including DVT, PE, and embolization associated with atrial fibrillation and prosthetic heart valves. May also be used after myocardial infarction to decrease reinfarction, stroke, venous thromboembolism, and death Prevention of myocardial infarction Prevention of thromboembolic disorders in clients with prosthetic heart valves or transient ischemic attacks Used with PTCA to prevent rethrombosis of treated arteries Intended for use with aspirin and heparin Essential thrombocythemia, to reduce the elevated platelet count, the risk of thrombosis, and associated symptoms IV bolus dose of 1 mg/kg followed by 4 h infusion at rate of 2.5 mg/kg/min Abdominal surgery, SC 2500 IU 1–2 h before surgery and then once daily for 5–10 days after surgery Hip replacement surgery, SC 2500 IU 1–2 h before surgery and the evening of surgery (at least 6 h after first dose) and then 5000 IU once daily for 5 days SC 750 IU twice daily, with first dose 1–24 h before surgery, then daily for 7–14 days after surgery DVT prophylaxis in clients having hip or knee replacement surgery, SC 30 mg twice daily, with first dose within 12–24 h after surgery and continued for 7–10 days Abdominal surgery, SC 40 mg once daily with first dose given 2 h before surgery, for 7–10 days DVT/pulmonary embolism management, outpatients, SC 1 mg/kg q12h; inpatients, 1 mg/kg q12h or 1.5 mg/kg q24h Unstable angina 1 mg/kg q12h in conjunction with oral aspirin (100–325 mg once daily) SC 2.5 mg daily, with first dose 6–8 h after surgery and continuing for a maximum of 11 days IV injection, 0.4 mg/kg over 15–20 sec, followed by continuous IV infusion of 0.15 mg/kg for 2–10 days or longer if needed SC 175 anti-Xa IU/kg daily for at least 6 days and until adequately anticoagulated with warfarin PO 2–5 mg/d for 2–3 days, then adjusted according to the international normalized ratio (INR); average maintenance daily dose, 2–5 mg PO 81–325 mg daily IV bolus injection, 0.25 mg/kg 10–60 min before starting PTCA, then a continuous IV infusion of 10 mcg/min for 12 h PO 0.5 mg 4 times daily or 1 mg twice daily initially, then titrate to lowest dose effective in maintaining platelet count <600,000/mm3 CHAPTER 57 DRUGS THAT AFFECT BLOOD COAGULATION 837 Drugs at a Glance: Anticoagulant, Antiplatelet, and Thrombolytic Agents (continued) Generic/Trade Name Indications for Use Routes and Dosage Ranges Cilostazol (Pletal) Intermittent claudication, to increase walking distance (before leg pain occurs) Clopidogrel (Plavix) Reduction of atherosclerotic events (myocardial infarction, stroke, vascular death) in clients with atherosclerosis documented by recent stroke, recent myocardial infarction, or established peripheral artery disease Prevention of thromboembolism after cardiac valve replacement, given with warfarin Same as above PO 100 mg twice daily, 30 min before or 2 h after breakfast and dinner; reduce to 50 mg twice daily with concurrent use of fluconazole, itraconazole, erythromycin, or diltiazem PO 75 mg once daily with or without food Dipyridamole (Persantine) Dipyridamole and Aspirin (Aggrenox) Eptifibatide (Integrilin) Ticlopidine (Ticlid) Tirofiban (Aggrastat) Treprostinil (Remodulin) Thrombolytic Agents Alteplase (Activase) Drotrecogin alfa, activated (Xigris) Reteplase, recombinant (Retavase) Streptokinase (Streptase) Tenecteplase (TNKase) Urokinase (Abbokinase) Acute coronary syndromes, including clients who are to be managed medically and those undergoing PTCA Prevention of thrombosis in clients with coronary artery or cerebral vascular disease (eg, clients who have had stroke precursors or a completed thrombotic stroke) Acute coronary syndromes, with heparin, for clients who are to be managed medically or those undergoing PTCA Acute myocardial infarction Pulmonary embolism Pulmonary arterial hypertension PO 25–75 mg 3 times per day, 1 h before meals PO 1 capsule (200 mg extended-release dipyridamole/25 mg aspirin) twice daily IV bolus injection, 180 mcg/kg, followed by continuous infusion of 2 mcg/kg/min. See manufacturer’s instructions for preparation and administration. PO 250 mg twice daily with food IV infusion, 0.4 mcg/kg/min for 30 min, then 0.1 mcg/kg/min. Patients with severe renal impairment (creatinine clearance <30 mL/min) should receive half the usual rate of infusion. See manufacturer’s instructions for preparation and administration. Continuous infusion by SC catheter and infusion pump at initial dose of 1.25 mg/kg/min, increasing by no more than 1.25 mg/kg/min per week for first 4 wks, and then by no more than 2.5 mg/kg/min per week for remaining duration of infusion Acute ischemic stroke Acute myocardial infarction Pulmonary emboli Reduction of mortality in severe sepsis IV infusion, 100 mg over 3 h (first hour, 60 mg with a bolus of 6–10 mg over 1–2 min initially; second hour, 20 mg; third hour, 20 mg) IV infusion of 24 mcg/kg/h for 96 h Acute myocardial infarction IV injection, 10 units over 2 min, repeated in 30 min. Inject into a flowing IV infusion line that contains no other medications. IV 250,000 units over 30 min, then 100,000 units/h for 24–72 h Management of acute, severe pulmonary emboli or iliofemoral thrombophlebitis Used to dissolve clots in arterial or venous cannulas or catheters May be injected into a coronary artery to dissolve a thrombus if done within 6 h of onset of symptoms Acute myocardial infarction Coronary artery thrombi Pulmonary emboli Clearance of clogged IV catheters IV bolus dose based on weight, 30 mg (for <60 kg) not to exceed 50 mg (>90 kg) IV 4400 units/kg over 10 min, followed by continuous infusion of 4400 units/kg/h for 12 h For clearing IV catheters, see manufacturer’s instructions aPTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; PE, pulmonary embolism; PTCA, percutaneous transluminal coronary angioplasty or atherectomy. fibrin, and inhibiting factor XIII (the fibrin-stabilizing factor). Other effects include inhibiting factors V and VIII and platelet aggregation. Heparin acts immediately after intravenous (IV) injection and within 20 to 30 minutes after subcutaneous injection. It is metabolized in the liver and excreted in the urine, primarily as inactive metabolites. Heparin does not cross the pla- cental barrier and is not secreted in breast milk, making it the anticoagulant of choice for use during pregnancy and lactation. Disadvantages of heparin are its short duration of action and the subsequent need for frequent administration, the necessity for parenteral injection (because it is not absorbed from the gastrointestinal [GI] tract), and local tissue reactions at injection sites. 838 SECTION 9 DRUGS AFFECTING THE CARDIOVASCULAR SYSTEM Prophylactically, low doses of heparin are given to prevent DVT and pulmonary embolism in clients at risk for development of these disorders, such as the following: 1. Those with major illnesses (eg, acute myocardial infarction, heart failure, serious pulmonary infections, stroke) 2. Those having major abdominal or thoracic surgery 3. Those with a history of thrombophlebitis or pulmonary embolism, including pregnant women 4. Those having gynecologic surgery, especially if they have been taking estrogens or oral contraceptives or have other risk factors for DVT 5. Those expected to be on bed rest or to have limited activity for longer than 5 days Low-dose heparin prophylaxis is either ineffective or contraindicated in major orthopedic surgery, abdominal prostatectomy, and brain surgery. Therapeutically, heparin is used for management of acute thromboembolic disorders (eg, DVT, thrombophlebitis, pulmonary embolism). In these conditions, the aim of therapy is to prevent further thrombus formation and embolization. Heparin is also used in disseminated intravascular coagulation (DIC), a life-threatening condition characterized by widespread clotting, which depletes the blood of coagulation factors. The depletion of coagulation factors then produces widespread bleeding. The goal of heparin therapy in DIC is to prevent blood coagulation long enough for clotting factors to be replenished and thus be able to control hemorrhage. Heparin is also used to prevent clotting during cardiac and vascular surgery, extracorporeal circulation, hemodialysis, blood transfusions, and in blood samples to be used in laboratory tests. Contraindications include GI ulcerations (eg, peptic ulcer disease, ulcerative colitis), blood dyscrasias, severe kidney or liver disease, severe hypertension, polycythemia vera, and recent surgery of the eye, spinal cord, or brain. It should be used with caution in clients with hypertension, renal or hepatic disease, alcoholism, history of GI ulcerations, drainage tubes (eg, nasogastric tubes, indwelling urinary catheters), and any occupation with high risks of traumatic injury. Low–Molecular-Weight Heparins Standard heparin is a mixture of high–and low–molecularweight fractions, but most anticoagulant activity is attributed to the low–molecular-weight portion. Low–molecularweight heparins (LMWHs) contain the low–molecularweight fraction and are as effective as IV heparin in treating thrombotic disorders. Indications for use include prevention or management of thromboembolic complications associated with surgery or ischemic complications of unstable angina and myocardial infarction. Currently available LMWHs (dalteparin, enoxaparin, tinzaparin) differ from standard heparin and each other; they cannot be used interchangeably (ie, unit for unit). LMWHs are given subcutaneously and do not require close monitoring of blood coagulation tests. These characteristics allow outpatient anticoagulant therapy, an increasing trend. The drugs are also associated with less thrombocy- Nursing Notes: Apply Your Knowledge You are caring for a patient who is in traction. He is receiving 5000 units of subcutaneous heparin bid. Discuss the reason why this patient is receiving heparin and how you will safely administer the medication. topenia than standard heparin. However, platelet counts should be monitored during therapy. Warfarin Warfarin is the most commonly used oral anticoagulant. It acts in the liver to prevent synthesis of vitamin K–dependent clotting factors (ie, factors II, VII, IX, and X). Warfarin is similar to vitamin K in structure and therefore acts as a competitive antagonist to hepatic use of vitamin K. Anticoagulant effects do not occur for 3 to 5 days after warfarin is started because clotting factors already in the blood follow their normal pathway of elimination. Warfarin has no effect on circulating clotting factors or on platelet function. Warfarin is well absorbed after oral administration. It is highly bound to plasma proteins (98%), mainly albumin. It is metabolized in the liver and primarily excreted as inactive metabolites by the kidneys. Warfarin is most useful in long-term prevention or management of venous thromboembolic disorders, including DVT, pulmonary embolism, and embolization associated with atrial fibrillation and prosthetic heart valves. In addition, warfarin therapy after myocardial infarction may decrease reinfarction, stroke, venous thromboembolism, and death. Smaller doses are being used now than formerly, with similar antithrombotic effects and decreased risks of bleeding. Like heparin, warfarin is contraindicated in clients with GI ulcerations, blood disorders associated with bleeding, severe kidney or liver disease, severe hypertension, and recent surgery of the eye, spinal cord, or brain. It should be used cautiously with mild hypertension, renal or hepatic disease, alcoholism, history of GI ulcerations, drainage tubes (eg, nasogastric tubes, indwelling urinary catheters), and occupations with high risks of traumatic injury. In addition, warfarin is contraindicated during pregnancy. Other Anticoagulant Drugs Danaparoid, a heparinoid, is a low–molecular weight, heparin-like drug derived from porcine mucosa. It has antithrombotic effects and is given subcutaneously to prevent postoperative thromboembolism in clients having hip replacement surgery, in the management of ischemic stroke, and as an alternative anticoagulant in clients who cannot tolerate heparin. Although related to heparin and LMWHs, it does not contain heparin and cannot be used interchangeably with standard heparin or LMWHs. CHAPTER 57 DRUGS THAT AFFECT BLOOD COAGULATION Fondaparinux produces anticoagulant effects by directly binding to circulating and clot-bound factor Xa, accelerating the activity of antithrombin and inhibiting thrombin production. It is used in the prevention of DVT in clients having surgery for hip fracture or joint replacement surgery of the knee or hip. Lepirudin, bivalirudin, and argatroban are direct thrombin inhibitors that prevent blood coagulation by inactivating thrombin. They are used as a heparin substitute for clients who need anticoagulation but have thrombocytopenia with heparin. Antiplatelet Drugs Antiplatelet drugs prevent one or more steps in the prothrombotic activity of platelets. As described previously, platelet activity is very important in both physiologic hemostasis and pathologic thrombosis. Arterial thrombi, which are composed primarily of platelets, may form on top of atherosclerotic plaque and block blood flow in the artery. They may also form on heart walls and valves and embolize to other parts of the body. Drugs used clinically for antiplatelet effects act by a variety of mechanisms to inhibit platelet activation, adhesion, aggregation, or procoagulant activity. These include drugs that block platelet receptors for thromboxane A2, adenosine diphosphate (ADP), glycoprotein (GP) IIb/IIIa, and phosphodiesterase. Thromboxane A2 Inhibitors Aspirin is a commonly used analgesic–antipyretic–antiinflammatory drug (see Chap. 7) with potent antiplatelet effects. Aspirin exerts pharmacologic actions by inhibiting synthesis of prostaglandins. In this instance, aspirin acetylates cyclooxygenase, the enzyme in platelets that normally synthesizes thromboxane A2, a prostaglandin product that causes platelet aggregation. Thus, aspirin prevents formation of thromboxane A2 and thromboxane A2–induced platelet aggregation and thrombus formation. A single dose of 300 to 600 mg or multiple doses of 30 mg (eg, daily for several days) inhibit the cyclooxygenase in circulating platelets almost completely. These antithrombotic effects persist for the life of the platelet (7 to 10 days). Aspirin may be used long term for prevention of myocardial infarction or stroke, and in clients with prosthetic heart valves. It is also used for the immediate treatment of suspected or actual acute myocardial infarction, for transient ischemic attacks (TIAs), or evolving thrombotic strokes. Adverse effects are uncommon with the small doses used for antiplatelet effects. However, there is an increased risk of bleeding, including hemorrhagic stroke. Because approximately 85% of strokes are thrombotic, the benefits of aspirin or other antiplatelet agents are thought to outweigh the risks of hemorrhagic strokes (approximately 15%). Nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen and many other aspirin-related drugs, inhibit cyclooxygenase reversibly. Their antiplatelet effects subside when the drugs are eliminated from the circulation and the 839 drugs usually are not used for antiplatelet effects. However, clients who take an NSAID daily (eg, for arthritis pain) may not need to take additional aspirin for antiplatelet effects. Acetaminophen does not affect platelets in usual doses. Adenosine Diphosphate Receptor Antagonists Ticlopidine inhibits platelet aggregation by preventing ADPinduced binding between platelets and fibrinogen. This reaction inhibits platelet aggregation irreversibly, and effects persist for the lifespan of the platelet. The drug is indicated for prevention of thrombotic stroke in people who have had stroke precursor events (eg, TIAs) or a completed thrombotic stroke. Ticlopidine is considered a second-line drug for clients who cannot take aspirin. The adverse effects (eg, neutropenia, diarrhea, skin rashes) and greater cost make it prohibitive for use by many clients. Contraindications include active bleeding disorders (eg, GI bleeding from peptic ulcer or intracranial bleeding), neutropenia, thrombocytopenia, severe liver disease, and hypersensitivity to the drug. Ticlopidine is rapidly absorbed after oral administration and reaches peak plasma levels about 2 hours after a dose. It is highly protein bound (98%), extensively metabolized in the liver, and excreted in urine and feces. As with other antiplatelet drugs, there is increased risk of bleeding with ticlopidine. Clopidogrel is chemically related to ticlopidine and causes similar effects. It is indicated for reduction of myocardial infarction, stroke, and vascular death in clients with atherosclerosis and reportedly causes fewer or less severe adverse effects than ticlopidine. Glycoprotein IIb/IIIa Receptor Antagonists Abciximab is a monoclonal antibody that prevents the binding of fibrinogen, von Willebrand factor, and other molecules to GP IIb/IIIa receptors on activated platelets. This action inhibits platelet aggregation. Abciximab is used with percutaneous transluminal coronary angioplasty or removal of atherosclerotic plaque to prevent rethrombosis of treated arteries. It is used with aspirin and heparin and is contraindicated in clients who have recently received an oral anticoagulant or IV Dextran. Other contraindications include active bleeding, thrombocytopenia, history of a serious stroke, surgery or major trauma within the previous 6 weeks, uncontrolled hypertension, or hypersensitivity to drug components. Eptifibatide and tirofiban inhibit platelet aggregation by preventing activation of GP IIb/IIIa receptors on the platelet surface and the subsequent binding of fibrinogen and von Willebrand factor to platelets. Antiplatelet effects occur during drug infusion and stop when the drug is stopped. The drugs are indicated for acute coronary syndrome (eg, unstable angina, myocardial infarction) in clients who are to be managed medically or by angioplasty or atherectomy. Drug half-life is approximately 2.5 hours for eptifibatide and 2 hours for tirofiban; the drugs are cleared mainly by renal excretion. With tirofiban, plasma clearance is approxi- 840 SECTION 9 DRUGS AFFECTING THE CARDIOVASCULAR SYSTEM mately 25% lower in older adults and approximately 50% lower in clients with severe renal impairment (creatinine clearance <30 mL/minute). The drugs are contraindicated in clients with hypersensitivity to any component of the products; current or previous bleeding (within the previous 30 days); a history of thrombocytopenia after previous exposure to tirofiban; a history of stroke within 30 days or any history of hemorrhagic stroke; major surgery or severe physical trauma within the previous month; severe hypertension (systolic blood pressure >180 mm Hg with tirofiban or >200 mm Hg with eptifibatide, or diastolic blood pressure >110 mm Hg with either drug); a history of intracranial hemorrhage, neoplasm, arteriovenous malformation, or aneurysm; a platelet count less than 100,000 mm3; serum creatinine 2 mg/dL or above (for the 180 mcg/kg bolus and the 2 mcg/kg/min infusion) or 4 mg/dL or above (for the 135 mcg/kg bolus and the 0.5 mcg/kg/min infusion); or dependency on dialysis (eptifibatide). Bleeding is the most common adverse effect, with most major bleeding occurring at the arterial access site for cardiac catheterization. If bleeding occurs and cannot be controlled with pressure, the drug infusion and heparin should be discontinued. These drugs should be used cautiously if given with other drugs that affect hemostasis (eg, warfarin, thrombolytics, other antiplatelet drugs). Phosphodiesterase Inhibitor Cilostazol inhibits phosphodiesterase, an enzyme that metabolizes cyclic adenosine monophosphate (cAMP). The inhibition increases intracellular cAMP, which then inhibits platelet aggregation and produces vasodilation. The drug reversibly inhibits platelet aggregation induced by various stimuli (eg, thrombin, ADP, collagen, arachidonic acid, epinephrine, and shear stress). It is indicated for management of intermittent claudication. Symptoms usually improve within 2 to 4 weeks, but may take as long as 12 weeks. The drug is contraindicated in clients with heart failure. Cilostazol is highly protein bound (95% to 98%), mainly to albumin, extensively metabolized by hepatic cytochrome P450 enzymes, and excreted in urine (74%) and feces. The drug and two active metabolites accumulate with chronic administration and reach steady state within a few days. The most common adverse effects are diarrhea and headache. Miscellaneous Agents Anagrelide inhibits platelet aggregation induced by cAMP phosphodiesterase, ADP, and collagen. However, it is indicated only to reduce platelet counts for clients with essential thrombocythemia (a disorder characterized by excessive numbers of platelets). Doses to reduce platelet production are smaller than those required to inhibit platelet aggregation. Dipyridamole inhibits platelet adhesion, but its mechanism of action is unclear. It is used for prevention of throm- boembolism after cardiac valve replacement and is given with warfarin. Thrombolytic Agents Thrombolytic agents are given to dissolve thrombi. They stimulate conversion of plasminogen to plasmin (also called fibrinolysin), a proteolytic enzyme that breaks down fibrin, the framework of a thrombus. The main use of thrombolytic agents is for management of acute, severe thromboembolic disease, such as myocardial infarction, pulmonary embolism, and iliofemoral thrombosis. The goal of thrombolytic therapy is to re-establish blood flow and prevent or limit tissue damage. Heparin and warfarin are given after completion of thrombolytic therapy. Thrombolytic drugs are also used to dissolve clots in arterial or venous cannulas or catheters. Alteplase, reteplase, and tenecteplase are tissue plasminogen activators used mainly in acute myocardial infarction to dissolve clots obstructing coronary arteries and re-establish perfusion of tissues beyond the thrombotic area. The drugs bind to fibrin in a clot and act locally to dissolve the clot. The most common adverse effect is bleeding, which may be internal (eg, intracranial, GI, genitourinary) or external (eg, venous or arterial puncture sites, surgical incisions). The drugs are contraindicated in the presence of bleeding, a history of stroke, central nervous system surgery or trauma within the previous 2 months, and severe hypertension. Streptokinase and urokinase are enzymes that break down fibrin. They are used mainly to lyse coronary artery clots in acute myocardial infarction. Streptokinase may also be used to dissolve clots in vascular catheters and to treat acute, severe, pulmonary emboli or iliofemoral thrombophlebitis. Urokinase is recommended for use in clients allergic to streptokinase. As with other anticoagulants and thrombolytic agents, bleeding is the main adverse effect. Drotrecogin alfa (Xigris) is a recombinant version of human activated protein C that is approved for use in severe sepsis or septic shock. Severe sepsis is characterized by an excessive inflammatory reaction to infection, inappropriate blood clot formation, and impaired breakdown of clots. Drotrecogin alfa is given for its thrombolytic effects, along with other therapies for inflammation and infection. The major adverse effect is bleeding. Drugs Used to Control Bleeding Anticoagulant, antiplatelet, and thrombolytic drugs profoundly affect hemostasis, and their major adverse effect is bleeding. As a result, systemic hemostatic agents (antidotes) may be needed to prevent or treat bleeding episodes. Antidotes should be used cautiously because overuse can increase risks of recurrent thrombotic disorders. The drugs are described in this section and in Drugs at a Glance: Systemic Hemostatic Drugs. CHAPTER 57 DRUGS THAT AFFECT BLOOD COAGULATION 841 Drugs at a Glance: Systemic Hemostatic Drugs Generic/Trade Name Indications for Use Dosage Aminocaproic acid (Amicar) Control bleeding caused by overdoses of thrombolytic agents or bleeding disorders caused by hyperfibrinolysis (eg, cardiac surgery, blood disorders, hepatic cirrhosis, prostatectomy, neoplastic disorders) Used in selected clients undergoing coronary artery bypass graft surgery to decrease blood loss and blood transfusions Treatment of heparin overdosage PO, IV infusion, 5 g initially, followed by 1.0 to 1.25 g/h for 8 h or until bleeding is controlled; maximum dose, 30 g/24 h Aprotinin (Trasylol) Protamine sulfate Tranexamic acid (Cyklokapron) Vitamin K (Mephyton) Control bleeding caused by overdoses of thrombolytic agents Prevent or decrease bleeding from tooth extraction in clients with hemophilia Antidote for warfarin overdosage Aminocaproic acid and tranexamic acid are used to stop bleeding caused by overdoses of thrombolytic agents. Aminocaproic acid also may be used in other bleeding disorders caused by hyperfibrinolysis (eg, cardiac surgery, blood disorders, hepatic cirrhosis, prostatectomy, neoplastic disorders). Tranexamic acid also is used for short periods (2 to 8 days) in clients with hemophilia to prevent or decrease bleeding from tooth extraction. Dosage of tranexamic acid should be reduced in the presence of moderate or severe renal impairment. Aprotinin is a natural protease inhibitor obtained from bovine lung that has a variety of effects on blood coagulation. It inhibits plasmin and kallikrein, thus inhibiting fibrinolysis, and inhibits breakdown of blood clotting factors. It is used to decrease bleeding in selected clients undergoing coronary artery bypass surgery. Protamine sulfate is an antidote for standard heparin and LMWHs. Because heparin is an acid and protamine sulfate is a base, protamine neutralizes heparin activity. Protamine dosage depends on the amount of heparin administered during the previous 4 hours. Each milligram of protamine neutralizes approximately 100 units of heparin or dalteparin and 1 mg of enoxaparin. A single dose should not exceed 50 mg. The drug is given by slow IV infusion over at least 10 minutes (to prevent or minimize adverse effects of hypotension, bradycardia, and dyspnea). Protamine effects occur immediately and last for approximately 2 hours. A second dose may be required because heparin activity lasts approximately 4 hours. Protamine sulfate can cause severe hypotensive and anaphylactoid reactions. Thus, it should be given in settings with equipment and personnel for resuscitation and management of anaphylactic shock. Vitamin K is the antidote for warfarin overdosage. An oral dose of 10 to 20 mg usually stops minor bleeding and returns the international normalized ratio (INR) (see section on Regulation of Heparin and Warfarin Dosage, later) to a normal range within 24 hours. See manufacturer’s literature Depends on the amount of heparin given within the previous 4 h PO 25 mg/kg 3 to 4 times daily, starting 1 d before surgery, or IV 10 mg/kg immediately before surgery, followed by 25 mg/kg PO 3 to 4 times daily for 2–8 d PO 10–20 mg in a single dose Herbal and Dietary Supplements Many commonly used herbs and supplements have a profound effect on drugs used for anticoagulation. Multivitamin supplements may contain 25 to 28 mcg of vitamin K and should be taken consistently to avoid fluctuating vitamin K levels. Doses of vitamin C in excess of 500 mg/d may lower INR and vitamin E in excess of 400 IU/d may increase warfarin effects. Herbs commonly used that may increase the effects of warfarin include alfalfa, celery, clove, feverfew, garlic, ginger, ginkgo, ginseng, and licorice. Clients taking warfarin should be questioned carefully about their use of herbs as well as vitamin or mineral supplements. Nursing Process Assessment Assess the client’s status in relation to thrombotic and thromboembolic disorders. • Risk factors for thromboembolism include: • Immobility (eg, limited activity or bed rest for more than 5 days) • Obesity • Cigarette smoking • History of thrombophlebitis, deep vein thrombosis (DVT), or pulmonary emboli • Congestive heart failure • Pedal edema • Lower limb trauma • Myocardial infarction • Atrial fibrillation • Mitral or aortic stenosis • Prosthetic heart valves • Abdominal, thoracic, pelvic, or major orthopedic surgery 842 SECTION 9 DRUGS AFFECTING THE CARDIOVASCULAR SYSTEM • Atherosclerotic heart disease or peripheral vascular disease • Use of oral contraceptives • Signs and symptoms of thrombotic and thromboembolic disorders depend on the location and size of the thrombus. • DVT and thrombophlebitis usually occur in the legs. The conditions may be manifested by edema (the affected leg is often measurably larger than the other) and pain, especially in the calf when the foot is dorsiflexed (Homans’ sign). If thrombophlebitis is superficial, it may be visible as a red, warm, tender area following the path of a vein. • Pulmonary embolism, if severe enough to produce symptoms, is manifested by chest pain, cough, hemoptysis, tachypnea, and tachycardia. Massive emboli cause hypotension, shock, cyanosis, and death. • Disseminated intravascular coagulation (DIC) is usually manifested by bleeding, which may range from petechiae or oozing from a venipuncture site to massive internal bleeding or bleeding from all body orifices. Nursing Diagnoses • Ineffective Tissue Perfusion related to thrombus or embolus or drug-induced bleeding • Acute Pain related to tissue ischemia • Impaired Physical Mobility related to bed rest and pain • Ineffective Coping related to the need for long-term prophylaxis of thromboembolic disorders or fear of excessive bleeding • Anxiety related to fear of myocardial infarction or stroke • Deficient Knowledge related to anticoagulant or antiplatelet drug therapy • Risk for Injury related to drug-induced impairment of blood coagulation Planning/Goals The client will: • Receive or take anticoagulant and antiplatelet drugs correctly • Be monitored closely for therapeutic and adverse drug effects, especially when drug therapy is started and when changes are made in drugs or dosages • Use nondrug measures to decrease venous stasis and prevent thromboembolic disorders • Act to prevent trauma from falls and other injuries • Inform any health care provider when taking an anticoagulant or antiplatelet drug • Avoid or report adverse drug reactions • Verbalize or demonstrate knowledge of safe management of anticoagulant drug therapy • Keep follow-up appointments for tests of blood coagulation and drug dosage regulation • Avoid preventable bleeding episodes Interventions Use measures to prevent thrombotic and thromboembolic disorders. • Have the client ambulate and exercise legs regularly, especially after surgery. • For clients who cannot ambulate or do leg exercises, do passive range-of-motion and other leg exercises several times daily when changing the client’s position or performing other care. • Have the client wear elastic stockings. Elastic stockings should be removed every 8 hours and replaced after inspecting the skin. Improperly applied elastic stockings can impair circulation rather than aid it. For clients on bed rest, intermittent pneumatic compression devices can also be used. • Avoid trauma to lower extremities. • Maintain adequate fluid intake (1500–3000 mL/day) to avoid dehydration and hemoconcentration. • Assist clients to promote good blood circulation (eg, exercise) and avoid situations that impair circulation (eg, wearing tight clothing, crossing the legs at the knees, prolonged sitting or standing, bed rest, and placing pillows under the knees when in bed). For the client receiving anticoagulant therapy, implement safety measures to prevent trauma and bleeding. • For clients who cannot ambulate safely because of weakness, sedation, or other conditions, keep the call light within reach, keep bedrails elevated, and assist in ambulation. • Provide an electric razor for shaving. • Avoid intramuscular injections, venipunctures, and arterial punctures when possible. • Avoid intubations when possible (eg, nasogastric tubes, indwelling urinary catheters). For the client receiving tirofiban or eptifibatide: • Monitor the femoral artery access site closely. This is the most common site of bleeding. • Avoid invasive procedures as much as possible (eg, arterial and venous punctures, intramuscular injections, urinary catheters, nasotracheal suction, nasogastric tubes). If venipuncture must be done, avoid sites where pressure cannot be applied (eg, subclavian or jugular veins). • While the vascular sheath is in place, keep clients on complete bed rest with the head of the bed elevated 30 degrees and the affected limb restrained in a straight position. • Discontinue heparin for 3 to 4 hours and be sure the activated clotting time is less than 180 seconds or the activated partial thromboplastin time (aPTT) is below 45 seconds before removing the vascular sheath. • After the vascular sheath is removed, apply pressure to the site and observe closely. For outpatients, be sure there is no bleeding for at least 4 hours before hospital discharge. For the client receiving a thrombolytic drug or a revascularization procedure for acute myocardial infarction: • Monitor closely for bleeding. • Assist the client and family to understand the importance of diligent efforts to reverse risk factors contributing to coronary artery disease (eg, diet and perhaps medication CHAPTER 57 DRUGS THAT AFFECT BLOOD COAGULATION to lower serum cholesterol to below 200 mg/dL and lowdensity lipoprotein cholesterol to below 130 mg/dL, weight reduction if overweight, control of blood pressure if hypertensive, avoidance of smoking, stress reduction techniques, exercise program designed and supervised by a health care provider). • Assist the client and family to understand the importance of complying with medication orders to prevent reinfarction and other complications, and continued medical supervision. Evaluation • Observe for signs and symptoms of thromboembolic disorders or bleeding. • Check blood coagulation tests for therapeutic ranges. • Observe and interview regarding compliance with instructions about drug therapy. • Observe and interview regarding adverse drug effects. PRINCIPLES OF THERAPY Drug Selection Choices of anticoagulant and antiplatelet drugs depend on the reason for use and other drug and client characteristics. 1. Heparin is the anticoagulant of choice in acute venous thromboembolic disorders because the anticoagulant effect begins immediately with IV administration. 2. Warfarin is the anticoagulant of choice for long-term maintenance therapy (ie, several weeks or months) because it can be given orally. 3. Aspirin has long been the most widely used antiplatelet drug for prevention of myocardial reinfarction and arterial thrombosis in clients with TIAs and prosthetic heart valves. However, clopidogrel may be more effective than aspirin. 4. When anticoagulation is required during pregnancy, heparin is used because it does not cross the placenta. Warfarin is contraindicated during pregnancy. 5. Various combinations of antithrombotic drugs are used concomitantly or sequentially (eg, abciximab is used with aspirin and heparin; thrombolytic drugs are usually followed with heparin and warfarin). Regulation of Heparin and Warfarin Dosage Heparin dosage is regulated by the activated partial thromboplastin time (aPTT), which is sensitive to changes in blood clotting factors, except factor VII. Thus, normal or control values indicate normal blood coagulation; therapeutic values indicate low levels of clotting factors and delayed blood coagulation. During heparin therapy, the aPTT should be maintained at approximately 1.5 to 2.5 times the control or 843 baseline value. The normal control value is 25 to 35 seconds; therefore, therapeutic values are 45 to 70 seconds, approximately. With continuous IV infusion, blood for the aPTT may be drawn at any time; with intermittent administration, blood for the aPTT should be drawn approximately 1 hour before a dose of heparin is scheduled. Monitoring of aPTT is not necessary with low-dose standard heparin given subcutaneously for prophylaxis of thromboembolism or with the LMWHs (eg, enoxaparin). Warfarin dosage is regulated according to the INR, for which therapeutic values are 2.0 to 3.0 in most conditions. An average daily dose of 4 to 5 mg maintains a therapeutic INR; stopping warfarin returns an elevated INR to normal in approximately 4 days in most clients. The INR is based on prothrombin time (PT). PT is sensitive to changes in three of the four vitamin K–dependent coagulation factors. Thus, normal or control values indicate normal levels of these factors; therapeutic values indicate low levels of the factors and delayed blood coagulation. A normal baseline or control PT is approximately 12 seconds; a therapeutic value is approximately 1.5 times the control, or 18 seconds. When warfarin is started, PT and INR should be assessed daily until a stable daily dose is reached (the dose that maintains PT and INR within therapeutic ranges and does not cause bleeding). Thereafter, PT and INR are determined every 2 to 4 weeks for the duration of oral anticoagulant drug therapy. If the warfarin dose is changed, PT and INR are needed more often until a stable daily dose is again established. For many years, the PT was used to regulate warfarin dosage. PT is determined by adding a mixture of thromboplastin and calcium to citrated plasma and measuring the time (in seconds) it takes for the blood to clot. However, values vary among laboratories according to the type of thromboplastin and the instrument used to measure PT. The INR system standardizes the PT by comparing a particular thromboplastin with a standard thromboplastin designated by the World Health Organization. Advantages of the INR include consistent values among laboratories, more consistent warfarin dosage with less risk of bleeding or thrombosis, and more consistent reports of clinical trials and other research studies. Some laboratories report both PT and INR. Warfarin dosage may need to be reduced in clients with biliary tract disorders (eg, obstructive jaundice), liver disease (eg, hepatitis, cirrhosis), malabsorption syndromes (eg, steatorrhea), and hyperthyroidism or fever. These conditions increase anticoagulant drug effects by reducing absorption of vitamin K, decreasing hepatic synthesis of blood clotting factors, or increasing the breakdown of clotting factors. Despite these influencing factors, however, the primary determinant of dosage is the PT and INR. Warfarin interacts with many other drugs to cause increased, decreased, or unpredictable anticoagulant effects (see Nursing Actions). Thus, warfarin dosage may need to be increased or decreased when other drugs are given concomitantly. Most drugs can be given if warfarin dosage is titrated according to the PT or INR and altered appropriately when an interacting drug is added or stopped. INR or PT measurements and vigi- 844 SECTION 9 DRUGS AFFECTING THE CARDIOVASCULAR SYSTEM CLIENT TEACHING GUIDELINES Drugs to Prevent or Treat Blood Clots General Considerations ✔ Antiplatelet and anticoagulant drugs are given to people who have had, or who are at risk of having, a heart attack, stroke, or other problems from blood clots. For prevention of a heart attack or stroke, you are most likely to be given an antiplatelet drug (eg, aspirin, clopidogrel) or warfarin (Coumadin). For home management of deep vein thrombosis, which usually occurs in the legs, you are likely to be given heparin injections for a few days, followed by warfarin for long-term therapy. These medications help to prevent the blood clot from getting larger, traveling to your lungs, or recurring later. ✔ All of these drugs can increase your risk of bleeding, so you need to take safety precautions to prevent injury. ✔ To help prevent blood clots from forming and decreasing blood flow through your arteries, you need to reduce risk factors that contribute to cardiovascular disease. This can be done by a low-fat, low-cholesterol diet (and medication if needed) to lower total cholesterol to below 200 mg/dL and low-density lipoprotein cholesterol to below 130 mg/dL; weight reduction if overweight; control of blood pressure if hypertensive; avoidance of smoking; stress reduction techniques; and regular exercise. ✔ To help prevent blood clots from forming in your leg veins, avoid or minimize situations that slow blood circulation, such as wearing tight clothing; crossing the legs at the knees; prolonged sitting or standing; and bed rest. For example, on automobile trips, stop and walk around every 1 to 2 hours; on long plane trips, exercise your feet and legs at your seat and walk around when you can. ✔ Following instructions regarding these medications is extremely important. Too little medication increases your risk of problems from blood clot formation; too much medication can cause bleeding. ✔ While taking any of these medications, you need regular medical supervision and periodic blood tests. The blood tests can help your health care provider regulate drug dosage and maintain your safety. ✔ You need to take the drugs as directed; avoid taking other drugs without the health care provider’s knowledge and consent; inform any health care provider (including dentists) that you are taking an antiplatelet or anticoagulant drug before any invasive diagnostic tests or treatments are begun; and keep all appointments for continuing care. How Can You Avoid This Medication Error? Helen Innes is admitted to your medical unit for management of bacterial pneumonia. She has been on oral antibiotics for 7 days but her respiratory condition has not improved. In addition to her intravenous antibiotics, you administer her usual dose of Coumadin that she takes for a history of pulmonary emboli. When you document the medications given, you notice that her international normalized ratio (INR) is 6. ✔ With warfarin therapy, you need to avoid walking barefoot; avoid contact sports; use an electric razor; avoid injections when possible; and carry an identification card, necklace, or bracelet (eg, MedicAlert) stating the name of the drug and the health care provider’s name and telephone number. Also, avoid large amounts of certain vegetables (eg, broccoli, brussels sprouts, cabbage, cauliflower, chives, collard greens, kale, lettuce, mustard greens, peppers, spinach, turnips, and watercress), tomatoes, bananas, or fish; these foods contain vitamin K and may decrease anticoagulant effects. ✔ For home management of deep vein thrombosis, both warfarin and enoxaparin (Lovenox) are given for 3 months or longer. With Lovenox, you need an injection, usually every 12 hours. You or someone close to you may be instructed in injecting the medication, or a visiting nurse may do the injections, if necessary. Even if a nurse is not needed to give the injections, one will usually visit your home each day to perform a finger stick blood test. The results of this test determine your daily dose of warfarin. Once the blood test and the warfarin dose stabilize, the blood tests are done less often (eg, every 2 weeks). ✔ Report any sign of bleeding (eg, excessive bruising of the skin, blood in urine or stool). If superficial bleeding occurs, apply direct pressure to the site for 3 to 5 minutes or longer if necessary. Self-Administration ✔ Take aspirin with food or after meals, with 8 oz of water, to decrease stomach irritation. However, stomach upset is uncommon with the small doses used for antiplatelet effects. Do not crush or chew coated tablets (long-acting preparations). ✔ Take cilostazol (Pletal) 30 minutes before or 2 hours after morning and evening meals for better absorption and effectiveness. ✔ Take ticlopidine (Ticlid) with food or after meals to decrease GI upset. Clopidogrel (Plavix) may be taken with or without food. ✔ With Lovenox, wash hands and cleanse skin to prevent infection; inject deep under the skin, around the navel, upper thigh, or buttocks; and change the injection site daily. If excessive bruising occurs at the injection site, rubbing an ice cube over an area before the injection may be helpful. lant observation are needed whenever a drug is added to or removed from a drug therapy regimen containing warfarin. Thrombolytic Therapy 1. Thrombolytic therapy should be performed only by experienced personnel in an intensive care setting with cardiac and other monitoring devices in place. CHAPTER 57 DRUGS THAT AFFECT BLOOD COAGULATION 2. All of the available agents are effective with recommended uses. Thus, the choice of a thrombolytic agent depends mainly on risks of adverse effects and costs. All of the drugs may cause bleeding. Alteplase may act more specifically on the fibrin in a clot and cause less systemic depletion of fibrinogen, but this agent is very expensive. Streptokinase, the least expensive agent, may cause allergic reactions because it is a foreign protein. Combination therapy (eg, with alteplase and streptokinase) may also be used. 3. Before a thrombolytic agent is begun, INR, aPTT, platelet count, and fibrinogen should be checked to establish baseline values and to determine if a blood coagulation disorder is present. Two or 3 hours after thrombolytic therapy is started, the fibrinogen level can be measured to determine that fibrinolysis is occurring. Alternatively, INR or aPTT can be checked for increased values because the breakdown products of fibrin exert anticoagulant effects. 4. Major factors in decreasing risks of bleeding are selecting recipients carefully, avoiding invasive procedures when possible, and omitting anticoagulant or antiplatelet drugs while thrombolytics are being given. If bleeding does occur, it is most likely from a venipuncture or invasive procedure site, and local pressure may control it. If bleeding cannot be controlled or involves a vital organ, the thrombolytic drug should be stopped and fibrinogen replaced with whole blood plasma or cryoprecipitate. Aminocaproic acid or tranexamic acid may also be given. 5. When the drugs are used in acute myocardial infarction, cardiac dysrhythmias may occur when blood flow is reestablished. Therefore, antidysrhythmic drugs should be readily available. Use in Children Little information is available about the use of anticoagulants in children. Heparin solutions containing benzyl alcohol as a preservative should not be given to premature infants because fatal reactions have been reported. When given for systemic anticoagulation, heparin dosage should be based on the child’s weight (approximately 50 units/kg). Safety and effectiveness of LMWHs (eg, enoxaparin) have not been established in children. Warfarin is given to children after cardiac surgery to prevent thromboembolism, but doses and guidelines for safe, effective use have not been developed. Accurate drug administration, close monitoring of blood coagulation tests, safety measures to prevent trauma and bleeding, avoiding interacting drugs, and informing others in the child’s environment (eg, teachers, babysitters, health care providers) are necessary. Antiplatelet and thrombolytic drugs have no established indications for use in children. 845 Use in Older Adults Older adults often have atherosclerosis and thrombotic disorders, including myocardial infarction, thrombotic stroke, and peripheral arterial insufficiency, for which they receive an anticoagulant or an antiplatelet drug. They are more likely than younger adults to experience bleeding and other complications of anticoagulant and antiplatelet drugs. For example, aspirin or clopidogrel is commonly used to prevent thrombotic stroke, but both drugs increase risks of hemorrhagic stroke. With standard heparin, general principles for safe and effective use apply. With LMWHs, elimination may be delayed in older adults with renal impairment and the drugs should be used cautiously. They should also be used with caution in clients taking a platelet inhibitor (eg, aspirin, clopidogrel) to prevent myocardial infarction or thrombotic stroke or an NSAID for arthritis pain. NSAIDs, which are commonly used by older adults, also have antiplatelet effects. Clients who take an NSAID daily may not need low-dose aspirin for antithrombotic effects. With warfarin, dosage should be reduced because impaired liver function and decreased plasma proteins increase the risks of bleeding. Also, many drugs interact with warfarin to increase or decrease its effect, and older adults often take multiple drugs. Starting or stopping any drug may require that warfarin dosage be adjusted. Use in Renal Impairment Most anticoagulant, antiplatelet, and thrombolytic drugs may be used in clients with impaired renal function. For example, heparin and warfarin can be used in usual dosages, and thrombolytic agents (eg, streptokinase and urokinase) may be used to dissolve clots in IV catheters or vascular access sites for hemodialysis. Dosage of LMWHs should be reduced in clients with severe renal impairment (creatinine clearance <30 mL/minute) because they are excreted by the kidneys and elimination is slowed. In addition, home management of DVT with LMWHs and warfarin is contraindicated in clients with severe renal impairment. Guidelines for the use of other drugs include the following: • Anagrelide may be given to clients with renal impairment (eg, serum creatinine <2 mg/dL) if potential benefits outweigh risks. Clients receiving this medication should be monitored closely for signs of renal toxicity. • Cilostazol is probably safe to use in clients with mild or moderate renal impairment. However, severe renal impairment alters drug protein binding and increases blood levels of metabolites. • Clopidogrel does not need dosage reduction in clients with renal impairment. • Danaparoid is excreted mainly by the kidneys and dosage may need to be reduced in clients with severe renal impairment. Monitor serum creatinine during therapy. 846 SECTION 9 DRUGS AFFECTING THE CARDIOVASCULAR SYSTEM • Eptifibatide does not need dosage reduction in clients with mild to moderate renal impairment. No data are available for clients with severe impairment or those on hemodialysis. • Lepirudin is excreted by the kidneys and may accumulate in clients with impaired renal function. Dosage should be reduced. • Ticlopidine may be more likely to cause bleeding in clients with renal impairment because the plasma drug concentration is increased and elimination is slower. • Tirofiban clearance from plasma is decreased approximately 50% in clients with severe renal impairment (eg, creatinine clearance <30 mL/minute), including those receiving hemodialysis. Dosage must be reduced by approximately 50%. Use in Hepatic Impairment Little information is available about the use of most anticoagulant, antiplatelet, and thrombolytic drugs in clients with impaired liver function. However, such drugs should be used very cautiously because these clients may already be predisposed to bleeding because of decreased hepatic synthesis of clotting factors. Additional considerations include the following: • Warfarin is more likely to cause bleeding in clients with liver disease, because of decreased synthesis of vitamin K. In addition, warfarin is eliminated only by hepatic metabolism and may accumulate with liver impairment. • Low–molecular-weight heparins are contraindicated for home management of DVT in clients with severe liver disease because of high risks of excessive bleeding. • Anagrelide is metabolized in the liver and may accumulate with hepatic impairment. Clients with evidence of impairment (eg, bilirubin or aspartate aminotransferase more than 1.5 times the upper limit of normal) should receive anagrelide only if potential benefits outweigh potential risks. When anagrelide is given, clients should be closely monitored for signs of hepatotoxicity. • Clopidogrel is metabolized in the liver and may accumulate with hepatic impairment. It should be used cautiously. • Dipyridamole is metabolized in the liver and excreted in bile. Home Care Antiplatelet agents and warfarin are used for long-term prevention or management of thromboembolism and are often taken at home. For prevention, antiplatelet agents and warfarin are usually self-administered at home, with periodic office or clinic visits for blood tests and other follow-up care. For home management of DVT, warfarin may be selfadministered, but a nurse usually visits, performs a finger stick INR, and notifies the prescriber, who then prescribes the appropriate dose of warfarin. Precautions are needed to decrease risks of bleeding. The risk of bleeding has lessened in recent years because of lower doses of warfarin. In addition, bleeding during warfarin therapy may be caused by medical conditions other than anticoagulation. Heparin may also be taken at home. Standard heparin may be taken subcutaneously, but LMWHs for home management of venous thrombosis are becoming standard practice. Enoxaparin is approved by the Food and Drug Administration for outpatient use. Daily visits by a home care nurse may be needed if the client or a family member is unable or unwilling to inject the medication. Platelet counts should be done before and every 2 to 3 days during heparin therapy. Heparin should be discontinued if the platelet count falls below 100,000 or to less than half the baseline value. Most home management regimens involve a structured protocol. Clients and family members should be educated about the disorder (usually DVT), including the potential consequences of either overcoagulation or undercoagulation, and the need for blood tests. The home care nurse needs to assess clients in relation to knowledge about prescribed drugs and ability and willingness to comply with instructions for taking the drugs, obtaining blood tests when indicated, and taking safety precautions. In addition, assess the environment for risk factors for injury. Interventions vary with clients, environments, and assessment data, but may include reinforcing instructions for safe use of the drugs, assisting clients to obtain laboratory tests, and teaching how to observe for signs and symptoms of bleeding. CHAPTER 57 DRUGS THAT AFFECT BLOOD COAGULATION NURSING ACTIONS 847 Drugs That Affect Blood Coagulation NURSING ACTIONS RATIONALE/EXPLANATION 1. Administer accurately a. With standard heparin: (1) When handwriting a heparin dose, write out “units” rather than using the abbreviation “U.” This is a safety precaution to avoid erroneous dosage. For example, 1000 U (1000 units) may be misread as 10,000 units. (2) Check dosage and vial label carefully. Underdosage may cause thromboembolism, and overdosage may cause bleeding. In addition, heparin is available in several concentrations (1000, 2500, 5000, 10,000, 15,000, 20,000, and 40,000 units/mL). (3) For SC heparin: (a) Use a 26-gauge, 1⁄2-inch needle. To minimize trauma and risk of bleeding (b) Leave a small air bubble in the syringe to follow dose Locks drug into subcutaneous space and minimizes trauma (c) Grasp a skinfold and inject the heparin into it, at a 90-degree angle, without aspirating. To give the drug in a deep subcutaneous or fat layer, with minimal trauma (d) Do not massage site after injection (4) For intermittent IV administration: (a) Give by direct injection into a heparin lock or tubing injection site. These methods prevent repeated venipunctures. (b) Dilute the dose in 50 to 100 mL of any IV fluid (usually 5% dextrose in water). (5) For continuous IV administration: This is usually the preferred method because it maintains consistent serum drug levels and decreases risks of bleeding. (a) Use a volume-control device and an infusioncontrol device. To regulate dosage and flow rate accurately (b) Add only enough heparin for a few hours. One effective method is to fill the volume-control set (eg, Volutrol) with 100 mL of 5% dextrose in water and add 5000 units of heparin to yield a concentration of 50 units/mL. Dosage is regulated by varying the flow rate. For example, administration of 1000 units/h requires a flow rate of 20 mL/h. Another method is to add 25,000 units of heparin to 500 mL of IV solution. To avoid inadvertent administration of large amounts. Whatever method is used, it is desirable to standardize concentration of heparin solutions within an institution. Standardization is safer, because it reduces risks of errors in dosage. b. With low–molecular-weight heparins: (1) Give by deep SC injection, into an abdominal skin fold, with the patient lying down, using the same technique as standard heparin. Do not rub the injection site. To decrease bruising (2) Rotate sites. c. After the initial dose of warfarin, check the international normalized ratio (INR) before giving a subsequent dose. Do not give the dose if the INR is above 3.0. Notify the health care provider. The INR is measured daily until a maintenance dose is established, then periodically throughout warfarin therapy. An elevated INR indicates a high risk of bleeding. d. Give ticlopidine with food or after meals; give cilostazol 30 min before or 2 h after morning and evening meals; give clopidogrel with or without food. e. With eptifibatide, tirofiban, and thrombolytic agents, follow manufacturers’ instructions for reconstitution and administration. These drugs require special preparation and administration techniques. (continued ) 848 SECTION 9 DRUGS AFFECTING THE CARDIOVASCULAR SYSTEM NURSING ACTIONS RATIONALE/EXPLANATION 2. Observe for therapeutic effects a. With prophylactic heparins and warfarin, observe for the absence of signs and symptoms of thrombotic disorders. b. With therapeutic heparins and warfarin, observe for decrease or improvement in signs and symptoms (eg, less edema and pain with deep vein thrombosis, less chest pain and respiratory difficulty with pulmonary embolism). c. With prophylactic or therapeutic warfarin, observe for an INR between 2.0 and 3.0. Frequency of INR determinations varies, but the test should be done periodically in all clients taking warfarin. d. With therapeutic heparin, observe for an activated partial thromboplastin time of 1.5 to 2 times the control value. e. With anagrelide, observe for a decrease in platelet count. Platelet counts should be done every 2 days during the first week of management and weekly until a maintenance dose is reached. Counts usually begin to decrease within the first 2 wk of therapy. f. With aspirin, clopidogrel, and other antiplatelet drugs, observe for the absence of thrombotic disorders (eg, myocardial infarction, stroke) g. With cilostazol, observe for ability to walk farther without leg pain (intermittent claudication). Improvement may occur within 2 to 4 wk or take as long as 12 wk. 3. Observe for adverse effects a. Bleeding: Bleeding is the major adverse effect of anticoagulant drugs. It may occur anywhere in the body, spontaneously or in response to minor trauma. With eptifibatide and tirofiban, most major bleeding occurs at the arterial access site for cardiac catheterization. (1) Record vital signs regularly. Hypotension and tachycardia may indicate internal bleeding. (2) Check stools for blood (melena). Gastrointestinal (GI) bleeding is fairly common; risks are increased with intubation. Blood in stools may be bright red, tarry (blood that has been digested by GI secretions), or occult (hidden to the naked eye but present with a guaiac test). Hematemesis also may occur. (3) Check urine for blood (hematuria). Genitourinary bleeding also is fairly common; risks are increased with catheterization or instrumentation. Urine may be red (indicating fresh bleeding) or brownish or smoky gray (indicating old blood). Or bleeding may be microscopic (red blood cells are visible only on microscopic examination during urinalysis). (4) Inspect the skin and mucous membranes daily. Bleeding may occur in the skin as petechiae, purpura, or ecchymoses. Surgical wounds, skin lesions, parenteral injection sites, the nose, and gums may be bleeding sites. (5) Assess for excessive menstrual flow. b. Other adverse effects: (1) With heparin, tissue irritation at injection sites, transient alopecia, reversible thrombocytopenia, paresthesias, and hypersensitivity These effects are uncommon. They are more likely to occur with large doses or prolonged administration. (2) With warfarin, dermatitis, diarrhea, and alopecia These effects occur only occasionally. Warfarin has been given for prolonged periods without toxicity. (3) With anagrelide, adverse cardiovascular effects (eg, tachycardia, vasodilation, heart failure) These effects are most likely to occur in clients with known heart disease. (4) With clopidogrel and ticlopidine, GI upset, skin rash, neutropenia, and thrombocytopenia Neutropenia and thrombocytopenia are more likely to occur with ticlopidine than clopidogrel. (continued ) CHAPTER 57 DRUGS THAT AFFECT BLOOD COAGULATION NURSING ACTIONS c. With thrombolytic drugs, observe for bleeding with all uses and reperfusion dysrhythmias when used for acute myocardial infarction. 849 RATIONALE/EXPLANATION Bleeding is most likely to occur at sites of venipuncture or other invasive procedures. Reperfusion dysrhythmias may occur when blood supply is restored to previously ischemic myocardium. 4. Observe for drug interactions a. Drugs that increase risks of bleeding with anticoagulant, antiplatelet, and thrombolytic agents: These drugs are often used concurrently or sequentially to decrease risks of myocardial infarction or stroke. (1) Any one of these drugs in combination with any other drug that affects hemostasis (2) A combination of these drugs b. Drugs that increase effects of heparins: (1) Antiplatelet drugs (eg, aspirin, clopidogrel, others) (2) Warfarin Additive anticoagulant effects and increased risks of bleeding (3) Parenteral penicillins and cephalosporins Some may affect blood coagulation and increase risks of bleeding c. Drugs that decrease effects of heparins: (1) Antihistamines, digoxin, tetracyclines These drugs antagonize the anticoagulant effects of heparin. Mechanisms are not clear. (2) Protamine sulfate The antidote for heparin overdose d. Drugs that increase effects of warfarin: (1) Analgesics (eg, acetaminophen, aspirin and other nonsteroidal anti-inflammatory drugs) (2) Androgens and anabolic steroids Mechanisms by which drugs may increase effects of warfarin include inhibiting warfarin metabolism, displacing warfarin from binding sites on serum albumin, causing antiplatelet effects, inhibiting bacterial synthesis of vitamin K in the intestinal tract, and others. (3) Antibacterial drugs (eg, aminoglycosides, erythromycin, fluoroquinolones, isoniazid, metronidazole, penicillins, cephalosporins, trimethoprim-sulfamethoxazole, tetracyclines) (4) Antifungal drugs (eg, fluconazole, ketoconazole, miconazole), including intravaginal use (5) Antiseizure drugs (eg, phenytoin) (6) Cardiovascular drugs (eg, amiodarone, beta blockers, loop diuretics, gemfibrozil, lovastatin, propafenone, quinidine) (7) Gastrointestinal drugs (eg, cimetidine, omeprazole) (8) Thyroid preparations (eg, levothyroxine) e. Drugs that decrease effects of warfarin: (1) Antacids and griseofulvin May decrease GI absorption (2) Carbamazepine, disulfiram, rifampin These drugs activate liver metabolizing enzymes, which accelerate the rate of metabolism of warfarin. (3) Cholestyramine Decreases absorption (4) Diuretics Increase synthesis and concentration of blood clotting factors (5) Estrogens, including oral contraceptives Increase synthesis of clotting factors and have thromboembolic effect (6) Vitamin K Restores prothrombin and other vitamin K–dependent clotting factors in the blood. Antidote for overdose of warfarin. f. Drug that may increase or decrease effects of warfarin: (1) Alcohol Alcohol may induce liver enzymes, which decrease effects by accelerating the rate of metabolism of the anticoagulant drug. However, with alcohol-induced liver disease (ie, cirrhosis), effects may be increased owing to impaired metabolism of warfarin. (continued ) 850 SECTION 9 DRUGS AFFECTING THE CARDIOVASCULAR SYSTEM NURSING ACTIONS RATIONALE/EXPLANATION g. Drugs that increase effects of cilostazol: (1) Diltiazem (2) Erythomycin (3) Itraconazole, ketoconazole Nursing Notes: Apply Your Knowledge Answer: Low-dose subcutaneous heparin is administered prophylactically to prevent deep vein thrombosis, which is associated with prolonged immobility. Activated partial thromboplastin time (aPTT) levels may be assessed before beginning therapy, but routine aPTT assessment and dosage adjustments are not required for low-dose heparin therapy. When giving the injection, take care to prevent trauma and subsequent bruising. A small, 26-gauge 1 ⁄2-inch needle is used. Leave a small air bubble in the syringe to follow the dose and lock the heparin into the subcutaneous space. The area is cleansed and grasped firmly and the needle is inserted at a 90-degree angle. Do not aspirate or rub the area because this fosters bruising. Avoid injections within 2 inches of incisions or the umbilicus and any areas that are scarred or abnormal. Although research indicates that various sites (abdomen, arms, and legs) can be used, the preferred site is the abdomen. Observe and report any signs of bleeding. How Can You Avoid This Medication Error? Answer: Ms. Innes’ INR is too high, which could significantly increase her risk for bleeding. Therapeutic INR levels are usually between 2 and 3. Before giving anticoagulants, it is important to check lab work (activated partial thromboplastin time for heparin, prothrombin time or INR for Coumadin) to determine whether the dose should be administered. For Ms. Innes, antibiotic therapy may have interfered with the synthesis of vitamin K in the intestine, thus increasing the risk of bleeding. Notify Ms. Innes’ physician. Because no signs of bleeding have been noted, he or she may decrease the Coumadin dosage. Review and Application Exercises 1. What are the major functions of the endothelium, platelets, and coagulation factors in hemostasis and thrombosis? 2. What are the indications for use of heparin and warfarin? 3. How do heparin and warfarin differ in mechanism of action, onset and duration of action, and method of administration? 4. List interventions to protect clients from anticoagulantinduced bleeding. 5. When is it appropriate to use protamine sulfate as an antidote for heparin? These drugs inhibit the main cytochrome P450 enzyme (CYP3A4) that metabolizes cilostazol. Grapefruit juice also inhibits drug metabolism and should be avoided. 6. When is it appropriate to use vitamin K as an antidote for warfarin? 7. How do antiplatelet drugs differ from heparin and warfarin? 8. For what conditions are antiplatelet drugs indicated? 9. When is it appropriate to use a thrombolytic drug? 10. How do aminocaproic acid and tranexamic acid stop bleeding induced by thrombolytics? 11. Compare and contrast nursing care needs of clients receiving anticoagulant therapy in hospital and home settings. SELECTED REFERENCES Activated protein C (Xigris) for severe sepsis (2002, Feb. 18). The Medical Letter on Drugs and Therapeutics, 44 (1124), 17–18. Cohen, M. (1999). Treatment of unstable angina: The role of platelet inhibitors and anticoagulants. Journal of Invasive Cardiology, 11(3), 147–159. Deblinger, L. (2000). The challenges of oral anticoagulation. Patient Care for the Nurse Practitioner, 3(12), 12–25. Drug facts and comparisons. (Updated monthly). St. Louis: Facts and Comparisons. Dunn, A. S. & Coller, B. (1999). Outpatient treatment of deep vein thrombosis: Translating clinical trials into practice. American Journal of Medicine, 106, 660–669. Duplaga, B. A., Rivers, C. W., & Nutescu, E. (2001). Dosing and monitoring of low-molecular-weight heparins in special populations. Pharmacotherapy, 21(2), 218–234. Ezekowitz, M. D. (2000). Use of anticoagulant drugs. In H. D. Humes (Ed.), Kelley’s Textbook of internal medicine, 4th ed., pp. 673–684. Philadelphia: Lippincott Williams & Wilkins. Gaspard, K. J. (2002). Alterations in hemostasis. In C. M. Porth, Pathophysiology: Concepts of altered health states, 6th ed., pp. 259–269. Philadelphia: Lippincott Williams & Wilkins. Haines, S. T, Racine, E. & Zeolla, M. (2002). Venous thromboembolism. In J. T. DiPiro, R. L. Talbert, G. C. Yee, G. R. Matzke, B. G. Wells, & L. M. Posey (Eds.), Pharmacotherapy: A pathophysiologic approach, 5th ed., pp. 337–373. New York: McGraw-Hill. Karch, A. M. (2003). Lippincott’s nursing drug guide. Philadelphia: Lippincott Williams & Wilkins. North American Nursing Diagnosis Association. (2001). Nursing diagnoses: Definitions & classification 2001–2002. Philadelphia: NANDA. Porsche, R. & Brenner, Z. R. (1999). Allergy to protamine sulfate. Heart and Lung, 28, 418–428. Sachdev, G. P., Ohlrogge, K. D., & Johnson, C. L. (1999). Review of the Fifth American College of Chest Physicians Consensus Conference on Antithrombotic Therapy: Outpatient management for adults. American Journal of Health-System Pharmacy, 56, 1505–1514. Skidmore-Roth, L. (2001). Mosby’s handbook of herbs & natural supplements. St. Louis: Mosby. White, R. H., Beyth, R. J., Zhou, H., & Romano, P. S. (1999). Major bleeding after hospitalization for deep-venous thrombosis. American Journal of Medicine, 107, 414–424.