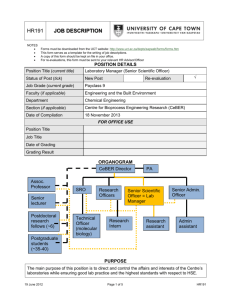
VAM Bulletin 24
advertisement

VAM BULLETIN An LGC publication in support of the National Measurement System MCERTS Ultrafine combustion particles Clinical molecular genetic testing The registration of forensic practitioners Scientists in the law courts Issue Nº 24 Spring 2001 C O N T E N T S Keith Marshall Editor Contents General enquiries about VAM to: VAM Helpdesk 020 8943 7393 vam@lgc.co.uk http://www.vam.org.uk Guest column LGC’s address: LGC, Queens Road TEDDINGTON Middlesex TW11 0LY Contributed articles MCERTS – Setting the standards for regulatory monitoring ...........................................3 Ultrafine particles from combustion sources ...................................................................6 Clinical molecular genetic testing – A total quality approach .........................................10 ISSN 0957-1914 Focus on forensic analysis The DTI VAM programme: The DTI’s programme on Valid Analytical Measurement (VAM) is an integral part of the UK National Measurement System. The VAM programme aims to help analytical laboratories demonstrate the validity of their data and to facilitate mutual recognition of the results of analytical measurements. The VAM programme sets out the following six principles of good analytical practice, backed up by technical support and management guidance, to enable laboratories to deliver reliable results consistently and thereby improve performance. 1. Analytical measurements should be made to satisfy an agreed requirement. 2. Analytical measurements should be made using methods and equipment, which have been tested to ensure they are fit for their purpose. 3. Staff making analytical measurements should be both qualified and competent to undertake the task. 4. There should be a regular independent assessment of the technical performance of a laboratory. Standards in evidence – The registration of forensic practitioners ..................................13 “We may have to go to court with this one…” ..............................................................16 Case study The development of an industry-based PT scheme and the benefits of participation ...................................................................................19 VAM in education Taking the VAM message from school to the workplace................................................23 VAM news Notes on applying ILAC Guidelines to microbiological PT schemes now available.........24 VAM products news Computer-based training package introduces market’s first measurement uncertainty module ................................................................................24 Buying analytical services.............................................................................................25 International news IMEP®: Bringing SI-traceable values to field laboratories ..............................................26 LGC hosts a week of international meetings .................................................................27 Reference materials update New reference materials now available .........................................................................28 5. Analytical measurements made in one location should be consistent with those elsewhere. Chemical nomenclature 6. Organisations making analytical measurements should have well defined quality control and quality assurance procedures. Forthcoming events........................................................................................................30 A trivial system for pharmaceutical products.................................................................29 Contact points ................................................................................................................32 Cover photograph by Andrew Brookes 2 V A M B U L L E T I N G U E S T C O L U M N MCERTS – Setting the standards for regulatory monitoring John Tipping Environment Agency John Tipping of the Environment Agency’s National Compliance Assessment Service gives an overview of the Agency’s Monitoring Certification Scheme and its growing role in setting the standards for regulatory monitoring. Introduction CERTS is the Environment Agency’s Monitoring Certification Scheme for instruments and monitoring services. The scheme is built on proven international standards to ensure that the quality of monitoring data is high. MCERTS consists of a product certification scheme, accredited under the EN 45000 series of European Standards, to deliver certification of instruments and monitoring services to a growing family of Agency performance standards. MCERTS promotes public confidence in monitoring data, and provides industry with a framework for choosing monitoring systems and services that meet the Agency’s performance specifications. M Background The Agency is moving towards increased reliance on self-monitoring by operators and on the use of continuous monitoring and sampling systems. This policy is well established for processes regulated under Integrated Pollution Control (IPC) but is now being extended into other regulatory regimes e.g. Integrated Pollution Prevention and Control (IPPC), Urban Wastewater Treatment Directive (UWWTD). The Agency needs to have confidence in the quality of operator monitoring, which is where MCERTS comes in. When operators demonstrate that their provisions for monitoring have been tested and certified to MCERTS standards, the Agency and the public can have greater confidence in the quality of the resulting data. MCERTS was developed to address: • the need identified by the Agency, for a product certification scheme to help industry select suitable monitoring systems, and to promote public confidence in operator monitoring data; • the desire for UK manufacturing companies to have independent, authoritative endorsement of their products, which would facilitate their access to international markets and increase take-up of their products in the UK. Benefits of the scheme The benefits of MCERTS are that it: • is a certification scheme that is accepted and formally recognised within the UK and internationally; • provides assurance to regulatory authorities that monitoring equipment and services approved to MCERTS standards are fit for purpose and capable of producing results of the required quality and reliability; • gives users of the monitoring equipment or services confidence that they are robust and conform to the Agency’s performance standards; • supports the delivery of accurate and reliable data to the public; • provides the framework for certifying further types of monitoring instrumentation and other aspects of compliance monitoring. Scope of the scheme The initial focus of MCERTS was on continuous emissions monitoring systems (CEMs) for chimneystacks. The performance standards for CEMs cover: • extractive stack emission-monitoring instruments, where a sample of gas is drawn from the stack, generally through a sample conditioning line, into the measuring cell; 3 V A M B U L L E T I N • cross-stack or in-situ emission monitoring instruments, where measurements of the target species are made within the gaseous atmosphere of the stack or duct. The determinands include sulfur dioxide (SO2), oxides of nitrogen (NO and/or NO2), carbon monoxide (CO), carbon dioxide (CO 2), hydrogen chloride (HCl), volatile organic compounds (expressed as total organic carbon (TOCs.), oxygen (O2), water vapour (H 2 O) and particulate material (dust). Instruments monitoring temperature, pressure and mass flow of the stack gas, are also included. The measurement ranges covered for each of the determinands depends on the specific, industrial process application for the CEM. The instrument supplier and certification body agree this scope before the system is evaluated. In December 2000 the Agency launched MCERTS for continuous ambient airquality monitoring systems (CAMs). The performance standards for CAMs cover systems that: • continuously monitor ambient pollutant concentrations in-situ and automatically produce results; • sample ambient air over an extended period (e.g. days or weeks) onto a filter for off-line analysis, e.g. for particulates, metals, or PAHs. G U E S T C O L U M N The determinands covered include sulfur dioxide (SO 2), nitrogen monoxide (NO), nitrogen dioxide (NO2), carbon monoxide (CO), ozone (O3), particulate matter (PM10 and PM2.5), lead, other metals (cadmium, arsenic, nickel, and mercury), benzene, and poly-aromatic hydrocarbons (PAHs). CAMs certified under MCERTS will meet the performance requirements of the Air Quality Directive and Daughter Directives and the forthcoming CEN standards for monitoring ambient air. The Agency is progressively expanding MCERTS to cover all regulatory monitoring activities. Future developments include: • manual stack emissions monitoring; • portable instruments for stack-emissions monitoring; • data acquisition and handling; • sampling equipment and instruments for water monitoring; • operators’ on-site arrangements; • contaminated land analysis. (See Future Developments) Currently MCERTS is a voluntary scheme. However, having good quality monitoring data is such a key element of regulation that the Agency is considering making MCERTS mandatory in the future for processes it regulates. In addition, the Agency is currently taking part in discussions with other EU countries and within CEN on the harmonisation of certification requirements across Europe. The aim of these discussions is to ensure that the requirements for certification in Europe are equivalent and avoid duplication of testing. In the medium term this could result in the development of a Europe-wide certification scheme. MCERTS performance standards The MCERTS performance standards are drawn from relevant CEN (European Committee for Standardization) and ISO (International Organization for Standardization) standards, where available. Prior to publication the Agency carries out extensive consultations on the proposed standards to ensure they are appropriate for the intended application. Once published, the Agency reviews the standards on a regular basis to keep them up to date. For CEMs and CAMs, the standards include: • linearity; • cross-sensitivity to interfering substances; • sample pressure and temperature; • delay time, response time; • lower detection limit; • repeatability; • environmental conditions; • susceptibility to physical disturbance; • evaluation of the accuracy; • reproducibility; • availability and maintenance interval; • long term zero and span drifts. Testing is organised in two parts: • laboratory based tests to ensure that instruments perform to the required specifications; • field trials over a three-month period, to ensure that the instruments are robust and continue to work in real applications. The structure of the scheme MCERTS consists of two main components; the MCERTS performance standards as discussed above, and secondly, and very importantly, a formally accredited product certification scheme, the ‘delivery vehicle’, operating under the requirements of the EN 45000 series of European standards. The Agency has appointed Sira Certification Service (SCS) as the certification body to operate the MCERTS scheme. SCS is independent of all the interested groups, including the instrument manufacturers and end users. The MCERTS scheme currently provides product certification to EN 45011 and is accredited by UKAS. Over the next 12 months it will be expanded to include personnel certification to EN 45013. There will also be a register of MCERTS equipment and services. The National Physical Laboratory and AEA Technology currently carry out laboratory and field testing. The Agency is also publishing test-house performance standards so that other organisations can 4 V A M B U L L E T I N offer testing services to MCERTS standards. All testing must be accredited to ISO/IEC 17025, the international standard for general operating criteria for testing laboratories, and meet the MCERTS standards. The evaluation of results obtained during the laboratory and field-testing is carried out by the scheme, using a group of independent, qualified people known as the Certification Committee. The importance of product certification Product certification under MCERTS requires an instrument manufacturer to demonstrate that the manufacturing process is controlled under a quality management system and produces instruments that deliver consistent performance. Once an instrument is certified the manufacturer has to inform the certification body running the Scheme of any planned design or manufacturing changes to the instrument that affect performance. The certification body then assesses the proposed changes, and carries out further tests if required, to ensure a modified instrument still meets the MCERTS performance standards. As a further check, the certification body also audits manufacturers each year. Design and manufacturing changes take place quite frequently in monitoring equipment. Product certification is critically important to track any changes and provide assurance to potential customers and regulators that instruments manufactured months or maybe years after the original instrument was certified, still meet the MCERTS standards. As an additional safeguard, the MCERTS certificate has a lifetime of five years, after which an instrument has to be resubmitted for more detailed assessment and re-testing (if required). Financing of the scheme MCERTS is self-financing with costs recovered from fees charged to applicants to the scheme. The fees cover: • the application for instrument certification; • laboratory and field tests; • preparation of test reports; • assessments by the Certification Committee; • preparation of the MCERTS certificate; • promotion and policing of the Scheme; • the costs of sustaining accreditation to EN 45011. G U E S T Instrument certification procedure The MCERTS certification procedure has been designed to be as simple and straightforward as possible. It consists of the following stages: Initial application The supplier of the equipment or service submits an application to SCS, together with a clear identification of instruments submitted, including two sets of drawings, a copy of any relevant control software, and evidence of quality control procedures, e.g. ISO 9001. Selection of the Certification Committee SCS then appoints a Certification Committee, which normally consists of three experts in the products or services under test. The committee members must be impartial; for example, they must not have been involved with the specific manufacturer or service supplier in the previous two years. Review of application The Certification Committee reviews the application and agrees the relevant performance standards and appropriate laboratory and field tests for the intended applications of the products or services. Quotation for testing SCS, in conjunction with the applicant, asks qualified test laboratories (initially NPL and AEA Technology) to quote. The client confirms the test programme, test schedule, and quotation, usually in a preliminary meeting with SCS and the test laboratories. The client places a contract with SCS to cover all testing and certification, after which SCS places contracts with the chosen laboratories. Laboratory and test methods The manufacturer installs and commissions the equipment at the test laboratories as required. After testing, the reports are sent to SCS and the manufacturer. The testing laboratory immediately informs SCS of any failures during testing, to allow the applicant to take corrective action. Review of test results The Certification Committee reviews all test results and decides to issue or refuse a certificate. The reasons for refusal will be reported, as well as any special conditions applying to the certificate. The certificate and C O L U M N Future Developments The Environment Agency is expanding MCERTS progressively to cover all regulatory monitoring activities. Future developments include: Manual stack emissions monitoring The extension of MCERTS to manual stack emissions testing will include two performance standards; one for organisations involved in testing, providing sectoral guidance to supplement the general requirements of ISO 17025, and the second for stack testers. Stack testers will be certified under the personal competency standard EN 45013. The Agency carried out a consultation on its proposals in 1999 and received widespread support. The work is a collaboration with the Source Testing Association (STA), the primary trade association for stack testing organisations in the UK. It is hoped to launch the Scheme in late 2001. Portable instruments for stackemissions monitoring Portable instruments are increasingly being used for stack monitoring. The MCERTS standards are being developed in collaboration with CoGDEM, the trade association representing instrument manufacturers and the instrument group of the STA. The consultation on the draft standards will take place in early 2001. Data acquisition and handling Continuous monitoring systems produce large amounts of data requiring processing and storage. It is important that these data are handled correctly to ensure reports submitted to the Agency are correct and meet the regulatory requirements. Increasingly these requirements are defined in EC Directives, e.g. Large Combustion Plant Directive, Hazardous Waste Incineration Directive. A performance standard for data acquisition and handling will be produced during 2001. The Agency is in the final stages of preparing a technical specification prior to issuing invitations to tender for the work. 5 V A M B U L L E T I N Sampling equipment and instruments for water monitoring MCERTS performance standards are in preparation for sampling equipment and instrument for water monitoring. These cover automatic waste water sampling equipment to support the Agency’s implementation of the Urban Waste Water Treatment Directive, and instruments measuring flow, turbidity, ammonia, and pH. Consultation on the draft standards is expected to take place in spring 2001. Further standards are planned for instruments measuring chemical oxygen demand, phosphate, dissolved oxygen, nitrate and total organic carbon. Operator on-site arrangements MCERTS certified monitoring and services can only deliver good quality monitoring data if the operators’ on-site arrangements are also of a high standard, e.g. sampling is carried out at a representative position in the stack, instruments are calibrated correctly on installation and annually, maintenance is carried out properly. This standard will address these issues and capture the requirements of the forthcoming CEN Standard on quality assurance of monitoring arrangement being produced by Working Group 9 of CEN Technical Committee 264. Contaminated land analysis The Agency is moving to the risk based regulation of contaminated land. In support of this policy operators are already required to submit test reports to the Agency from analytical laboratories accredited by UKAS to ISO 17025. The MCERTS standard in development will provide sectoral guidance to supplement the general requirements of ISO 17025. The MCERTS standard will not specify the test methods themselves but set the minimum performance requirements for the test methods used by the laboratories. A consultation on the draft standard is planned for spring 2001. G U E S T C O L U M N the accompanying drawing schedules defining the instrument will list the valid range of applications and processes. These can be extended beyond the test application or process by agreement with the Certification Committee. An appeals procedure can be invoked in the event of any disagreement. Further information For general questions about MCERTS, please contact: John Tipping Environment Agency National Compliance Assessment Service Cameron House C O N T R I B U T E D White Cross Industrial Estate South Road LANCASTER LA1 4XQ, UK Tel +44 (0)1524 581901 Fax +44 (0)1524 842709 Email John.Tipping@ environment-agency.gov.uk For information on MCERTS certification, or applications, then please contact: Ian Knott Sira Certification Service South Hill CHISLEHURST Kent BR7 5EH, UK Tel +44 (0)20 8467 2636 Fax +44 (0)20 8467 6515 Email Idknott@siratc.co.uk General email: sales@siratc.co.uk Further information on the certification process can be found within the SCS publication, ‘A Guide for Certification of Continuous Emission Monitoring Systems under the Environment Agency’s MCERTS Scheme’, from the SCS web site, http://www.sira.co.uk. Both this web site, and the Agency’s web site, http://www.environment-agency.gov.uk contain more information about MCERTS, and an up-to-date list of certified instruments. A R T I C L E S Ultrafine particles from combustion sources John McAughey & Ian Marshall AEA Technology ombustion has long been known as a source of soot and sulfate-based particulate material. As combustion sources have become more efficient, measurements have shown that significant numbers of submicron diameter particles may be formed. Recent epidemiological evidence has implicated these particles as a key factor in cardiac and respiratory disease in susceptible population sub-groups. Thus, there has been a significant increase in measurement of submicron (or ultrafine particles), with respect to combustion emissions, air quality and toxicology. To date, most world-wide activity has focused on vehicle emissions. This article describes standards and calibration activities supporting the automotive and oil industry, and government. C Background In recent years, there has been increasing interest in the properties and applications of nanoparticles, particularly in the fields of materials, environmental health and combustion emissions. Despite this increasing interest, there is little consensus on a size definition of nanoparticles or ultrafine particles, with them being described variously as < 10 nm, < 100 nm, and sub-micron (< 1000 nm). This article will address activities in the fields of combustion emissions from mobile sources, where the particles of interest are < 100 nm diameter. Combustion emissions from mobile sources It is recognised that all combustion related activities generate particles. This is most noticeable with open fires, which may produce a sooty flame, but is also true for more controlled combustion sources, where the particles are less visible. To date most research and measurement activity has focussed on vehicle emissions. The measurement of particles from vehicle emissions is a comparatively new field, which has originated in response to concerns with the health effects of respirable particles in the ambient environment. Respirable particles are commonly referred to as PM10, that is, particles smaller than 10 µm aerodynamic diameter. New air quality standards for PM10 have been promulgated in the EU and US in response to concern 6 V A M B U L L E T I N over health effects attributed to particles, although the US regulations have since been contested and are under review. In the EU, the Daughter Directive (90/30/EC) sets targets for 2005 and 2010, but these are subject to an on-going review process. It is recognised that an important part of this review process is to better define particulate matter. Whilst all other air quality standards are based on defined chemical entities, particulate matter is known to be a complex mixture with respect to particle size and chemical composition. Particle size is a key determinant of the environmental behaviour, persistence and fate of particles in the environment, and also influences the regional deposition of particles in the human lung. Chemical composition is likely to influence the potential toxicity of these particles. However, there is a growing recognition that other particle characteristics may have a more significant impact on health than total particulate mass, including particle diameter, surface area, number concentration and composition. It is also recognised that over 90% of the number of diesel particles reside in the range below 100 nm while only 1–20% of the mass typically resides in the same ultrafine particle range. The parameters used to characterise diesel particles need to reflect these new concerns. C O N T R I B U T E D Particle emissions from diesel engines have been studied in the greatest depth and typically comprise 3 modes: • An accumulation mode consisting of a complex mixture of agglomerated solid carbonaceous material and ash; this mode appears to be stable and reproducible, with typical diameters in the region of 50 – 300 nm. Solid carbon is formed during combustion in local fuel-rich regions. Much of the carbon is subsequently oxidised and emitted as solid agglomerates. • A volatile nucleation mode comprising sulfate and condensable organic compounds as a particle/droplet; this mode has a typical diameter in the region of 5 – 30 nm, although smaller particles have also been observed. These particles are formed by the homogenous nucleation of sulfate (emitted SO 2 converts to SO 3, which in turn forms sulfate droplets). Volatile or soluble organic compounds derived from unburned fuel and evaporated lubricating oil (SOF), condense upon these seed nuclei. This mode has been shown to be labile to heat and can be removed by heating in the presence of a thermal denuder. Formation of this mode can be reproducible under controlled conditions, but is highly dependent on dilution ratio, dilution rate, temperature, humidity and the residence time prior to tail-pipe dilution. • A coarse mode comprising aggregates of accumulation mode particles, which are believed to have been deposited and reentrained in the exhaust system; particle diameters may be in the region of 1–2.5 µm. This mode tends to show poor reproducibility under measurement and in practice many automotive sampling systems cut-off below this mode. A series of typical particle size distributions with different sample temperatures and sample residence times from a diesel engine is shown in Figure 1, as measured by a Scanning Mobility Particle Sizer (SMPS), which measures the electrical mobility diameter. It is notable that the carbon based accumulation mode is reproducible under the 3 sampling conditions, with a median diameter of 50 nm. In contrast, the sulfate based nucleation mode increases in number concentration as sampling temperature A R T I C L E S Figure 1: Particle size distributions from a diesel engine as measured by SMPS. decreases; and with residence time in the system, consistent with more favourable conditions for homogeneous nucleation of sulfate1. In this case the median diameter is 8 – 15 nm, increasing with increasing residence time. Particles from spark-ignition engines are qualitatively similar to those from diesel, most prominently in the volatile nucleation mode. Particles from spark-ignition engines are less well understood, but are believed to comprise more volatile SOF and sulfate equivalent components. However, emission factors are two to three orders of magnitude lower than for diesel. For particle size measurement, new specialist sampling and measurement techniques have been deployed, generally under existing test cycles for regulated emission measurements, where sampling is carried out on chassis dynamometer systems from full-flow constant volume sampling systems. It is notable that in a review conducted for the US Environmental Protection Agency1, only eight laboratories in the world were conducting such particle size measurements on a routine basis. This number has increased greatly in the subsequent period, and as such, this has been an important period to validate sampling and measurement for both industry and regulators. Historically, early work on the measurement of diesel aerosols was conducted in the laboratory and on the roadway2,3. More recently there have been further review papers addressing measurement of differing aspects of the emitted particles4. Much of the current interest in further characterisation of particulate emissions arose from Bagley et al 5 , who described an increase in the formation of ultrafine particles in a new technology engine, where particle mass emissions were being reduced. Recent papers have also described low-concentration measurement of spark-ignition engine emissions 6,7,8 and measurement of diesels fitted with particulate traps9,10. The most significant issue in this field is the nature of the nucleation mode particles formed as a consequence of the diluting conditions and residence time in the measurement system1. As noted above, these ‘new’ particles are typically less than 5 – 30 nm diameter and consist of sulfate and condensable organic material. There is an on-going research effort to determine the nature of these particles. This relates principally to determining whether they are formed in real-life roadway conditions, or whether they exist solely as an artefact of the existing sampling system. If these sub-10 nm particles are observed by roadways under ambient conditions, they must be further assessed as to their potential health implications. (Note: an ambient particle mode of 20 nm has been observed at or near roadways in a number of cities; however there are currently no composition data available to confirm that these are derived directly from nuclei-mode diesel emissions). I 7 V A M B U L L E T I N II Normalised particle number concentration Particle diameter C O N T R I B U T E D Health Interest in this field is driven by concerns that exposure to combustion emissions may be a significant source of poor cardiorespiratory health in susceptible population groups. A number of studies11 have variously concluded that in the larger countries of Western Europe, exposure to ambient particles contributes each year to many thousands of acute deaths from respiratory or heart disease in the days immediately following pollution episodes, with pro rata effects in other countries. The associated loss of life is strictly unknown, but believed to be in weeks or months rather than years, on average. There is less well-established information about the additional effects of longer-term exposure to ambient particles, and these are less easily to quantify reliably. In the UK, the Committee on the Medical Effects of Air Pollutants (COMEAP)12, citing Brunekreef 13, concluded that these ‘chronic’ effects might be the dominant ones. A summary table (Table 1) summarises briefly the various particle metrics (size and composition) considered with respect to health effects, confirming the complexity of the problem of attributing risk from the particle mixture. It is noted however, that the estimated health costs of increased particle exposures with respect to mortality and morbidity, may be equivalent to up to 1.7% of Gross Domestic Product 11. It is within this context, that there are significant research programmes by industry and government seeking to modify engine and fuel technologies and further reduce emissions. Hence, there is a pressing requirement for sampling and measurement standards. Measurement A number of techniques have been applied to the measurement of particle size of vehicle particle emissions. The most commonly adopted techniques are those which are able to conduct near real-time measurements. These include electrical mobility analysers such as the Scanning Mobility Particle Sizer (SMPS: TSI Inc., MN – Figure 2 illustrates size fractionation via an electrostatic classifier; subsequent measurement is conducted by a Condensation Particle Counter). Inertial impaction instruments such as the Electrical A R T I C L E S Metric Evidence Source Strength of Evidence PM Mass Epidemiology Consistent association between PMx and reported health effects – a useful unifying PM measure PM Particle Size Epidemiology Dosimetry Toxicology Indications from epidemiology and toxicology that fine PM2.5 is more potent than coarse PM on a mass concentration basis (although ambient composition will vary). Finer particles penetrate more readily into lungs, cells and through tissue barriers PM Surface Area Toxicology Finer particles have greater surface area per unit of mass; Oberdorster data implies toxicity for a known material is consistent with available surface area Ultrafine PM Epidemiology Dosimetry Toxicology Growing recent epidemiological database suggesting that this fraction may be of importance. Toxicology – inflammatory reponse on ultrafine exposure Particle number concentration is also a metric of interest Metals & Compounds Toxicology Have cytotoxic and inflammatory properties. The “metals hypothesis” associated with the soluble metal fraction of ROFA and may be related to the ability of these metals to catalyze production of free radicals in tissues. Limited epidemiology data from Utah steel works studies Acids Toxicology Human effects observed in laboratory but significant neutralising capacity in lung Organics Toxicology Compound-specific effects – particular concern for lung cancer Sulfate / Nitrate Toxicology Human effects observed in laboratory; metric is often under-reported in ambient measurements Peroxides Toxicology Plausible toxicology route but ambient concentration low Elemental Carbon and Soot Epidemiology Toxicology Soot has irritant, mutagenic, and carcinogenic properties that vary with delivered dose and the properties of the sorbed materials. It is plausible that it could exert both short-term (irritant) and long-term (carcinogenic) effects. Co-factors (SO2, NOx, O3, CO) Epidemiology Significant differences in health markers for different gaseous co-pollutants with location Table 1: Summary of particle metrics. Low Pressure Impactor (ELPI : Dekati, Finland) have also been used widely. The SMPS instrument operates in the size regions from 7 – 300 nm or 15 – 700 nm and has been adopted widely for the measurement of particle size distribution under steady-state conditions, or to monitor specific size ranges throughout transient cycles. The ELPI operates from 35 – 10,000 nm and has been used widely to measure size distribution data during transient cycles. Isokinetic sampling is achieved readily, for the particle sizes of interest in the sub-10 µm region. Sample losses have been assessed by theory and measurement 1,14,15. Losses are 8 V A M B U L L E T I N principally by thermophoresis with minimal losses by diffusion and impaction. VAM Programme Within the VAM Programme, a programme of work has been conducted aimed at providing industry with improved tools and ‘know-how’ for the calibration for aerosol instrumentation that measures the number concentrations of ultrafine (<100 nm) particles. This has comprised two parts: • A Design Standard has been proposed and tested successfully for calibrating the linearity of aerosol number-based instrumentation. C O N T R I B U T E D Figure 2: Electrostatic classifier for discrimination of sub-micron particles by electrical mobility. • The Vehicle Particulates Emission Club (VPEC) has been established as a subscription-based research club, and as a partnership between industry and government, to conduct research with respect to generic sampling and measurement issues. Number calibration standard A programme of work has been performed to develop a design standard for calibrating number concentration measuring instrumentation, for aerosol instrumentation in the ultrafine region. Emphasis has been on achieving a robust and cost-effective capability that can be used both in specialist calibration laboratories as well as being able to be taken to the point of measurement. The potential of using accurate dilution (over a wide concentration range) as a means of providing a traceable and costeffective means of calibrating the linearity of number-measuring aerosol instrumentation was identified as a key component of this study. An absolute dilution performance of better than 10% was identified as a design target. A Sample Conditioning System (SCS) meeting this target has been designed and manufactured. The SCS has undergone comprehensive calibration and validation in terms of temperature and pressure sensor calibration, temperature compensation of measured pressures, flow calibration, pressure stability, leak-tightness and dilution verification with a CO gas standard16. An example calibration of a TSI Condensation Nucleus Counter was also undertaken utilising the SCS. Vehicle Particle Emissions Club The Vehicle Particle Emissions Club (VPEC) is a shared-cost research club established in the UK in 1999, in a collaboration of industry and Government, with a view to assessing priorities in generic measurement issues and establishing a research programme to address these areas. The founder members are shown in Table 2. The Club has to date commissioned studies to calibrate the Scanning Mobility Particle Sizer with respect to size standards and the draft number standard. In parallel, a roundrobin exercise is being conducted at a number of chassis dynamometer facilities in the UK, using a reference vehicle and SMPS, versus local SMPS instruments (http://www.bigfoot.com/~vpec). It is likely that the remit of the Club will widen to address other combustion sources of particles. A R T I C L E S and the US, with good links between all parties. In the US, a large programme of work has been sponsored by EPA and conducted by University of Minnesota (http://www.me.umn.edu/centers/cdr/Proj_E PA.html). Most recently, the Co-ordinating Research Council (CRC) conducted a sampling and measurement workshop (Paris – May, 2000) with details reported at http://www.crcao.com. In Europe, another intercomparison programme has been conducted for the SMPS instrument by a number of German and Swiss laboratories, co-ordinated by the Institut für Gefahrstoff-Forschung der BBG. A further programme on particle emission factors is being conducted within the European Commission 5th Framework Programme, co-ordinated by University of Thessaloniki. Conclusion This is an interesting period for measurement of ultrafine particles; the number of measurements will increase as legislators move to introduce new standards, and as industry moves to improve existing products and introduce new technologies. It is clear that the need for improved sampling and measurement validation has been recognised, and that significant progress is being made at an international level. REFERENCES 1. Kittelson, D. B. (1999), Review of diesel particulate matter sampling methods, USEPA, Downloadable from WWW at Centre for Diesel Research, University of Minnesota. 2. Kittelson, D. B., Doan, D. F., and Verrant International activities As well as UK-based activities, there are significant activities in Europe J. A. (1979), Investigation of a diesel exhaust aerosol, SAE Technical Paper Series No. SAE 780109. Warrendale PA Government Industry Research Organisations Department of Trade & Industry, UK (VAM) Castrol, UK AEA Technology, UK Department of Environment, Transport & the Regions, UK Ford, UK Motor Industry Research Association (MIRA), UK US Department of Energy, via the National Renewable Energy Laboratory, Co Shell Global Solutions, UK Ricardo Consulting Engineers, UK Table 2: Founder Members of the Vehicle Particle Emissions Club (VPEC) 9 V A M B U L L E T I N C O N T R I B U T E D A R T I C L E S 3. Dolan, D. F. and D. B. Kittelson (1979), ignition engine, SAE Technical Paper Quantification of the Effects of Air Roadway measurements of diesel Series No. SAE 980528. Warrendale PA Pollution on Health in the United exhaust aerosols, SAE Technical Paper Series No. SAE 790492. Warrendale PA Kingdom. UK Department of Health. 8. Maricq, M., Chase, R. E., Podsiadlik, D. HMSO, London H. and Vogt, R. (1999). Vehicle Exhaust 4. Lepperhoff, G. et al. (1994), Methods to Particle Size Distributions: A Comparison 13. Brunekreef B. (1997), Air pollution and analyse non-regulated emissions from of Tailpipe and Dilution Tunnel life expectancy: is there a relation?, diesel engines. SAE Technical Paper Measurements, SAE Technical Paper Occup Environ Med; 54: 781-784. Series No. SAE 941952. Warrendale PA series 1999-01-1461. Warrendale, PA 5. Bagley, S. T., Baumgard, K. J., Gratz, L. D., 9. Baumgard, K. J. and Johnson, J. H. Johnson, J. H., and Leddy, D. G. (1996), (1992). The effect of low sulfur fuel and Characterization and a ceramic particle filter on diesel Aftertreatment Device Effects on Diesel exhaust particle size distributions, Emissions, Health Effects Institute Technical Paper Series, No. 920566, Research Report Number 76, 88 pp. SAE: Warrendale, PA 14. Dickens, C. J., Ball, M. H. E., Booker, of Fuel 6. Rickeard, D. J., Bateman, J. R., Kwon, 10. Mayer, A. et al (1997). VERT- Clean Y. K., McAughey, J. J. and Dickens, C. J. Diesel engines for tunnel construction, (1996), SAE 970478, Warrendale PA Exhaust particulate size Paper 961980, Warrendale PA Hughes, M. (1997), Evaluation of Instruments for Vehicle Emission Particle Sizing, AEA Technology Report AEAT-1180, 69 pp. (Unclassified). 15. Ball, M. H. E. (1998), Measurement of Ultrafine Particles – Guidance Notes, AEA Technology Report AEAT-3936 (Unclassified). distribution: vehicle and fuel influences in light-duty vehicles, SAE Technical D., Donald, J. R., Tope, A. M. and 11. World Health Organisation (1999), downloadable from www.who.dk/ London99/WelcomeE.htm 16. Marshall, I. A. and Booker, D. R. (1999), Development of an Ultrafine Number Concentration 7. Graskow, B. R. and Kittelson, D. B. (1998), Characterisation of exhaust 12. COMEAP (1998), Committee on the particulate emissions from a spark- Medical Effects of Air Pollutants, Standard, AEA Technology Report No. AEAT/EEQA0028 (Unclassified). Clinical molecular genetic testing – A total quality approach Rob EllesI, Simon RamsdenII, Simon PattonI, II, Su StenhouseII, David BartonI EMGQNI & UK NEQAS for Molecular GeneticsII egional Genetics Centres in the NHS have introduced molecular genetic testing associated with inherited disease over the last 15 years. Currently there are 24 laboratories in the UK offering a service portfolio for the major single gene disorders. In the early days few inherited diseases were open to a diagnosis and the techniques such as Southern blotting were very cumbersome R I II EMGQN: European Molecular Genetics Quality Network UK NEQAS: UK National External Quality Assessment Scheme and laborious, requiring a great deal of technical and scientific skill to achieve accuracy, reliability and a timely response to clinical need. The introduction of PCRbased techniques, along with the possibility of a diagnosis for most of the common genetic conditions, transformed the landscape of genetic testing. During this period the professional groups in the UK and elsewhere began to focus their attention on quality issues relevant to all areas of the analytical process. In the last five years the recognition of 1 0 V A M B U L L E T I N genes pre-disposing individuals to some common diseases (breast/ovarian and bowel Molecular genetic testing ‘Molecular genetic testing’ is a term used to describe many diagnostic activities relevant to sporadic cancers and infectious disease. However this article is restricted to genetic testing for inherited disease in humans with DNA as the normal analyte. C O N T R I B U T E D cancer), the availability of better instrumentation – fluorescent DNA sequencers for example – have set the pace towards a more service-orientated approach. The next five to ten years will see genetic testing take its proper place in the armoury of medicine. We can foresee an increasing understanding of disease processes, a need to stratify the disease population by genotype and the use of pharmacogenetic testing to improve clinical management. In hardware terms the introduction of high throughput (capillary electrophoresis DNA sequencing) and later parallel processing (gene chip) technologies will mean that access to genetic testing is less limited by cost and reporting time constraints. Genetic testing has a high media profile and the consequent raised expectations by the public place a responsibility on service providers to retain public confidence and introduce quality systems in line with current clinical analytical standards. Although genetic testing for inherited disease is not unique in the ethical and social issues that it presents, it does have features that emphasise the need for high quality standards. The establishment of a person’s genotype is usually a ‘one off’ test and is unlikely be repeated in their life-time. Furthermore, the results may not only be highly predictive of their own health status, it may also have important implications for the health of their immediate and extended family. Errors in molecular genetic testing are therefore perceived to have serious and long-term consequences. For these reasons a strong link has been established between genetic testing in the laboratory and the availability of genetic counselling. Pre- and post- test genetic counselling may be as important a part of the quality of care package for the genetics patient as an examination before the procedure and subsequent follow-up is for a surgical patient. Genetic testing laboratories, working closely with clinical colleagues, place a strong emphasis on providing full interpretations of their data and consider that the output from the laboratory is not limited to the technical genotype but includes a modified genetic risk presented to inform the counselling process (Figure 1).1 A total quality approach must start with the promotion of an appropriate skill and knowledge base in the staff and attention to their motivation and confidence in career progression. The professional societies representing scientists in the field have been active since 1990 in developing training structures. In the last few years they have strongly supported state registration of staff, which is designed to ensure a basic level of competence and promote public confidence in groups with a critical role in patient care. The statutory register for clinical scientists came into force in October 2000 (Table 1). While clinical scientists involved in genetic testing have always been aware of the importance of quality control in their laboratories, independent assessment of this A R T I C L E S Figure 1: Added value in genetic testing. quality first began with a trial External Quality Assessment (EQA) scheme organised by the Clinical Molecular Genetics Society in 1990–91. Within 3 years this scheme received Department of Health funding as a pilot programme and in 1995 it came under the UK National External Quality Assessment Scheme umbrella. UKNEQAS covers most UK laboratory proficiency schemes in clinical analysis. The molecular genetics scheme has been accredited by Clinical Pathology Accreditation Ltd. The EQA scheme for molecular genetics has introduced some advanced features in an attempt to assess the whole analytical process, including not only the technical aspects of genotyping but elements of pre-test handling and the post-analytical interpretation of data. In brief – centres receive validated clinical DNA samples from the UKNEQAS for molecular genetics, forming a mock clinical case, complete with clinical details, patient identifiers and a request for a REGULATORY BODY/ROLE FUNCTION Clinical Molecular Genetics Society Recruitment, appointments and grading Royal College of Pathologists Guidance to Department of Health on grading Post-graduate basic training 2-year super-numary training in centres accredited by the Training Accreditation Board Career-grade training Preparation programmes for membership of the RCPath Possible 2/3 years after entry to an assessed post Scientists not in training are registered for CPD Table 1: Quality and training in the scientific work-force 1 1 V A M Department of Health Guidance to Hospital Trusts, National panel of external assessors Examinations leading to Membership of the RCPath Statutory registration Continued Professional Development (CPD) Council for Professions Supplementary to Medicine B U L L E T I N C O N T R I B U T E D disease-specific analysis. Laboratories are asked to genotype the sample(s) and return a normal laboratory report including their interpretation of the data in the light of the clinical information supplied. Reports are marked by expert assessors and assigned a numerical score. This process assesses pretest handling (transcription of correct patient identifiers) and the accuracy of the genotype (mutation identified/no mutation detected, marker alleles scored). In addition, assessors look for mention of key points of interpretation that may include the significance of the test result in light of the clinical details, limits of sensitivity or specificity of the test, key recommendations to the clinicians, implications of the results for other family members and requirements for further testing. 1997 Hereditary bowel cancer 1998 Huntington’s disease 1999 Fascio Scapulo Humeral Dystrophy 2000 Familial breast/ovarian cancer Spinal Muscular Atrophy Friedreich’s ataxia Meeting for EQA scheme organisers and assessors Charcot Marie tooth disease Y-chromosome microdeletions 2001 Internal Quality Control Meeting for EQA scheme organisers and assessors Retinoblastoma Fragile X disease Prader Willi syndrome/ Angelman syndrome Table 2: European best practice meetings held or planned 1997–2001 Studies showing variation in the performance of laboratories across Europe, as well as the demand from European centres to be included in the UKNEQAS, led to a successful bid to the European Union’s Standards, Measurement and Testing Programme for a pilot Quality Network.2, 3. This European Molecular Genetics Network (EMQN) of 18 partners is funded until the end of 2001, to evaluate disease-specific EQA schemes being offered to approximately 350 centres, from six European EQA scheme providers. To date nearly 400 European centres have participated in schemes A R T I C L E S promoted or associated with EMQN most of which are closely modelled on the UKNEQAS system (Table 3).4 An emphasis on the interpretation of data inevitably introduces a subjective element to a Laboratory Proficiency Scheme. Early in the development of the UKNEQAS system, this led to a recognition of a need to develop a consensus amongst the laboratory community on what constituted ‘best practice’. The other main driver for action was the recognition, in looking at EQA returns, of a great divergence in methodologies and practice (for instance, in a single disease test, up to ten different genetic markers might be in use in different centres). Bringing scientists together to discuss best practice not only resulted in a convergence of methodologies (without necessarily imposing rigidity in a fast moving area of technology), but also in agreement on guidelines that could inform both the participants in EQA schemes and the expert assessors, who mark the returns from the laboratories. Guidelines were first published by the CMGS (www.cmgs.org) and included advice specific to a disease diagnosis and also elements of internal quality control, for instance handling data and samples in the laboratory and good reporting practice. Subsequently the EMQN was funded to continue this process at European level and has taken on board publishing the resulting guidelines through its web site (www.emqn.org). Fourteen best practice meetings will be held over three years. The link with EQA gives guidelines an authority (laboratories need to follow the guidelines to perform well in EQA) whilst the consensus mechanism from which guidelines are produced prevents them from assuming an authoritarian or arbitrary character. A key element of standardised testing systems is the availability of Certified Reference Materials (CRMs) that are traceable, stable and exhibit known variance. Molecular genetics, in common with other analytical disciplines, requires reference materials5. These would consist of materials with a known genotype (mutation or variant carrier), or ‘wild type’ (free of pathogenic variants). Reference materials of this sort are entirely lacking or poorly controlled in this area and the stability and variability parameters have not been explored at all. In response to an 1 2 V A M B U L L E T I N UKNEQAS EMQN Cystic Fibrosis ✓ ✓* Duchenne Muscular Dystrophy ✓ ✓ Huntington’s disease ✓ ✓ ✓ ✓ ✓ ✓ Prader Willi/ Angelman’s syndrome ✓ ✓ Familial Breast/ Ovarian cancer Freidriech’s ataxia Y-chromosome micro-deletion Charcot Marie Tooth Disease Fragile X disease ✓** ✓ ✓ Retinoblastoma Mitochondrial dseases Spinal Muscular Atrophy Spino Cerebellar Ataxiss Myotonic Dystrophy ✓ ✓ ✓ ✓ ✓ ✓ ✓ * in association with the European Concerted Action on Cystic Fibrosis. ** in association with the European Academy of Andrology. Table 3: External Quality Assessment Schemes for molecular genetic testing available in Europe 2000–2001. ‘Expression of Interest’ submitted by a partnership including EMQN, the EU recognised this need in a call for proposals under its 5th Framework Measurement and Testing programme and the proposed project to develop CRMs is currently under negotiation. Important elements of total quality assurance in the analytical laboratory are provided by workforce considerations, external checks on performance and the availability of traceable calibrants and controls (reference materials) but the assessment of all of these features is provided by good management practice. Accreditation for service is therefore a key to the compliance of the laboratory in all elements of internal quality control and external oversight that together assure the validity of the test result for the public. A number of bodies are recognised as competent to accredit, and many industrial and public analytical services are familiar with the ISO 9000 series of standards, against which the British C O N T R I B U T E D Standards Institute (BSI) is the inspecting agency in the UK. In the world of medical laboratories the main accrediting agency is Clinical Pathology Accreditation (UK) Ltd (CPA). CPA has developed specific standards relevant to diagnostic laboratories and has been accrediting centres since 1992. In the next two years, revised standards harmonised with ISO/IEC17025 will be introduced. These new standards introduce a more client-centred approach, and the concepts of a quality manual and the introduction of a quality manager will be central to their operation and the inspection process. To date only about 20% of UK Genetics laboratories have been accredited but most are in the process of applying or have been inspected and are meeting conditional requirements. In summary, as a discipline, Clinical Molecular Genetics in the UK has recognised the need to assure the quality of genetic testing in the transition from research to service. It has adopted all the available systems designed to assure quality including accreditation, and registration of personnel. In addition it has developed some systems with advanced features including interpretative laboratory proficiency schemes and linked ‘best practice’ guidelines. Finally it has helped to spread and harmonise these systems in Europe and to develop previously unexplored areas such as the production of Certified Reference Materials for genetic testing. REFERENCES 1. Stenhouse, S., and Middleton-Price, H. (1996) Quality Assurance in Molecular Diagnosis. 341-353 in Elles, R. (ed) Molecular Diagnosis of Genetic Diseases. Humana, Totowa. 2. Dequeker, E., and Cassiman, J.J. (1998) Evaluation of CFTR gene mutation testing methods in 136 diagnostic laboratories: report of a large European external quality assessment. Eur J Hum Genet 6(2):165-75. 3. Dequeker, E., and Cassiman, J.J. (2000) Genetic testing and quality control in diagnostic laboratories. J.J. Nat. Genet. 25: 259-260. 4. Losekoot, M., Bakker, B., Laccone, F., Stenhouse, S., Elles, R. (1999) A European pilot quality assessment scheme for molecular diagnosis of Huntington’s disease. Eur. J. Hum. Genet. 7(2): 217-22. A R T I C L E S Addresses European Molecular Genetics Quality Network c/o Regional Molecular Genetics Laboratory St Mary’s Hospital MANCHESTER M13 OJH UK National Centre for Medical Genetics Our Lady’s Hospital for Sick Children CRUMLIN Dublin IRELAND UK National External Quality Assessment Scheme for Molecular Genetics c/o Regional Molecular Genetics Laboratory St Mary’s Hospital MANCHESTER M13 OJH UK Northern Regional Genetics Service Claremont Place NEWCASTLE NE1 7RL UK 5. Jeffrey, G.P., Chakrabarti, S., Hegele, R.A., Adams, P.C. (1999) Polymorphism in intron 4 of HFE may cause overestimation of C282Y homozygote prevalence in haemochromatosis. Nat. Genet.22(4):325-6. F O C U S O N F O R E N S I C A N A L Y S I S Standards in evidence – The registration of forensic practitioners Alan Kershaw Council for the registration of Forensic Practitioners guess I should hesitate to offer a paper to a scientific publication. I am not, and never will be, a scientist. But I am conducting an experiment – one which has a good chance of succeeding and which represents some front line thinking in professional regulation I and in the setting of standards for forensic practice. Miscarriages of justice There must be few things that can as easily go undetected in a criminal trial as a flaw in the 1 3 V A M B U L L E T I N evidence of a forensic expert. And few things that will as readily bring the criminal justice system, and the name of science itself, into disrepute as the revelation that a forensic practitioner has been incompetent in bringing their findings to court. Some high profile cases in the 1970s, F O C U S O N F O R E N S I C The author. where flaws came to light many years later, resonate still in the public imagination. It would not be an exaggeration to say that they shook the criminal justice system to its foundations. Certainly, they cast a long shadow over the credibility and authority of forensic experts and the evidence they give in court. From them there is an unbroken line to the Council for the Registration of Forensic Practitioners (CRFP), which has now opened the first ever register of forensic practitioners. Restoring confidence Trust had to be restored. The courts and the public deserved the reassurance that the forensic process was all it should be. The “Increasingly courts rely on the objectivity of forensic science. It is essential that the profession ensures it provides it. Safer science means sustainable verdicts. The more powerful forensic science becomes, the more confidence society needs in its practitioners. Currently any Tom, Dick or Harriet can purport to be a forensic expert, often providing advice which is unhelpful at best, and positively misleading at worst. This register means something because, unlike all the others, it is based on peer review and not customer perceptions.” Dr Angela Gallop Director, ‘Forensic Alliance’ and President Elect of the Forensic Science Society A N A L Y S I S forensic science community, much to their credit, were quick to see the need for this and took steps to introduce the systematic practice of quality assurance which is now an ever-present feature of forensic laboratories, running through their management and their processes. But there was a piece missing, a task perhaps more difficult to perform but no less essential to restoring confidence: ensuring that the individual scientists and others who use professional skills to produce evidence for court are, and remain, fit for their job. The forensic professions settled for external accreditation by way of a structured, external assessment of the current competence of individual practitioners. That is what CRFP now offers. What CRFP is for CRFP has been set up with a single, overriding objective: to promote public confidence in forensic practice in the UK. This includes not just scientists but all the specialist groups involved in the whole chain of the forensic process, from the crime scene to the courtroom: scene examiners, fingerprint examiners, laboratory scientists, police surgeons, pathologists, dentists, IT specialists, archaeologists, anthropologists and all professionals who may contribute to an investigation and to the presentation of a successful case. The public demand – and deserve – to know that every practitioner in that chain is competent, and committed to professional values against which they are prepared to be judged if something goes wrong. What CRFP will do CRFP has three main functions: • publishing a register of competent forensic practitioners • ensuring through periodic revalidation that practitioners keep up to date and maintain competence • dealing with registered practitioners who fail to meet the necessary standards. The first of those is the central function, from which everything else flows. A register puts a boundary around a profession, showing who has been assessed as competent in the field. The second function is the public’s assurance that registered practitioners remain fit to do their job. Registration will be time 1 4 V A M B U L L E T I N limited, running for four years at a time. Before the end of that period each person on the register will have to satisfy us that they have learned positively from their experience, kept abreast of developments in their field and pursued a policy of continuous improvement. We will fulfil the third function by dealing, firmly and fairly, with any information we receive which raises a question about an individual’s fitness to stay on the register. There may be an allegation of misconduct, or a problem of ill health or poor professional performance. We will deal with each of these in the way that suits them best. Standards of conduct Professional regulation is often caricatured as the business of striking dysfunctional practitioners off the register. In fact, it is much more about helping good practitioners to stay good and become better through their careers. That is why we have published a Code of Conduct as the foundation of the register. This is not a guide on how to get into trouble. It is a clear, positive statement of professional values. Everyone applying for registration will have to show they understand those values and state that they will adhere to them in their everyday work. The Code focuses on the forensic practitioner’s overriding duty to the court and the administration of justice. Among other things we highlight the principles of: • putting justice and the needs of the court first; • honesty, integrity, objectivity and impartiality; • confidentiality and freedom from discrimination; • understanding the limits of professional confidence – even, perhaps especially, when under pressure in the witness box. How registration will work The register is now open to scientists, scene examiners, and fingerprint examiners. We have divided scientists into eight groups, designed to reflect broadly the thought processes involved in each specialist area. The eight groups are drugs, toxicology, marks, firearms, particulates and other traces, human contact traces, incident reconstruction and questioned documents. F O C U S For each group we are training a team of specialist assessors, each of them a competent practitioner in their field. They will assess the current competence of applicants from their professional group, along with their ability to practise safely and independently. The assessments will be structured and the system is designed to secure consistency and fairness across the sector. We have provided for second opinions and appeals against a refusal of registration and the whole process is subject to external validation by respected academics. We are asking each applicant for information about themselves, their qualifications and their professional experience; for a brief portfolio of recent casework; professional references; and declarations about their past record and willingness to adhere to the Code of Conduct. All this evidence will be judged against the criteria we have identified to define competence in each speciality. The scope of the register The register will be inclusive, covering practitioners throughout the forensic process – from the recovery of material, through its identification, examination and analysis, to the presentation of evidence in court. It will be a single register, identifying the specialist fields in which each practitioner works – and, in the case of scientists, the sub-specialties in which they have their particular interests. “The Courts have been and are likely to continue to be increasingly dependent on the quality of forensic practitioners who are involved in proceedings in the Courts. CRFP are to be warmly congratulated on the efforts which they are making to encourage forensic practitioners to adopt the necessary standards and good practice. The establishment of a register is very welcome. It should ensure that those practitioners who fulfil the required standards can be identified. It should encourage those who do not meet those standards to improve their practice.” The Rt Hon Lord Woolf of Barnes Lord Chief Justice of England and Wales O N F O R E N S I C A N A L Y S I S We have set the fee at £125 a year for registration in one speciality, plus £15 for each additional specialty, recognising the multi-skilling that is a feature of some forensic organisations. Some of the major employers have decided to pay the fees on behalf of their employees who apply for registration. A new professionalism At this stage we are focusing on those who work for the criminal courts. We will consider later whether to extend our scope to those working in civil litigation, where different issues arise and where the case for accreditation – though strong in our eyes – is not yet universally accepted. Who can apply? The register is open to scientists and scene examiners who are: • actively practising in their fields - that is, not simply organising a department or managing or training others; • using professional skills to produce evidence – oral or written – for use in court; • practising in their own right – that is, not working solely under supervision or carrying out others’ instructions. Who pays? CRFP’s start up costs are being funded by the Home Office – a powerful indicator of government support at a time when public expenditure is under constant scrutiny. In time they expect us, like other regulatory bodies, to fund ourselves by making a charge for registration and the retention of a name on the register. This is an important principle in professional regulation: independence from government is essential if a scheme is to retain the confidence of those on the register. 1 5 V A M B U L L E T I N In the current climate, professionals of all kinds are having to come to terms with the relatively new, but plain, fact that their expertise will no longer be taken for granted. They and their decisions must bear scrutiny in return for the great trust which society places in them. This is not something to be feared. It calls for a professionalism that welcomes external assessment, critical review and the systematic application of quality assurance techniques. This is how it should be: regulation is not something a regulatory body can come out of the blue and ‘do’ to a profession. Regulation starts with the individual practitioner and extends outwards to the teams they work in, their local and national associations, the bodies that represent them and the centres of excellence in which they are trained. “High quality evidence, professionally presented, is the key to an effective and efficient criminal justice system. That demands high professional standards in the multitude of specialist disciplines that come together as ‘forensic practitioners’. The Government wants to encourage the best use of expert evidence, which is why we are supporting the establishment of CRFP, which is a unique concept and a real world leader. I believe it will make an important contribution and wish it well.” Charles Clarke MP Minister of State at the Home Office CRFP has a crucial part to play, both directly and as a catalyst. We offer forensic practitioners the opportunity to demonstrate, to anyone who needs to know, their current fitness for the job they do. Registration is far more than a bureaucratic process. It is the expression of a standard and of a renewed professionalism. F O C U S O N F O R E N S I C A N A L Y S I S We may have to go to court with this one… Ric Treble & Fraser Nicholson LGC rofessional forensic scientists and expert witnesses expect their work to be used in legal proceedings, and are trained in how to conduct their work appropriately, to prepare statements or reports for court, and to present their evidence in person when this is necessary. However, any analyst could find themselves required or requested to present their results as evidence in a court case, criminal or civil. Sometimes the analyst is warned in advance that a court case is expected. On other occasions, what at the time appeared to be a routine analysis can subsequently become a component of a legal case. Whatever the circumstances, there can be understandable concern for an analyst with little experience of legal proceedings when a customer announces that “we may have to go to court with this one…” P …the scientist becoming involved in legal proceedings for the first time may feel they are being invited to enter this unfamiliar territory without a map There are a number of issues to be taken into consideration when analytical measurement data is to be used in court, some procedural, some legal and some practical. Apart from general conformance with the VAM Principles, many producers of analytical measurements for the courts work to specific guidelines or codes of practice for undertaking particular types of forensic analyses. Sector-specific organisations often align their recommendations with the specific regulation or legislation for which they are providing evidence. Many sector-specific and cross-sectoral organisations also have generic best practice guidance for their members. Although there is a range of such best practice guidance available, the scientist becoming involved in legal proceedings for the first time may feel they are being invited to enter this unfamiliar territory without a map. After consultations both with scientific experts with experience in this field and with legal practitioners who make use of expert witnesses, it was decided to produce a short guide 1 to explain some of the key issues involved and, more practically, to provide directions to suitable sources of advice and expertise. Some key issues Key background issues of which the expert needs to be aware when they take on forensic commitments include: • The requirement for expert evidence and opinion to be reliable. The privileged status and weight accorded to expert evidence makes a clear moral case for any expert evidence presented to be dependable. More practically, any court case, no matter how dignified the procedures appear, is a fight between two sides and all evidence is open to challenge. A scientist offering expert evidence or opinion must therefore be able and prepared to demonstrate, sometimes under aggressive questioning, that their work was valid. • The overarching requirement for the expert to be impartial. The expert’s responsibility is to the court, not to the side providing their instruction and/or payment. This principle has been clarified in a number of key cases, including ‘the Ikarian Reefer’2 (which was a ship, not an exotic cigarette) in the civil courts and the Judith Ward judgement3 in the criminal courts. It is now also spelt out in a number of legal and professional Codes of Conduct. • The need for timely provision of evidence. Delays, and subsequent costs, have been identified as key problems within the legal system. Major initiatives, including the 1 6 V A M B U L L E T I N Woolf Reforms in the civil courts and the Narey Reforms in the criminal system, have been established to tackle these problems. The expert has to play their role by agreeing a time-scale for their work and ensuring that this time-scale is met. Expecting to be challenged and the importance of validity In an adversarial legal system, such as ours, analytical measurement evidence being presented in court must be expected to be subject to challenge, in exactly the same way that all other types of evidence can be challenged. This has traditionally been achieved by the appointment of a suitably qualified expert to act for the other side in the case. It is therefore vital that analytical measurement data produced for use in court are as valid and reliable as possible. The producers of analytical measurement evidence that may be used in legal proceedings therefore need to know what standards of validity will be expected of them. The producers of analytical measurement evidence that may be used in legal proceedings therefore need to know what standards of validity will be expected of them. In addition, the users of the analytical results, that is the legal profession and the judiciary, also need to be aware of any factors that may increase, or decrease, confidence in the reliability of results. Legal professionals can become extremely annoyed if the expert they are using to develop their case decides to backtrack on the reliability of the evidence, or to start qualifying their evidence at a late stage. The basis of analytical evidence therefore needs clear explanation so that its validity and significance can be considered while the case is being prepared. In practice, analytical measurement is currently only rarely challenged or questioned F O C U S in depth in court. There is increasing reliance on pre-trial disclosure of expert evidence, so that issues can be identified and addressed by the two sides before the court convenes. If agreement can be reached on the majority of technical matters outside the court, only genuine issues of dispute between the two sides need to be decided by the court. In addition, the expert often finds that it is issues other than the measurements themselves, which are being questioned, such as chain-ofcustody details or possible alternative explanations for the findings. Although an expert might hope that their evidence will be unchallenged, this approach is potentially dangerous in that the court will be asked to draw conclusions based on information, the detail and reliability of which may not be fully explored. There is therefore a responsibility on the expert to ensure that any findings to be used in a court case are valid. Contents of the guide The contents of the guide are summarised below. It is structured as a series of sections, setting out key issues and directing the interested reader to appropriate sources of further information and advice. Where possible, the guide’s references are to suitable websites, in order to try to provide rapidly accessible information and advice for analysts who find themselves faced with the prospect of involvement in a legal case and who may not have time to seek out the more traditional paper-based resources. The English legal system The guide opens by addressing the English legal system. This is an extremely complex subject, so readers are directed to some of the more approachable introductions that have been prepared for the benefit of the novice expert about to become involved in legal proceedings. The guide does however explain some of the more common terms used in the Magistrates, Crown, County and High Courts. The role of the expert The guide then moves on to address the role of the expert within the legal system. The key legal difference between the expert witness and all other witnesses in a case is that the expert is allowed to present evidence of opinion, as well as evidence of fact. They O N F O R E N S I C are allowed to do this in order to assist the court to understand matters that are not within the common knowledge of the court. …there is a particular responsibility placed on the expert, in that they have to regard their role as acting for the court, irrespective of which side in the case is instructing them This means that there is a particular responsibility placed on the expert, in that they have to regard their role as acting for the court, irrespective of which side in the case is instructing them – and is paying their fees. There are other issues that the expert witness has to consider, and the guide provides references to a number of detailed codes of conduct for expert witnesses which have been published by professional bodies. How experts are identified and engaged If a court case develops from analytical work carried out under an existing contract, the expert and their work are subsumed into the legal process. However, where expert witnesses are sought by solicitors to assist in cases, they are usually identified either by entries in directories or registers of experts, or by word of mouth. As well as advertising their services by means of one or more of the directories, an expert who acquits themselves well in court will be remembered by the solicitor employing them and can usually expect to be re-engaged by the same and other solicitors when similar cases arise. When invited to assist in a case, the ‘terms of engagement’ need to be carefully agreed – even when there is pressure for a quick response. Issues to be addressed include the timetable required and the suitability and limits of the expert’s knowledge for the issues in question. Sources of advice on engagement issues and standard contracts for engagement are available. Chain-of-custody and contemporaneous note keeping There are a number of particular requirements for work to be used in court 1 7 V A M B U L L E T I N A N A L Y S I S particularly in criminal cases where the standard of proof required is ‘beyond reasonable doubt’. (In civil courts, by contrast, cases are decided ‘on the balance of the probabilities’). One of the most important requirements is the ability to demonstrate chain-of-custody. For analytical work, this refers to documentary evidence that the data presented refer to the original item of interest, and that the item has been in safe custody throughout the process, so that its integrity has been maintained. One of the most important requirements is the ability to demonstrate chain-of-custody. The guide reviews the various aspects of this process and the requirement for the keeping of contemporaneous records of work carried out, either by means of written notes and laboratory records or, in circumstances where making written records is not practical, by audio or video-tape records. Selection of methodology Use of validated methodology is extremely important as a means to demonstrate to the court that findings and opinions are soundly based. The more robust the evidence of the method’s conformity with the VAM principles, the less likelihood there is of the other side’s expert recommending a challenge over the validity of results. For routine testing, using methods subject to independent accreditation by bodies such as UKAS provides strong evidence of validity. For nonroutine work, where accreditation is not appropriate or practical, the use of standard published methods which are accepted in the relevant sector is advisable. However, in those cases where methods have to be adapted or developed specifically for the task, appropriate validation evidence will need to be compiled, and the EURACHEM Guide to method validation can provide useful advice. Retention and disposal of material The retention and disposal of evidential material and records needs to be carefully managed, material may be needed for subsequent appeal or review. As a general rule, no evidential documentation or material should ever be disposed of without the customer’s written authority, and any F O C U S O N F O R E N S I C disposal of material should be documented, including the return of items to customers. Explaining the significance of findings The significance of results should be explained by placing the findings into context. This is particularly true where ‘matches’ or similarities between items are being reported, and where population statistics therefore become important. For example, finding that two pieces of commercially available adhesive tape are made of the same materials is unlikely to be of any great significance, as millions of rolls of such tape may be in circulation. However, establishing an exact physical match between two torn ends of tape in different items in a case could be extremely significant. In recent years, Bayesian statistics has become increasingly widely applied to clarify such issues, led particularly by the need to explain the significance of DNA results. This powerful technique, based on the comparison of probabilities, is strongly recommended where the significance of results is likely to be an issue, and suitable reference sources are recommended in the guide. However, this is not a simple technique to master and the assistance of a statistician may have to be sought to clarify the complexities involved. Uncertainty and proof Associated with the issue of significance is the question of analytical uncertainty. Analysts are expected to be aware of the degree of uncertainty inherent in their results, and should clearly communicate this to the lawyers engaging their services so that they can decide on the use which can be made of the results. Similarly, an expert acting for the other side in a case can be expected to assess the degree of uncertainty in the results as part of their review of the findings. A common concern is that results which are to be reported as having a 95% or 99% confidence level may therefore be regarded as unsuitable for use in a criminal case where the standard of proof is to be ‘beyond reasonable doubt’ . However, this standard refers to the need to establish proof beyond reasonable doubt based on the whole of the evidence produced by both parties in the case – not for each individual item of evidence involved. A N A L Y S I S Sampling Sampling issues can vary from the need to collect a truly representative sample from a bulk consignment – for which statistically based guidance is available – to collecting an unique item from a crime scene. In both cases, contemporaneous documentation of the procedures followed to collect the sample and to avoid any contamination needs to be retained for subsequent examination if required. Sealing of items In order to demonstrate that the integrity of materials that have been sampled or submitted has been maintained, it is common to use tamper-evident methods of sealing or securing items. These allow the analyst to attest that the items have not been interfered with while they were in transit to or within the laboratory, or while stored under their control. Advice is included as to how this can be achieved in practice, either by using custom-designed ‘tamper evident evidence bags’ or by techniques such as sealing under signature. Again, detailed record keeping is advisable, so as to be able to show who had access to the sample, and at what time. Creating a case file It is good practice to create an indexed file containing all the paperwork relating to a sample or case, including notes, instrument output, calculations etc. This not only helps to avoid any loss of material, but also facilitates disclosure of case material . Preparing reports and statements The guide provides directions to sources of information on how statements and reports should be drafted for use in criminal and civil courts. The need to balance clarity for the non-scientific reader with a full exposition of the analytical issues requires careful composition. The courts prefer experts to adopt a standardised format to assist readers of such documents to find their way around the text. Analysts are also subject to some specific legal requirements covering issues such as hearsay. In practice, this requirement can usually be met by disclosure of the role played by assistants who have worked under the direction of the 1 8 V A M B U L L E T I N analyst. The other side can then decide whether they wish to question the assistants as well as the analyst. Disclosure As mentioned previously, pre-trial disclosure of expert’s findings is now routine. Examination of evidence and, where necessary, pre-trial meetings of the experts from the two sides are intended to settle as many areas of disagreement as possible before the trial, so that only any remaining areas of disagreement have to be presented to and decided by the court. The procedure for disclosure differs between civil and criminal cases, and the details are normally attended to by the lawyers involved in the case. However, the responsibility remains with the analyst to ensure that the lawyer acting for their side of the case is aware of the existence of disclosable material. Preparing for Court Appearances The final and, for the novice, often the most worrying, stage in the process is to prepare oneself to present evidence in court. The guide includes directions to sources of advice and, for those who anticipate a number of appearances as expert witnesses, organisations providing training courses in the ‘art’ of court presentation. It is hoped that the guide will form a useful introduction to the issues involved in taking analytical measurements to the court, and provide analysts unfamiliar with the requirements of court work with directions to suitable sources of references to prepare them for their role in the dispensation of justice. REFERENCES 1. VAM Report LGC/VAM/2000/062, Legal aspects of analytical measurement, undertaking analytical measurement for court – A good practice guide for scientists, (2000) 2. The Ikarian Reefer (1993), 20 FSR 563 3. R v Ward (1993), 1 WLR 619, 96 Cr. App. Ref. 1, 2 All ER 577, Court of Appeal (criminal Division). F O C U S O N F O R E N S I C A N A L Y S I S Useful web sites for background information Organisations supporting forensic experts: The Academy of Experts The Expert Witness Institute The Council for the Registration of Forensic Practitioners The Forensic Science Society The British Academy of Forensic Sciences The Society of Expert Witnesses www.academy-experts.org.uk www.ewi.org.uk www.crfp.org.uk www.demon.co.uk/forensics/ www.lawsoc.org.uk www.sew.org.uk Directories of Expert Witnesses: Expert Witness – Expert Consultant The Law Society of England and Wales UK Register of Expert Witnesses www.expertwitness.co.uk www.lawsoc.org.uk www.jspubs.com (Includes links to useful sites for experts) Court Skills Training: Bond Solon Training Professional Solutions and Services Ltd www.bondsolon.com www.prosols.uk.com Other useful sites: The Criminal Justice Joint Planning Unit (CJJPU) Eurachem website Valid Analytical Measurement (VAM) RSC Code of Conduct and Guidance on Professional Practice www.criminal-justice-system.gov.uk www.vtt.fi/ket/eurachem www.vam.org.uk www.rsc.org C A S E S T U D Y This issue of the VAM Bulletin contains the seventh of a series of case studies, demonstrating the business benefits of VAM. Companies often perceive the merit in sharing their own stories of the tangible benefits of the VAM approach. It is hoped that this articles will provided enough detail to be of value beyond the industrial sector directly involved, to a wider range of businesses engaged in analytical science. The development of an industry – based PT scheme and the benefits of participation Dr Roger S. Brown Source Testing Association Rod A. Robinson National Physical Laboratory Summary T he Source Testing Association (STA) has adopted a proficiency-testing (PT) scheme for gas measurement as a service to its membership. This scheme will help ensure that members offering gas measurements meet the minimum requirements of its code of practice. It will also satisfy the requirements of Quality Assurance Schemes accredited by UKAS. The PT scheme has been developed for the STA by the National Physical Laboratory (NPL) and has been underpinned by the DTI’s Valid Analytical Measurement (VAM) Programme. This article puts the Association into perspective and discusses the logistics of the scheme that has been subsequently 1 9 V A M B U L L E T I N developed from an initial trial to a fully operational scheme offered to our membership. The scheme reassures the clients of our members that the data submitted to legislative bodies has validity. This article discusses the fruitful partnership the STA has formed with NPL in developing a Gas Analysis Proficiency Testing Scheme. The Source Testing Association To understand the reasons why a proficiency testing scheme is important to the members of the STA it is important to understand a little of the STA’s history, structure, its codes of practice and its endorsement programmes. C A S E S T U D Y We initially took as our model the Source Evaluation Society (from the USA), and developed a Code of Practice that members must sign up to on joining the Association. We established a constitution, set rules for membership, established an organisational structure, and launched the Association in 1996 as a non-profit making organisation. Although the Association started life as a trade association for those directly involved in source (stack) testing our recruitment drive gave us a much wider membership and our current membership consists of the following groups: • test Houses (50%); • regulators (5%); • process Operators (15%); • instrumentation Companies (25%); • gas suppliers (5%). The STA was set up in 1995 to meet a need to improve the quality of emissions testing and monitoring in the UK. Industrial air pollution has been regulated in the UK since the first Clean Air Act of the 1950s. The Environmental Protection Acts of 1990 and 1995 require operators to prove to the Enforcement Authorities that their process complies with the relevant Prescribed Processes Regulations. This principle continues with the latest Integrated Pollution Prevention and Control (IPPC) regulations, which came into force this year. In all these regulatory instruments the ‘Polluter Pays’ concept operates. It is crucial that valid measurements are made, and that process operators and regulators have confidence in the data they receive from the test houses (i.e. organisations who undertake emission measurements). The mission statement of the Source Testing Association is to: “Advance the science and practice of emission monitoring and to develop and maintain a high quality of service to customers.” I II SEPA: Scottish Environment Protection Agency NIHES: Northern Ireland Industrial Pollution and Radiochemical Inspectorate The STA has developed a programme of seminars and workshops and through these and other activities has developed links with: • The Department of Trade & Industry (& in particular the VAM Programme); • Department of Environment, Transport & the Regions (DETR); • The Environment Agencies EA, SEPAI, NIHESII; • The United Kingdom Accreditation Service (UKAS); • The Chartered Institute of Environmental Health Officers (CIEHO). The STA is organised such that each area that is important to the practice of emission monitoring has its own task group (working party). This ensures that key matters within that area are addressed. The task groups currently in operation are listed in Table 1. Task Group Date Formed Technical Task Group (TTG) 1996 Endorsement Sub Group 1997 Standard Review Sub Group 1999 An Instrumentation Task Group 2000 Health & Safety Task Group 1997 Quality Task Group 1996 Marketing Group 1999 Small Business Task Group 1996 Table 1: Task groups currently in operation. 2 0 V A M B U L L E T I N Each Task Group has its own remit and develops strategies that are considered by the management group before being put forward to the membership. The Drive For Quality To satisfy the need to improve quality, in 1996 the STA Technical Task Group & Quality Group approached the VAM key players (NPL, LGC, UKAS) and on November 7, an STA workshop was arranged in Stoke on Trent. The key point, which arose from the VAM meeting, was that our members and the Association should adopt the VAM principles [See Page 2]. In general, these tenets were mirrored by some of our own stated codes of practice, which are that members who conduct stack testing should: 1. use the correct method; 2. use the correct equipment; 3. use equipment which is calibrated; 4. utilise competent personnel; 5. operate to documented protocols; 6. operate the equipment correctly; 7. operate to a quality system; 8. implement risk assessment & health & safety policies; 9. participate in proficiency testing schemes. Following the meeting in Stoke, the STA committed itself to establishing a series of endorsement programmes and starting a trial proficiency testing scheme. The Endorsement and Gas Analysis Proficiency Testing Scheme The STA was founded on the principle that its members must abide by its code of practice and achieve at least minimum standards of conduct (which are documented). To assist in demonstrating this to clients of our members, it was decided to operate a number of Quality Assurance endorsement programmes. These consisted of peer review of the member applying for endorsement for a class of tests, be it for dust or gaseous measurement. The STA then convened a panel to consider the submitted protocols against BS CEN and US EPA methods and arranged a site appraisal of the sampling teams on a rolling basis. C A S E The STA set up a series of endorsement schemes to allow on-site peer review of members. In 1998 the STA launched its first endorsement programme for particulate testing of source emissions from industrial facilities. Currently thirty member companies participate in this scheme. In 1999 we launched our measurement scheme by instrumental means for carbon monoxide (CO), carbon dioxide (CO 2), nitric oxide (NO), oxides of nitrogen (NOx), sulfur dioxide (SO2) and volatile organic compounds (VOCs) and we currently have 11 member companies who have enrolled in this latter scheme. The verification of quality in source testing is very difficult because, in general, the emissions on a process can vary due to a number of different factors. These include, changing feedstock, varying operating conditions, changes in flue gas velocity etc. Unless a series of simultaneous trials with co-location of sampling probes is conducted, it is almost impossible to verify data directly; even then across a stack there can be significant variations in concentration levels. Tests such as these are very expensive and few clients would be willing to pay the costs. One way of addressing these difficulties is to operate one or more PT schemes. These allow inter-comparison against round robin samples and give an indication of inter-company variations. This can then provide input to the endorsement schemes. Schemes such as this also have significance to UKAS accredited companies, as UKAS accreditation to ISO 17025 requires participation in PT schemes where they exist. In the future, PT schemes will undoubtedly play their part in personnel competency schemes such as the Environment Agency’s, “MCERTS Scheme for Manual Stack Testing”. In order to trial a PT scheme the STA formed a link with the National Physical Laboratory (NPL) and asked Dr Peter Woods’ group to run a trial proficiency testing scheme for gas analysis. This trial was operated by NPL and funded as a part of the VAM Programme by the DTI. In this trial scheme a set of 5 commercial gas cylinders were labelled by NPL traceable to UK national standards. The STA approached its members to seek participation in the scheme, eighteen companies within the STA agreed to participate and in the end sixteen companies returned results for statistical analysis. The logistical arrangements of preparing, shipping, supplying regulators etc., cannot be under estimated and in many ways has turned out to be more taxing for the STA and NPL than the science involved. The approximate gas concentration was identified to the company but not all companies analysed all cylinders due to different capabilities and the availability of equipment and staff. The gases that were circulated are shown in Table 2. Species Nominal Concentration CO in Nitrogen 2%I, 1000 ppmII, 100 ppm NO in Nitrogen 500 ppm SO2 in Nitrogen 1000 ppm Table 2: Gas cylinders distributed by NPL. The scheme was in the process of being developed during the trial and a fully defined protocol was not available. It had not been stipulated how participants should introduce the gases to their analysers. Thus some companies would introduce the gases directly to a gas analyser, whilst others would introduce the test gases to the analysers via a gas conditioning system. Some systems needed less than one litre of gas per minute of gas whilst others would require several litres of gas per minute from the supplied gas cylinders. In addition it must be remembered that the gases shipped were very different, in their presentation, from those sampled in the field. The gases in a stack are often hot and may be present in conditions ranging from relatively dry to extremely wet. Condensation combined with high levels of dust and the presence of many other species can often cause sampling and analysis complications. However, the pure binary mixture gases were a first attempt to consider the intervariability of companies involved in the source-testing field. The gases were distributed around the participating members free of charge over a period of 6–12 months, and the results collected and collated by NPL. What happened next – The good, the bad and the ugly? The overall results from the scheme were encouraging with only one organisation falling into an unacceptable category. The data were processed and analysed by NPL and a summary of the data is shown in Figure 1. As an example, NPL also processed the data on the basis of z-scores as this enables criteria of acceptability to be set more easily and allows interpretation of the data at low concentrations to be assessed more fairly. A small error in measurement is more apparent at the lower concentration levels and results in a relatively higher percentage difference. In this study the z score was defined as: x–T z = σ Where: z = the z score x = the value obtained by participant T = the true value for test sample σ = the assigned value for the expected standard deviation (for the purposes of this example this was derived from the requirements of the EU Hazardous Waste Directive) The criteria for acceptability in the PT Scheme was set as: |z| ≤ 2 Satisfactory 2 >|z|< 3 Questionable |z| ≥ 3 Unsatisfactory I 2 1 V A M B U L L E T I N S T U D Y II % ≡ 10 mmol mol-1 ppm ≡ µmol mol-1 C A S E S T U D Y Figure 1: Percentage difference in gas concentration of the measured result versus the true level in the cylinder. Figure 2: The range of variability in the ‘Trial Gas Proficiency Scheme’ in terms of z-scores. The data are represented in this format in Figure 2. As mentioned earlier, the data analysis showed that only on one occasion was one member company yielding an unacceptable result. Full details were not available, from many of the companies, on the procedure they adopted in analysing the gases shipped to them, so further comment was not possible. The STA/NPL had asked the participating companies to quote uncertainties associated with their analyses but most companies did not provide this on submission of their data. Even so, the data were sufficiently encouraging, and the response from our members overwhelmingly supportive, that the STA has adopted a full PT scheme for gas analysis. This scheme is operated by NPL and the Gas Species Nominal Concentration 1 Sulfur Dioxide 1000 ppmI 2 Sulfur Dioxide 100 ppm 3 Carbon Monoxide 1000 ppm 4 Carbon Monoxide 100 ppm 5 Oxides of Nitrogen 500 ppm 6 Oxygen 11%II 7 VOC control mixture ~ 4 mg m-3 (TOC) Table 3: Species to be measured in NPL/STA PT scheme. The STA will continue to manage and refine the gas analysis PT scheme in partnership with VAM and NPL to meet the needs of regulators and members alike. It is essential to ensure that the NPL/STA schemes can be harmonised with Environment Agency schemes such as MCERTS and also UKAS requirements to minimise any needless expenditure by our members. The STA has decided that the scheme is certainly a useful mechanism in demonstrating proficiency in gas analysis and is currently developing a PT scheme for flow measurement to assist in other STA endorsement programmes. The NPL/STA PT scheme has provided a further method by which the STA can continue to provide value-added services to its membership. STA and it receives some DTI support through the current VAM programme. To date fifteen companies have agreed to join and currently two sets of gases are being circulated. These two sets of gases have been certified by NPL and are being shipped by BOC to STA participants. UKAS are following the progress of the scheme and our members recognise the value of the scheme in helping to maintain credibility to clients and their peers. A fully operational PT scheme has now been established for the STA. Following discussions within the STA the range of species has been altered slightly and the nominal values for the scheme are shown in Table 3. I 2 2 V A M B U L L E T I N II ppm ≡ µmol mol-1 % ≡ 10 mmol mol-1 V A M I N E D U C A T I O N Taking the VAM message from school to the workplace t is important that the UK has a competent workforce able to meet the challenges posed by new technologies and enable sustainable development of the chemical industry. Analytical scientists are a pivotal part of this workforce. The new VAM programme hopes to address some of the issues and facilitate improvements where they are necessary. The need for reliable measurements and the means by which they are obtained should be incorporated early in the education process; therefore the projects span from school level to the professional analysts. How early is a matter of debate now that science forms an integral part of primary education. It is important to make students aware of the contribution that chemical measurements make to the national economy and the impact they have on our quality of life. Within the programme there is provision to address these issues. There will be events for teachers to enhance their awareness of how to obtain valid results and then how to demonstrate their reliability. We will continue to organise with Nuffield curriculum projects an annual Proficiency Testing competition for students studying chemistry at A level. This titration exercise has proved very popular in the past and we have over 80 schools/colleges showing interest this year. We are starting to explore the possibility of a similar competition in biology. A number of teachers have indicated their interest in developing a suitable competition that will fit in with the syllabus. Increasingly University departments are including Quality Assurance and Quality Control topics in their course programmes. I We are pleased about this. However, in most cases these are at the Master’s level and only in analytical chemistry MSc programmes. We hope to increase the coverage into chemistry courses. Another new venture for MSc students, in analytical and environmental science, is an opportunity for them to take part in a round of a national Proficiency Testing scheme. This will provide them with valuable experience with very little cost to the university, as entry will be free. They will be sent samples of solids and solutions to analyse and their results will be compared with those from commercial laboratories. We will provide the universities with detailed feedback in the hope that it will help in course development and assessment. During the 1997–2000 VAM programme a network group was formed from analysts in SOCSA (Specialised Organic Chemicals Sector Association) companies. The ‘SOCSA Analytical Networking Group’ (SANG) meets about 4 times a year. The meetings have followed a variety of formats but currently one of the main items on the agenda is a discussion of analytical results obtained for a sample distributed between meetings. This is a simple benchmarking exercise that helps identify best practice and resolve the problems that may arise during the analysis. This group of companies has accrued many benefits from the network. These include: • development of training packages; • setting acceptable and achievable performance parameters; • access to documentation to help set up their own quality system; 2 3 V A M B U L L E T I N • involvement of junior analysts with analysts in other companies; • benchmarking of skills; • identification of analytical experts; • advice on new equipment and servicing; • best practice in many areas of analytical science. In the current programme there is an opportunity to set up other similar networks either in different regions (SANG members are mainly in the North East) or in a different sector. Identifying SMEs that would like to be involved in the project is not straightforward. We are very anxious to hear from anyone who would like to participate in such networks. We hope that university departments will also be part of the new networks. It is important that there is a good dialogue between academia and industry. The intention is to identify training needs, develop new training materials and set up a coaching system. It is important that academia are in on the discussions. Method development and validation are important and time-consuming tasks in analytical science. There is a programme that will investigate cost effective methodology, again the needs of industry and particularly SMEs will be sought. The programme is varied but has the general theme of quality of measurement and dissemination of best practice. The output of a programme is always more valuable to users of that output if they are involved during the programme. Many of the contacts are made by word of mouth so if you know of anyone who can contribute to any of the projects or wish to take part in the events please contact us through the VAM Helpdesk (page 2 and 32). V A M N E W S Notes on applying ILAC guidelines to microbiological PT schemes now available roficiency testing has become a principal quality assurance tool for many laboratories. Most laboratory accreditation bodies now expect, if not require, participation in a relevant proficiency testing scheme if one is available. Proficiency testing results have become an important factor in the assessment of a laboratory’s performance by accreditation bodies, the laboratory’s customers, and the laboratory itself. While proficiency testing schemes have become key to assessing the competence of analysts, until recently there has not been any widely accepted detailed guidance on competence for the providers of those schemes. ISO Guide 43-1:1997 and elements of other standards gave general guidance, but there has not been anything suitable for accreditation bodies to assess proficiency testing scheme providers. The recently published ILAC-G13:2000, “Guidelines for the Requirements for the P V A M P R O D U C T Competence of Providers of Proficiency Testing Schemes”, has remedied this deficiency. The ILAC guidelines have been well received. A number of accreditation bodies, including UKAS, are using them as a basis to develop accreditation services for proficiency testing scheme providers. One of the advantages of the Guidelines is that they are not specific to any field, applying as well to chemistry as to physics. This writer has recently raised issues that are special to microbiological analysis, making it difficult to apply criteria in the same ways as to other disciplines1. He has suggested that such issues do not absolve microbiology laboratories from requirements to adhere to criteria, but that they must be taken into account when assessing how requirements are met. At the request of the VAM Proficiency Testing Working Group the Campden & Chorleywood Food Research Association convened an ad hoc working group to consider special issues in the application of the ILAC Guidelines to microbiological proficiency testing schemes. They produced a short document to be read in conjunction with the ILAC Guidelines. The topics covered include: • homogeneity of microorganisms in the sample; • stability; • sample matrix; • statistical design. The notes should help those applying the ILAC Guidelines to microbiology take account of appropriate special issues, and are intended to be widely available via the VAM Bulletin and the VAM web site. REFERENCES 1. Microbiological Proficiency Testing: A Personal Perspective, Jewell, K., Accreditation and Quality Assurance, In Press. N E W S Computer-based training package introduces market’s first measurement uncertainty module GC has recently launched VAMSTAT II: a new edition of its successful, computer-based learning package for teaching statistics to analytical chemists. The new version benefits from a complete revision and update, and is designed to run under MS Windows 9x/NT. A key innovation is the inclusion of a measurement uncertainty module, a first in the market and introduced as a direct response to the growing pressures from accreditation bodies to see labs making this information available for customers and for audit purposes. Other L new features include more, improved animations, interactive techniques, an extensive glossary and a bookmark facility. “Escalating demands from customers and regulators for quality in analysis have increased the need for analytical scientists to be proficient in the use of basic statistics,” said Steve Ellison – Head of LGC’s Statistics and Chemometrics group, and a co-author of VAMSTAT II. “Building on the sound statistical information and effective self-test facilities of the previous version, the new VAMSTAT release adds better graphics and 2 4 V A M B U L L E T I N more interaction to improve learning, simpler navigation for ease-of-use, and new information on analytical quality and measurement uncertainty to help analysts meet regulatory and customer requirements in full.” The principal technical author of VAMSTAT II was Michael Thompson, Professor of Analytical Chemistry at Birkbeck College (University of London), who originally conceived the VAMSTAT programme. An acknowledged expert in applications of statistics in analytical science V A M and quality issues, Prof. Thompson has twenty years experience in teaching statistics to analytical chemists. With additional input from practising analytical chemists, and the inclusion of ‘true-to-life’ examples, P R O D U C T VAMSTAT II provides grounding in all the statistics needed in the modern analytical chemistry laboratory. It requires minimal prior knowledge from the student and is suitable for workplace self-instruction or as a supplement to formal teaching courses and the remote-learning format makes for a costeffective solution to increase staff proficiency in the use of statistics. VAMSTAT II is divided into seven sections, each of which contains a number of self-contained sub-sections dealing with specific topics. The main areas covered are: • Basic concepts; • Significance tests; • Handling non-normal data and outliers; • Analysis of variance; • Quality of analytical data; N E W S • • Regression and applications; Measurement uncertainty. The package is suitable for use by those with little or no experience of statistics as well as the more experienced practitioner. The techniques, practical examples and questions presented are all strictly related to the needs of the analytical chemist. Most sections are supplemented by questions that test the user’s understanding of the material presented and the user is free to follow the topics and build up concepts in their natural sequence or to pick and choose topics as required. For further information contact the VAM Helpdesk (Pages 2 and 32) or the VAM web site, where a demonstration version of VAMSTAT II has been posted by LGC. Buying analytical services egular visitors to the VAM web site will have noticed that a new item appeared on the navigation menu, late last year. Clicking on this new arrival, the ‘Buyer’s Guide’ takes the visitor to a satellite web called ‘Buying Analytical Services’. This has been created as a resource to assist those who need to purchase such services. One important piece of advice constantly reiterated is the need for analysts to engage in dialogue with their customers in order to obtain a clear understanding of the customers’ requirements (VAM Principle 1 – Page 2). Advice of this nature is encapsulated in the VAM Principles, which form the bedrock upon which the entire VAM Programme is built. The VAM Programme has hitherto concentrated on providing tools and support to analysts to help them maintain or improve the quality of their measurements and this is still a primary function. However since, as the saying goes, ‘it takes two to tango’, it seems appropriate to offer some complementary advice to potential customers for analytical services to help them become better informed about the nature and process of analysis. This will hopefully benefit both customers and analysts since it will guide customers into thinking along similar lines to the analyst, in terms of establishing requirements. This should make for a more efficient discussion of the analytical problem and its possible solutions. A further benefit to R buyers/customers of measurements is that the Guide should help them to avoid overspecifying their requirements and could, in some cases, lead to a reduction in the cost to them of analysis. The Buyer’s Guide encourages users to think about purchasing analysis in terms of four main strands: • defining the purpose – clarifying why analysis is required; • defining the requirement – stating what is to be measured; • defining any constraints – what could limit the extent or type of analysis performed; 2 5 V A M B U L L E T I N • selecting a laboratory – how to find a suitable laboratory to carry out the work. The guide also contains a ‘Resources’ section which includes a risk assessment test, advice on the nature of analysis, information on useful contacts and links to other organisations, a model ‘requirements’ form that can be downloaded and a glossary. In addition, a feedback form allows users to comment about the site and it is hoped that they will take the opportunity to do so. The Buyer’s Guide will be an integral part of the new VAM website, which is currently under construction, and may be further developed in the light of user responses. I N T E R N A T I O N A L N E W S IMEP®: Bringing SI-traceable values to field laboratories Jørgen V. Nørgaard Lutgart Van Nevel Yetunde Aregbe Ioannis Papadakis Philip D. P. Taylor IRMM, Belgium nowledge of the degree of equivalence of analytical measurement results is important for economical, environmental and political reasons world-wide. The International Measurement Evaluation Programme (IMEP) offers to field laboratories reference values traceable to Le Système International d’unités (SI) of measurements and hence participating laboratories can evaluate their degree of equivalence independently from other participants results. The programme is coordinated by the Institute for Reference Materials and Measurements (IRMM) and is run under the auspices of IUPAC, EURACHEM, EUROMET, CITAC and EA. The certified test samples with undisclosed values are sent to interested laboratories. The participating laboratories are asked to return their measurement result with a statement of uncertainty. By displaying, in pictures, the results from the participants against the IMEP SI-traceable reference values, IMEP demonstrates the State of Practise (SoP) of chemical amount measurements in various fields. An example is given for IMEP-14, Trace Elements in Sediments (Figure 1). IMEP has different objectives, on purpose, than traditional round robin studies or proficiency testing schemes. The values, which will serve as references, are obtained by means of well-understood measurement processes. These reference values are not derived from and therefore do not depend on the participant measurement results1. The IMEP reference values are established mainly by Isotope Dilution Mass Spectrometry K I II ISO International Organisation for Standardisation BIPM Bureau International des Poids et Mesures (IDMS) applied as a Primary Method of Measurement (PMM2). The IMEP reference laboratories are selected specifically for each IMEP round depending on the material, matrix and elements under investigation. All IMEP reference laboratories have shown in previous work their capability, e.g. by publications in renowned international scientific journals. Other characteristics of the IMEP programme are that participating laboratories work under normal conditions with their choice of techniques, procedures and instrumentation and are requested to report their results with a best estimate of combined uncertainty according to ISO I /BIPM II guidelines3. IMEP is open to all laboratories Figure 1 2 6 V A M B U L L E T I N on a voluntary basis and full confidentiality with respect to the link between results and identity of each participant is guaranteed. IMEP is focused as much as possible to ‘real life’ samples and IMEP-rounds are organised in cases where the objective evaluation of measurement results is important and critical. IMEP aims at result-oriented evaluation of the measurement capability of participating laboratories. The European Accreditation (EA) has recognised the characteristics of IMEP, and as a result, IRMM now offers IMEP as a tool to accreditation bodies. IMEP rounds are open to all interested laboratories world-wide, which is also reflected in the number of participants. The various rounds have at times several hundred participants. IMEP also operates with Regional Co-ordinators (RC) in various countries. The RCs are very valuable to the programme, since information can be distributed and discussed at local level without language barriers. It makes the rounds faster in terms of logistics and in return the various countries obtain valuable information concerning the State of Practise in their country through the RCs. Since 1988 IMEP rounds have mainly concentrated on trace elements in various matrices such as water4,5,6, polyethylene7,8,9, I N T E R N A T I O N A L serum10,11,12 and car catalysts13. Carbon and oxygen isotope ratios in carbon dioxide14,15 and trace elements in sediments16 have been successfully completed. At present, the round IMEP-12, Trace Elements in Water, is ongoing and interested laboratories are welcome to contact the IMEP office at IRMM. The deadline for registration to IMEP-12 is March 2001. IMEP-16, Lead in Wine, is closed for reporting results and the round is now in the evaluation phase. IMEP-16 had 160 participating laboratories. Furthermore, IMEP-17, Trace and Minor Constituents in Human Serum, is in the planning stage. Samples are being certified and the round will be open to interested laboratories in the autumn 2001. Future planned rounds are IMEP-18, Sulfur in Oil and IMEP-19, which will focus on a rice material. A web site devoted entirely to IMEP has been designed and is currently running as a sub site at the IRMM home page (see address below). On the new IMEP web site, detailed information can found concerning ongoing rounds, future rounds but also completed rounds. All documentation can be downloaded including various publications. Meeting, ed. by BIPM, Sèvres, Cedex, France (1995). 3. Guide to the Expression of Uncertainty in Measurement, ISO, Geneva, (1995). Uldall, M. Loikkanen, M.M. Müller, J.-C. Libeer, H. Steensland, K. Hellsing, A. Squirrell, L.A. Penberthy, D. Schiel, T. Tamberg, T. Walczyk and J.W.H. Lam (1999), Accred. Qual. Assur., 4, 463-472. 4. A. Lamberty, L. Van Nevel, J. R. Moody and P. De Bièvre (1996), Accred. Qual. Assur., 1, 71-82. 13. A. Held, L. Van Nevel, E. Poulsen and P. D. P. Taylor, IMEP-11 participants report GE/R/IM/1/99, EUR 18735 EN. 5. L. Van Nevel, P.D.P. Taylor, U. Örnemark, J.R. Moody, K.G. Heumann and P. De Bièvre (1998), Accred. Qual. Assur., 3, 56-68. 14. J. V. Nørgaard, H. Kipphardt, S. Valkiers and P. D. P. Taylor (1999), “IMEP-8: Carbon and Oxygen Isotope ratios in CO2 – Certification Report”, EUR 19061 EN, EC-JRC-IRMM, Geel, Belgium. 6. I. Papadakis, J.V. Nørgaard, E. Vendelbo, L. Van Nevel and P.D.P. Taylor, (2001), Analyst, 126, 228-233. 7. A. Lamberty, P. De Bièvre and A. Götz, (1993) Fres. J. Anal. Chem, 345, 310-313. 8. L. Van Nevel, E. Poulsen, I. Papadakis and P.D.P. Taylor, EC, JRC, IRMM internal report, IMEP-10 participants report GE/R/SIM/11/98. 9. L. Van Nevel, E. Vendelbo and P.D.P. Taylor (2000), “The IRMM International Measurement Evaluation Programme, IMEP-13: Trace Elements in Polyethylene – Report to Participants”, EUR 19562 EN, EC-JRC-IRMM, Geel, Belgium. 10. P. De Bièvre, J. Savory, A. Lamberty and G. Savory (1988), Fres. Z. Anal. Chem, 332, 718-721. 1. I. Papadakis and P.D.P. Taylor, (2000) Accred. Qual. Assur., 5, 331 11. A. Lamberty, J. Savory, J.R. Moody, P. De Bièvre, K. J. R. Rosman and J. W. Gramlich (1998), Accred. Qual. Assur, 3, 447-458. 2. Comité Consultatif pour la Quantité de Matière (CCQM), Report of the 1st 12. U. Örnemark, L.Van Nevel, P.D.P. Taylor, P. Robouch, P. De Bièvre, A. REFERENCES: N E W S 15. L. Van Nevel, E. Poulsen and P.D.P. Taylor (1999), “IMEP-8: n(13C)/n(12C) and n( 18 O)/n( 16 O) in CO 2 – Report to Participants”, EUR 19060 EN, EC-JRCIRMM, Geel, Belgium. 16. I. Papadakis, E. Vendelbo, L. Van Nevel and P.D.P. Taylor (2000), “The IRMM International Measurement Evaluation Programme, IMEP-14: Trace Elements in Sediments – Report to Participants”, EUR 19595 EN, EC-JRC-IRMM, Geel, Belgium. For further information about the IMEP programme, please contact: European Commission Joint Research Centre Institute for Reference Materials and Measurements Retieseweg B-2440 GEEL BELGIUM Tel +32 14 571 682 or 702 Fax +32 14 571 865 Email imep@irmm.jrc.be Website www.irmm.jrc.be/imep LGC hosts a week of international meetings uring November LGC welcomed over 30 delegates from around the world to a series of meetings in the United Kingdom. The delegates were all collaborators in CCQM activities. The CCQM (Consultative Committee on Amount of Substance) is the steering committee for international chemical metrology and has recently extended its activities to address biometrology. The CCQM aims to resolve the practical difficulties of achieving comparable measurements and to provide an international structure of national and regional laboratories. These will demonstrate D the equivalence of their measurement data through a series of key comparisons, which reflect applications relevant to industry, trade, health, environment, etc. The main event of the week was a two – day meeting of the CCQM Inorganic Working Group, chaired by Dr Mike Sargent (LGC). The four working groups of the CCQM are the main routes for taking forward key comparisons and other experimental studies. They provide a more informal forum than the CCQM itself (which meets annually in Paris) and provide the scientists involved in CCQM activities with an opportunity to discuss their 2 7 V A M B U L L E T I N work. It was also agreed to host a meeting of the CCQM Electrochemical Analysis Working Group, chaired by Dr Wolfgang Richter (PTB, Germany). Many of the delegates attend both working groups, so a joint session covering topics of common interest was arranged. For the same reason LGC also agreed to host a meeting of the IUPAC Commission on Isotope Specific Measurements. Following submission of a paper by LGC to the CCQM meeting held in April 2000, it was agreed to form an ad hoc Biometrology Task Group. In order to facilitate the work of the Group, LGC I N T E R N A T I O N A L hosted the first meeting on November 15. The CCQM President, Robert Kaarls, and colleagues from BIPM, DTI, IRMM, KRISS, LGC, NIST, NMi, NRC and NPL were able to spend a full day discussing priorities for CCQM biometrology activities and formulated an initial work plan which will be undertaken this year. R E F E R E N C E N E W S In view of the number of overseas visitors, LGC also arranged a number of events including a tour of its Teddington facilities (in particular, the team undertaking high accuracy analysis by mass spectrometry) a formal dinner, and a scientific seminar. The seminar was a meeting on metals speciation, with several M A T E R I A L S CCQM speakers and delegates, as well as LGC’s research team and collaborators from Plymouth and De Montfort Universities, and Birkbeck College. Dr Gerry Newman, chairman of the AMC sub-committee on ‘High Accuracy Analysis by Mass Spectrometry’, represented the RSC. U P D A T E New reference materials now available L GC continues to supply an expanding range of reference materials to assist analysts throughout the world. Use of reference materials increases confidence in analytical results and is a useful tool for laboratories seeking accreditation. A new selection of materials is now available from LGC. Analysis of environmental and food samples is particularly important and the new selection of materials reflects this (Table 1). LGC7200 – LGC 7209 (meat species) are particularly exciting as it is thought that this is the first time such materials have been produced. There have been increasing problems with misleading descriptions of meat samples, and meat mixtures are difficult to detect. These materials are intended for use as quality control standards in the identification and determination of species, and will also assist in method development. LGC prepared the materials from prime cuts and confirmed the identification with several techniques including DNA analysis. The samples are moist to simulate the sort of samples usually encountered. A processed pork material will be available soon, certified for proximates, and with indicative values for soya and casein. For information on the types of reference materials available or to order, please contact: The Reference Materials Team LGC (Address on Pages 2 and 32) Tel: 020 8943 7565 Email: rmsales@lgc.co.uk For free advice on the use or world-wide location of specific reference materials, contact the VAM Helpdesk (page 2 and 32) and ask for the ‘Reference Materials Advisory Service (REMAS)’. Catalogue Number Material Unit Analytes LGC1204 Dimethoate 250 mg Purity LGC1205 Malathion 250 mg Purity LGC1820 3,3’,4,4’-Tetrachlorobiphenyl 0.02 g Purity LGC1821 3,3’,4’4’,5-Pentachlorobiphenyl 0.02 g Purity LGC1822 3,3’4,4’,5,5’-Hexachlorobiphenyl 0.02 g Purity LGC6016 Estuary water 50 ml Metals LGC6113 (RM) Soil 4 x 50 g PCBs LGC6114 (RM) Harbour sediment 4 x 50 g PCBs LGC6181 Sewage sludge 100 g Leachable Metals LGC6182 (RM) Sewage sludge 30 g PAHs LGC6187 River sediment 80 g Leachable Metals LGC6188 River sediment 30 g PAHs LGC7107 (RM) Madeira cake 160 g Proximates LGC7151 (RM) Processed meat 5 x 250 g Proximates & nitrate LGC7200 (RM) Beef - raw 30 g Species Authenticity LGC7201 (RM) Beef - cooked 30 g As LGC7200 LGC7202 (RM) Lamb - raw 30 g As LGC7200 LGC7203 (RM) Lamb - cooked 30 g As LGC7200 LGC7204 (RM) Pork - raw 30 g As LGC7200 LGC7205 (RM) Pork - cooked 30 g As LGC7200 LGC7206 (RM) Chicken - raw 30 g As LGC7200 LGC7207 (RM) Chicken - cooked 30 g As LGC7200 LGC7208 (RM) Turkey - raw 30 g As LGC7200 LGC7209 (RM) Turkey - cooked 30 g As LGC7200 LGC7301 Butylated hydroxyanisole (BHA) 0.5 g Purity LGC7305 Potassium sorbate 0.5 g Purity Table 1: New reference materials produced by LGC. 2 8 V A M B U L L E T I N C H E M I C A L N O M E N C L A T U R E A trivial system for pharmaceutical products Kevin Thurlow LGC P eople frequently question the value of systematic chemical nomenclature, assuming they give it any thought at all. However, it was slightly surprising to find the following passage in Agatha Christie’s, “A Caribbean Mystery”. One character tells us the victim was poisoned with “…what sounded like di-flor, hexagonalethylcarbenzol… The police doctor put it that way so that nobody should know, I suppose, what it really was. The stuff’s probably got some quite simple nice easy name…” Maybe nomenclators should be grateful for any mention of their activities in a popular novel, but it does help illustrate some of the usual misconceptions. The novelist worked in a dispensary in a hospital during the First World War, so doubtless had practical experience of the difficulty of some chemical names. Pharmaceutical products have a variety of names. There will be systematic names (necessary for notifying new chemicals), official ‘trivial’ names, and trade names. Systematic names give complete structural information. Official ‘trivial’ names, like International Nonproprietary Names (INN)1 will frequently contain some structural information. Trade names will probably give no structural information, although they may allude to the expected effects of the drug. Consider the well known ‘sedative-hypnotic’ depicted in Figure 1. The IUPAC name for this is: 7-chloro1,3-dihydro-1-methyl-5-phenyl-1,4benzodiazepin-2-one. This describes the structure unambiguously, and allows the structure to be deduced rapidly. This gives absolutely accurate information, rather than trying to keep it secret. However, it does have the obvious drawback that you would not want to ask for it by that name in your local pharmacy. It is clear that some sort of short name is preferable for everyday use, but this does have to be regulated. The World Health Organisation started the INN system in 1950 to fill this need. The idea was to have registered ‘trivial’ names, which would not clash with trade names or other generic names. You really want one authorised name. Proposed INNs are published, allowing interested parties to raise objections. If there are no objections, the INNs become recommended. The INN secretariat reviews any objections and takes appropriate action. Roger Trigg of British Pharmacopoeia Commission has explained the procedure in some detail2. INNs should be distinctive in sound and spelling, and should not be too long. It is useful for the name to reflect membership of pharmacologically related groups, not necessarily a great similarity in chemical structure. The aim is not to give large amounts of chemical information, or else you might as well use the systematic name. Anyway, it is easy to look up an INN and find out what it is. Figure 1, depicts diazepam(INN). The ‘-azepam’ tells the reader that it is a member of the ‘diazepam group’, with the characteristic ‘benzodiazepine’ fused ring structure. Figure 2 shows the ‘-azepam’ structure. R , R and R represent the various substituents on the ‘benzodiazepine’ ring structure. It will not be a surprise then that flunitrazepam(INN) also has a ‘benzodiazepine’ structure, with ‘fluoro-’ and ‘nitro-’ groups attached. Similarly, Nitrazepam(INN) has a ‘nitro-’ group. However, although temazepam(INN) has the characteristic structure shown in Figure 2, the name gives no hints regarding the nature of the other substituents. You might think this is 1 Figure 1: Diazepam. 2 9 2 3 V A M B U L L E T I N Figure 2: -azepam structure. a failure of the system, but the INN secretariat found that some prefixes were appearing much too frequently, so they now recommend using a neutral prefix, which does not give too much chemical information. The important idea is to have a short, unique, distinctive name. Lorazepam(INN) has ‘chloro-’ groups attached. You might expect this to be called ‘clorazepam’ or ‘chlorazepam’, but there was already a ‘clorazepate’, which also has a ‘benzodiazepine’ structure. The structural differences are sufficient to demand a different ending to the name. The INN secretariat is careful not to allow names that are too similar, particularly if they have similar medical actions. It is essential to avoid confusion over names, as the use of the wrong drug could lead to the death of a patient. Hence anything ending ‘-azepam’ will have related medical effects. More recent INNs do not necessarily give this much structural information, but ‘-caines’ are local anaesthetics, like lidocaine (formerly known as lignocaine), and ‘-profens’ are antiinflammatory drugs, like ibuprofen. Coming right up to date, anything ending ‘-mab’ will be a monoclonal antibody, from the brave new world of biotechnology. These generic names are very useful, particularly in the pharmaceutical industry and in medicine. However, manufacturers are going to want catchy trade names, to increase sales. Diazepam’s most common proprietary name is Valium, but there are many others. These include Serenack, Stress-Pam, Calmpose, Notense, Evacalm and Tensium. It is difficult to deduce any structural information from these names. You might guess they are benzodiazepines, but you could C H E M I C A L N O M E N C L A T U R E not be sure. However, it is fairly obvious what the intended effect of the drug is. The plethora of different trade names for diazepam does highlight the problem outlined earlier. It is quite usual for patients to grumble that their doctor has changed a medicine, and they hope the new drug will be as effective. The patient may well be receiving the same drug under a different name. The use of a variety of trade names for the same drug can cause confusion. Nobody will be F O R T H C O M I N G able to remember all of them. So all the types of names have their uses. The systematic name gives specific chemical information, but might be complicated. The INN gives general pharmacological information, with a simplified name. The trade name may give few clues as to the drug’s use, but should at least be easy to pronounce. REFERENCES 1. International Nonproprietary Names (INN) for Pharmaceutical Substances, Cumulative List No. 9, (1996) published by WHO, ISBN 92 4 0560165 2. Chemical Nomenclature, edited by K J Thurlow, published by Kluwer Academic, (1998), ISBN 0-7514-0475-6 For advice on chemical nomenclature, contact the VAM Helpdesk (page 2 and 32) and ask for the ‘Chemical Nom-enclature Advisory Service (CNAS)’. E V E N T S Training courses at LGC Good scientific practice for chemical analysis April 10–11, 2001 October 9–10, 2001 It is essential that analytical results are fit for purpose and meet the needs of those requesting the analysis. Implementing the six VAM Principles is one approach towards achieving this aim. This course is designed for laboratory managers and senior analysts, who are involved, or about to be involved, with the specification of analytical requirements and the selection of analytical methods to meet the needs of a client. It illustrates ways of implementing these principles and ensuring customer satisfaction cost-effectively. Statistics for analytical chemists May 9, 2001 September 25, 2001 January 10, 2002 This one-day training course is aimed at analytical chemists who need to use statistics and need a better understanding of the packages they use. The course starts from looking at the data and explaining what the statistical parameters that describe the data mean, as well as how to evaluate them. Theory is kept to a minimum but there will be ample opportunity for practice. Measurement Uncertainty (MU) Principles of MU June 6, 2001 November 13, 2001 February 5, 2002 Implementing MU principles in chemical testing June 7, 2001 November 14, 2001 February 6, 2002 The ability to estimate measurement uncertainty will give you and your customers CONFIDENCE in your results. These courses are ideally suited to analytical chemists who are involved in method development and method validation. The concepts of measurement uncertainty will be explained, as stated in the new EURACHEM/CITAC Guide, “Quantifying Uncertainty in Analytical Measurement”. The lectures and workshops will show how to calculate and apply measurement uncertainty by step-bystep instructions and clear worked examples. Method validation July 3–5, 2001 December 11–13, 2001 March 5–7, 2002 Method validation is a process that provides evidence that a given analytical method, when correctly applied, produces results that are fit for purpose. The course is designed for laboratory managers and analytical chemists, who are involved in method development and method validation. 3 0 V A M B U L L E T I N It will provide an understanding of method validation; its requirements and tools needed to carry it out. It will also demonstrate the link between method validation with measurement uncertainty and equipment qualification. ISO 17025 June 21, 2001 September 11, 2001 February 19, 2002 The standard ISO/IEC 17025: 1999: General requirements for the competence of testing and calibration laboratories is replacing ISO/IEC Guide 25, M10 and EN 45001. Accreditation bodies, such as UKAS in the UK, are using it as the basis of their accreditation. This course is designed to help testing laboratories that are currently accredited to manage the change. It also will help laboratories, considering accreditation, to plan their activities. These courses can be customised to suit an individual company’s needs and delivered in-house, if six or more staff require training. Further information is available from both the VAM and LGC’s websites (page 2 & 32) or by contacting: Lorraine Didinal, LGC (Address on Pages 2 and 32), Tel: 020 8943 7631, Fax: 020 8943 2767, Email: lad@lgc.co.uk. On-line registration is also available on the VAM web site. A D V E R T I S E M E N T “YOUR GATEWAY TO RELIABLE RESULTS” Launch of the new VAM web site By the time this edition of the VAM Bulletin goes to print, initial work should have been completed and the new VAM web site launched. This new site provides a vital source of information for anyone interested in valid analytical measurements. The site has been considerably updated with a wealth of new information and a new easy to use navigation system. Check out the site for yourself at www.vam.org.uk and register as a VAM member on-line (free of charge): to access even more information. Listed below is just some of the functionality and content you will discover when you enter this new gateway to reliable results. About VAM News ❖ find out more about VAM ❖ learn how to implement the ❖ ❖ ❖ ❖ VAM Principles ❖ check out the technical projects being undertaken read the latest VAM Bulletin on-line read the latest news review the Bulletin and news archive check out forthcoming events Communities Advice & Information ❖ give your views on one of the ❖ ask a question and seek bulletin boards technical advice ❖ find out the latest from any clubs ❖ review frequently asked or networks you belong to questions (FAQs) ❖ find help on a particular topic ❖ use the interactive guidance on buying analytical services & selecting laboratories Training & Education Publications ❖ search the library of reports, papers, ❖ find a suitable training course or seminar books and audio visual aids ❖ download many reports & papers for free ❖ order books and other ❖ make an on-line booking ❖ get guidance on laboratory skills and competencies products on-line ❖ obtain training & teaching resources ❖ enter PT competitions for schools ❖ check out the latest events for universities, colleges & schools 3 1 V A M B U L L E T I N F O R T H C O M I N G E V E N T S Other events National measurement conference – BEMC 2001 November 6–8, 2001 Majestic Hotel, Harrogate This conference is a 3-day parallel meeting devoted to advances in measurement technology and research practices within the UK. Workshops and seminars will be run during the conference to address common issues. Alongside technical sessions there will be an exhibition of measurement providers, instrument suppliers and accredited calibrated laboratories. Those interested in presenting a paper at the conference may, in the first instance, submit an abstract of not more than 200 words. Further information is available from: Hannah Edmunds National Physical Laboratory (Address on back cover) Tel: 020 8943 6260 Email: hannah.edmunds@npl.co.uk Web: www.nmpuk.co.uk C O N T A C T S Contact points For advice on: For information and advice on: • • • • • • Gas analysis; • ‘Availability of Gases Awareness Club’ Analytical quality assurance; Chemical nomenclature; Proficiency testing; Reference materials; Statistics. Contact: Paul Holland Environmental Standards Group Centre for Environmental and Optical Metrology NPL Tel: 020 8943 7174 Email: paul.holland@npl.co.uk Contact the VAM Helpdesk Tel: 020 8943 7393 Email: vam@lgc.co.uk Web site: www.vam.org.uk LGC Queens Road TEDDINGTON Middlesex, TW11 0LY Tel: 020 8943 7000 (switchboard) Fax: 020 8943 2767 Web: www.lgc.co.uk National Physical Laboratory (NPL) Queens Road TEDDINGTON Middlesex, TW11 0LW Tel: 020 8977 3222 (switchboard) 020 8943 6880 (NPL Helpline) Fax: 020 8943 2155 Web: www.npl.co.uk Aerosol Science Centre AEA Technology plc E6 Culham, ABINGDON Oxfordshire, OX14 3DB Tel: 01235 463677 Fax: 01235 463205 Email: aerosols@aeat.co.uk Produced by Horrex Davis Design Associates 3/01 3 2 V A M B U L L E T I N