KIDNEY INFECTIONS IN DIABETES MELLITUS
advertisement

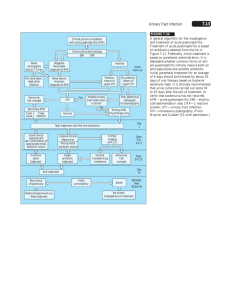
1 2 Review 12, Parliament Road, Middlesbrough, TS1 4LF United Kingdom Vuk Vrhovac Institute, University Clinic for Diabetes Endocrinology and Metabolic Diseases, Dugi dol 4a, HR-10000 Zagreb, Croatia KIDNEY INFECTIONS IN DIABETES MELLITUS Mahadeva Santhakumar Balachandar1, Pajica Pavkoviæ2, @eljko Metelko2 INTRODUCTION It is widely held that urinary tract infection (UTI) is more common in subjects with diabetes mellitus. Although there are relatively few recently published data, there is evidence suggesting that bacteriuria is more common in females but not in males with diabetes. Certain renal tract infections, including emphysematous pyelonephritis and cystitis, perinephric abscess, and candidiasis, show close association with diabetes mellitus. These, together with renal papillary necrosis, form the basis of UTI (1). Urinary tract is the most important and most common site of infection in diabetic patients. Diabetic patients have been found to have 5-fold frequency of acute pyelonephritis at autopsy than nondiabetics (2). The incidence of bacteriuria in diabetic men with good control of blood sugar is reported to be similar as in nondiabetic men, however, in pregnant diabetic women it is 2-4 times as common as in control groups (3,4). Most urinary tract infections in diabetic patients are relatively asymptomatic. The presence of diabetes predisposes to much more severe infections, especially in patients with poor diabetic control, acute ketoacidosis, or diabetic complications such as nephropathy, vasculopathy and neuropathy. This asymptomatic infection can lead to severe kidney damage and cause renal failure (5). Diabetologia Croatica 31-2, 2002 In Wheat’s review of the issue of infections and diabetes from 1980, 72% of 22 patients with emphysematous pyelonephritis, 80% of 19 patients with emphysematous cystitis, 57% of 250 patients with papillary necrosis, 36% of patients with prenephrotic abscess, and 10% of 130 patients with metastatic infection had diabetes (6). Bacteriuria is very common among diabetic patients because if unrecognizable and inadequately treated, it can lead to low grade foci of inflammation that can ultimately result in serious renal damage (7). Therefore, investigation of bacteriuria in diabetic patients by screening for urinary tract infection is very important to enable it to be properly treated to prevent the development of renal complications of diabetes and eventually severe renal damage and failure (8). CLASSIFICATION OF URINARY TRACT INFECTIONS ACCORDING TO TYPE OF INFECTION (9-11) Symptomatic UTI Asymptomatic UTI • • • • • acute recurrent chronic complicated uncomplicated 85 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS Localization of UTI Factors predisposing to reinfection A. Infections of upper urinary tract (12,13) • acute pyelonephritis • chronic pyelonephritis B. Infections of lower urinary tract (12,13) • cystitis • urethritis 1. incomplete voidance of urinary bladder Diabetes can increase proneness to various infections • Figure 1. Classification of uncomplicated and complicated UTI (9,12,13) Renal imaging No associated disease Cause of symptoms No kidney damage Abnormal Normal or abnormal with associated disease *Diabetes mellitus *Sickle-cell disease/trait *Analgesic abuse *NSAID abuse *Stones *Obstruction *Vesico-ureteric reflux reflux *Vesicourethral 'Complicated' FACTORS INFLUENCING RESISTANCE TO URINARY TRACT INFECTION (14-16) 1) hyperglycemia 2) ketoacidosis 3) neutrophil function 4) immune response 5) influence of endocrine systems 6) vascular insufficiency 7) neuropathy FACTORS CAUSING COMPLICATIONS IN DIABETIC PATIENTS WITH UTI (17,18) Factors predisposing to renal impairments 1. 2. 3. 4. calculus obstruction vesico-ureteric reflux papillary necrosis Factors predisposing to recurrent infections 1. 2. 3. 4. 5. 6. 86 • Impaired monocyte function Immune disorders (decrease in the levels of complement /C4 / and T-helper lymphocytes) (20) Infections can cause poor diabetes regulation (21) Risk of kidney damage and septicemia 'Uncomplicated' Impaired chemotaxis, phagocytosis, adhesion, intercellular destruction of microorganisms • Normal Impaired neutrophil function (15-17) calculus scars/spongy medulla prostatitis foreign bodies in the urinary tract urinary tract fistula (vesicoenteral) congenital anomalies • increased secretion of counterinsular hormones (glucagon, cortisol, growth hormone and catecholamines) • insulin secretion inhibition (sympathicus) • insulin resistance of peripheral tissues (increased cytokine secretion) (22) PATHOPHYSIOLOGY OF URINARY TRACT INFECTION Infection pathways (23) - ascending - hematogenic Infection factors (23,24) - number of microorganisms - virulence of microorganisms Defense mechanisms of the host (23,24) - length of the urethra - vesicourethral valvula - increased liquid intake and frequent urination - complete voidance of urinary bladder Abnormalities of urinary tract (19,23,24) - obstruction - vesico-ureteric reflux - foreign bodies - incoplete voidance of urinary bladder M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS The etiology of urinary tract infection includes transfer of organisms from remote foci in the body, from adjacent sources via bloodstream or lymph channels, or through urinary orifices; urinary tract obstruction by stone or other urinary bladder abnormality due to autonomic neuropathy or after catheterization of the urethra; residual urine in the urinary bladder is a medium favoring the growth of organisms (23,24). Acute urinary tract infection is more common in diabetic women due to the short urethra readily accessible to organisms from the vagina and rectum (23,24). Bladder has been confirmed to possess an inherent resistance factor, which is necessary for the maintenance of uninfected state. The frequency of bladder emptying helps reduce the bacterial factor in the bladder and is thought to be essential. Pyelonephritits usually develops secondarily to lower urinary tract infection, as shown in Table 1 (25). The most common agents of UTI in diabetic patients are: - bacteria - viruses - fungi - tuberculosis Table 1. Bacterial infections Fungal infections Viral infections Cystitis Invasive candidiasis * Cystitis * Emphysematous cystitis * Renal actinomycosis Pyelonephritis Pyelonephritis Vulvovaginal candidiasis * Emphysematous pyelonephritis* Papillary necrosis Perinephritic abscess * close association with diabetes mellitus Bacteria Many different microorganisms can infect urinary tract in diabetic patients, but the most common agents of bacteria are gram-negative bacilli; Escherichia coli causes approximately 90% of acute UTI in diabetic patients without urologic abnormality or calculi. The other bacilli involved, i.e. Protease, Klebsiella, Enterobacter, Serratia and Pseudomonas, account for a lower proportion of uncomplicated infections (26,27). These organisms are of increasing importance in recurrent infection of UTI in diabetic patients, Diabetologia Croatica 31-2, 2002 especially after urologic manipulation such as the placement of catheters for urine retention due to autonomic neuropathy and urinary bladder vasculopathy (25,28,29). The Protease species by virtue of urea production and Klebsiella species through production of extracellular slime and polysaccharides predispose to stone formation (23,29). Gram-positive cocci play a less important role in UTI in diabetic patients. Enterococci and Staphylococcus aureus can cause bacteremic infection of the kidneys and consequential renal damage. A saprophytic novobiocin resistant coagulase negative staphlycoccus has been recognized as an important cause of acute symptomatic UTI in a young diabetic female (30). Chlamydia trachomatis appears to be an important etiologic agent (23). Viruses Viruses can cause pyelonephritits in animals and may increase the susceptibility of the kidneys to infection with coliform bacteria (23). In humans, viruses are most commonly found in urine samples without evidence of acute UTI in diabetic patients, although some adenoviruses have been implicated as a cause of cystitis (31). Fungi Fungal infection of UTI in diabetic patients is important but clinically insignificant. Diabetic patients with urinary tract Torulopsis globrata infection account for 20%-90% of all infections with this Candida species. Torulopsis globrata can cause cystitis, pyelonephritis, renal or perirenal abscess, fungus ball, and a picture of gram-negative sepsis (33,34). The presence of candida at a rate of >10000 colonies/m2 urine indicates candidal infection, but diabetic patients with indwelling catheters may have higher counts with no significant evidence of infection by candida, which may lead to chronic infection with severe renal damage (29). Tuberculosis Tuberculosis is a serious comorbidity in diabetics, in whom it is more extensive and 3-16 times more common than in nondiabetics (24). The association of UTI and tuberculosis is very common in diabetic patients due to the impaired body defense 87 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS mechanisms. Not all diabetic patients with microscopic hematuria should be automatically regarded as glomerulonephritis as a diabetic complication before renal tuberculosis has been excluded (23,35). It may still be advisable to recommend periodic tuberculin test in diabetic patients. Those who have never been tested should receive underdose medical supervision, prophylactic treatment with isoniazid pyridoxine for a year due to the greater severity of tuberculosis in diabetic patients (24). BACTERIURIA Bacteriuria is the presence of bacteria in the urine. The concept of significant bacteriuria was introduced to distinguish between the bacteria that are actually multiplying in the urine from the contaminants in collecting vessels, periurethral tissues and urethra, and gross fecal or vaginal contamination (36). The criterion for significant bacteriuria is the presence of 100,000 colonies/ml urine or more, and is an excellent operational definition. When the clean void method is used for sample collection, it is based on the finding of contaminants at a number ranging from 1000 to 100,000 colonies/ml urine. The organisms found in urinary tract infection grow well in urine and may reach from more than 100,000 colonies/ml to 10 million colonies/ml, however, with proper instructions, cleaning and refrigeration of urine samples, and with prompt processing of the culture it is usually not difficult to obtain reliable results and make an accurate diagnosis of UTI in diabetic patients with asymptomatic infection (37). The examination and screening procedure for bacteria generally require 2 or 3 consecutive positive specimens, indicating that the patient has a persistent significant bacteriuria. A bacterial count lower than 100,000 colonies/ml urine may occur in patients with bacteriuria, but when the clean void method is used these counts become valid when shown to be persistent and when the same species of bacteria are present. The bacterial count becomes higher when the urine is allowed to incubate for long time, so the first morning specimen is preferred but not essential. Low or borderline counts may be due to diabetes in properly hydrated patients. Aseptic methods of urine collection from renal pelvis or urethra, or by bladder puncture in specific situations permit the diagnosis of significant bacteriuria in diabetic patients regardless of the organism count (38,39). 88 Differentiation between the upper and lower urinary tract infection is performed by studying the antibodies coating the bacteria in urine specimens. Urine specimens are tested for the presence of antibody coated bacteria by direct immunofluorescence with the use of fluorescein-conjugated horse antiserum to human globulin (40). Gram-positive bacteria are antibody coated to a lesser extent than gram-negative urinary pathogens, because of the less frequent renal parenchymal invasion or lower immunogenicity. The bacteria infecting the kidneys are only coated by antibody and this infection does not occur in the lower urinary tract segment (41). Causes of bacterial persistence (42) - infected renal calculi - chronic bacterial prostatitis - unilateral infected reflux nephropathy - medullary sponge kidneys - infected necrotic papillae - infected urachal cysts - vesicovaginal fistulae - vescointestinal fistulae - ectopic ureter draining dysplastic renal segment - foreign body Several controlled studies have examined the prevalence of bacteriuria in diabetic compared with nondiabetic subjects (43,44). Bacteriuria predisposes to cystitis and upper UTI (8). Of 12 studies, 75% reported a higher (2- to 4-fold) prevalence of bacteriuria in diabetic subjects. Nearly all these studies chose cases among diabetic subjects attending outpatient clinics. Since clinic attendance is probably related to the underlying disease severity, it is possible that these studies included diabetic subjects with a more severe disease and comorbid conditions, who were therefore at a higher risk of bacteriuria. One study that sampled diabetic and control nondiabetic subjects from a defined community still found a higher prevalence of bacteriuria associated with diabetes (5). Thus, these data support the concept of the higher prevalence of bacteriuria in diabetic subjects. There are no data on the incidence of bacteriuria associated with diabetes, as presented in Table 2. M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS Studies on the association between diabetes and bacteriuria 1989 – 1991 Table 2. Outcome Outcome Description of subjects prevalence (%) Definition Diabetic Adjustment Nondiabetic Diabetic Nondiabetic Factors Asymptomatic Outpatients; Outpatients; F:18.0 F:6.0 Bacteriuria F:54, M:37 F:337, M:102 M:5.0 M:4.0 Bacteriuria Outpatients; Casualty dept; F:19.8 F:18.7 Similar age and F:91, M:59 F:91, M:59 M:1.7 sex distribution M:3.3 None Several uncontrolled case series demonstrate a low prevalence of bacteriuria in children with IDDM. In two studies, the prevalence of bacteriuria was 1.6%2.0% among 266 girls aged 6-15 years attending a diabetes summer camp (45), and 1% in 304 girls and 0 in 337 boys attending regular follow-up appointments at a diabetic clinic (46). Although these female rates may exceed those of nondiabetic children, the data suggest that bacteriuria is quite uncommon in girls and boys with IDDM. Longer duration of diabetes but not of glucose control is associated with higher bacteriuria prevalence. A statistically significantly longer diabetes duration was recorded in diabetic subjects with than in those without bacteriuria (47). The prevalence of bacteriuria increased 1.9-fold for every 10 years of diabetes duration (5). However, there was no association between longterm glucose control as reflected by the glycosylated hemoglobin level, and the prevalence of bacteriuria (5,47). and, with two exceptions (34,35), is two- to threefold that in nondiabetic females. As for males, only Bahl et al. (1970) (53) recorded a difference. From a restrospective hospital-based survey of infection, MacFarlane et al. (1986) (54) have concluded that diabetes mellitus increases the risk of bacteremia. Escherichia coli was the most common pathogen and the principal source was urinary tract. In a similar prospective study in diabetic individuals, Leibovici et al. (1991) (55) confirmed the importance of urinary tract as a source for bacteremia. In this study, Klebsiella spp. were more frequent isolates in diabetic (25%) than in nondiabetic individuals (12%) when urinary tract was the source of infection. In an uncontrolled study, Forland et al. (1977) (40) investigated the localization of infection in the renal tract of diabetic subjects and found 19% of women and 2% of men to have UTI. Using antibody-coated bacteria, 43% of a selected group of 42 patients had evidence of renal parenchymal diseases, which rose to nearly 80% over a week period in the absence of treatment. Out of the total of 333 patients, 22 had recurrent infections following treatment, i.e. 14 cases of reinfection (recurrence with a different organism or serotype) and 8 cases of recurrent infection with the same organism or serotype. Table 3. Summary of available studies on urinary tract infection in diabetic subjects Study Diabetic Nondiabetic Total Bacteriuric Total (%) Bacteriuric (%) Women CLINICAL PICTURE OF KIDNEY INFECTIONS IN DIABETES Urinary tract infection Autopsy studies suggest that urinary tract infection is more common in diabetes mellitus (48,49). Such studies, which primarily examined upper renal tract disease are, by virtue of case selection, open to bias. In one study, 6.8% of diabetic subjects had pyelonephritis, as compared to 1.6% of normal subjects (48). Even this difference may be an overstimate as some other conditions, e.g., ischemia, renal papillary necrosis, and reflux nephropathy, may mimic pyelonephritis (50). Controlled clinical studies suggest that bacteriuria is more common in diabetic females but not in males. Table 3, adapted from Wheat (6), summarizes these studies. The proportion of females with diabetes and bacteriuria is consistent in these studies (9%-20%) Diabetologia Croatica 31-2, 2002 Kass HE (1957) /43/ 54 337 6 20 91 19 19 114 8 11 100 3 81 18 81 4 44 16 27 15 341 9 100 5 2 O’Sullivan DJ, Fitzgerald MG, Meynell MJ et al. (1961) /51/ 91 Vjelsgaard (1966a) /61/ 128 Bahl AL, Chugh RN, Sharma KB (1970) /53/ 97 Ooci BS, Chen B, Yu M. (1974) /66/ Williams DN, King H, Harris DM (1975) /52/ Schmitt JK, Fawcett CJ, Gullickson G (1986) /47/ 18 Men O’Sullivan et al. (1961) 59 3 59 Vjelsgaard (1966a) 141 1 146 2 Bahl et al. (1970) 97 11 100 3 Ooi et al. (1974) 67 8 67 3 Harkonen et al. (1977) 37 5 102 4 Schmitt et al. (1986) 411 1 100 0 89 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS Prior instrumentation may increase the risk of bacteriuria in diabetic and nondiabetic patients (56), this paper suggesting doubling of the risk. All UTI may be asymptomatic (45), however, a significant proportion of these may lead to a more severe infection (6). antibiotics is recommended for pyelonephritis (64). A cephalosporin, given i.v. for 48 hours, is suitable. Where relapse occurs, a six-week treatment is advocated (65). The factors that predispose diabetic individuals to infection are ill-understood. Many factors may combine to increase the frequency of infection, including autonomic neuropathy leading to delayed bladder emptying (57), diabetic nephropathy, and impaired host defense mechanisms (58). The prevalence of UTI in diabetic nephropathy is reported to be 13% (59). Sawers et al. (60) found bacteriuria to be more common in diabetic women with autonomic neuropathy, but surprisingly they found no evidence for increased residual urine volume after micturition. The duration of diabetes (61) and the presence of complications (47) have been related to increased bacteriuria in diabetes. Asymptomatic bacteriuria is common in diabetic women, particularly in the older age group, with Escherichia coli being the most common isolate. Untreated infections may lead to renal parenchymal infection which may impair renal function (66). Accordingly, all UTIs should be treated regardless of symptoms. In case of recurrent UTI, imaging of the renal tract should be done to exclude underlying pathology (e.g., renal papillary necrosis); it may indicate the need of prolonged antibiotic therapy. Treatment for at least 6 weeks may be required. In alloxan-induced diabetic rats, Obana et al. (62) found greater adherence of Serratia marcescens to bladder epithelial cells than in control animals. Contrary to what one might expect, poor glycemic control does not contribute to the frequency of UTI. Schmitt et al. (47) found no relationship between HbA1c and bacteriuria in 752 NIDDM patients. In another study, the mean HbA1c was 11.5% in bacteriuric and 11.4% in non-bacteriuric diabetic women (58). Pathogens Escherichia coli is the most common organism isolated. Schmitt et al. (47) found it to account for 75% of isolates from female diabetics. There is no significant difference in bacterial isolates between diabetic and control subjects, although bacterial counts are generally higher in the former (6). Group B streptococcus is said to be more common in diabetic subjects with pyelonephritis (59). Therapy Positive urine cultures (i.e. >10 organisms/ml) should be treated in diabetic individuals even if asymptomatic. The choice of antibiotic should reflect the sensitivity of the organism, and treatment does not differ between diabetic and nondiabetic individuals, although some authors prefer longer duration of treatment in diabetic patients (63). A 14-day course of 90 Conclusion INFECTIVE TUBULOINTERSTITIAL NEPHRITIS This poorly recognized cause of infection and occasionally of acute renal failure in unobstructed kidneys was initially documented in two series by Richet and Mayaud (1978) in 30 cases (67), and by Baker et al. (1979) in five cases (68). The larger series included histologic evidence for an acute infection with microabscesses. The condition was reviewed by Cattell (1992) (69). From the published series it appears that diabetes is a risk factor for acute renal failure in this group (69,70), along with analgesic abuse, nonsteroidal drug use, sickle-cell disease (or trait), and possibly alcoholism. ACUTE RENAL FAILURE Although definite data are lacking, there is an impression that diabetic patients may be more likely than nondiabetics to suffer renal failure as a result of UTI and subsequent pyelonephritis and septicemia. Apart from infective tubulointerstitial nephritis described above, this may result from the failure of autoregulation of renal blood flow with falls in blood pressure in patients with even moderate degrees of diabetic nephropathy (71). RENAL TRACT CANDIDIASIS Diabetes appears to be more common in individuals with fungal UTI. It is present in up to 90% of patients with Candida glabrata (formerly Torulopsis glabrata) (72,73). Candida albicans was cultured from the urine of M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS 35% of patients with diabetes mellitus as compared to only 9% of controls without glycosuria (74). Candida gives rise to a wide variety of infections of the renal tract. Although many are asymptomatic, they may lead to cystitis, pyelonephritis, renal abscesses, and fungal ball formation (72,75). It has been concluded that a significant proportion of candidal infection in the urinary tract remains to be fully established (76). A value of >10000 organisms/ml is currently taken to reflect infection (77,78). Infection may originate from the genital region, gastrointestional tract, or by hematogenous spread. There is no agreement as to whether all infections in diabetic patients should be treated. Indeed, control of blood sugar may be all that is required to clear the urine. Symptomatic individuals and those with candiduria accompanied by pyuria should receive antifungal therapy. Patients with indwelling catheters that cannot be removed may be treated with intermittent instillations of amphotericin B. Where oral therapy is adequate, flucytosine is advocated (79). Alternatively, fluconazole may be used (80). Most lower UTIs clear within two to three weeks (76). Renal candidiasis often requries a more aggressive approach, including irrigation of the renal pelvis with antifungal drugs, oral or parenteral therapy, and sometimes surgical intervention. Amphotericin B alone or in combination with flucytosine is the treatment of choice (81). PERINEPHRIC ABSCESS This type of abscess generally develops in renal parenchyma, spreading therefrom to the surrounding fascia. Diabetes is present in 30%-40% of cases (82-84). Bilateral abscesses are rare, occurring in only 2% in one series (83). A single case of association with type 1 diabetes mellitus has been described (85). The infection may arise from an ascending UTI or by hematogenous spread. The onset is frequently insidious with symptoms often present for more than five days. These include flank discomfort, nausea, vomiting, dysuria, and occasionally hematuria. Two thirds of patients have flank tenderness or a mass (86). Laboratory investigations may show an elevated white cell count or erythrocyte sedimentation rate (ESR) but are not helpful in diagnosis. If the abscess, blood and urine cultures are examined, then more than 90% show positive culture (84). Diabetologia Croatica 31-2, 2002 Intravenous urogram (IVU) is often abnormal, but ultrasound (US) or computed tomography (CT) scan offer the best hope for a positive diagnosis. Sometimes a delayed and repeat scanning is necessary before the abscess becomes apparent. US or CT scan-guided aspiration of the abscess may then follow. As in other renal tract diseases, gram-negative organisms are most commonly isolated. Anaerobes and gram-positive cocci, including Staphylococcus aureus, are also frequently found. Treatment includes intravenous antibiotics and control of hyperglycemia if present. Surgery includes percutaneous drainage of the abscess, or nephrectomy. Relief of obstruction caused by renal calculi may be required. The mortality associated with this condition is now less than 20% (59). Early diagnosis with the increasing use of isotope scans and direct imaging techniques should help reduce this figure further, but increased awareness of the diagnosis by a nonspecialist is required. RENAL PAPILLARY NECROSIS The pathogenesis of this condition is poorly understood. Infarction of the renal papilla is most likely involved, with infection probably as a contributory factor. Autopsy studies indicate that renal papillary necrosis (RPN) is more common in diabetic subjects (48,87). Of 859 diabetic patients autopsied in the first series, 29 (3.4%) and 16 of 307 (5.2%) in the second study showed papillary or pyramidal necrosis. The prevalence in cases of pyelonephritis was much higher, reaching 25% (87,88). The prevalence of RPN in nondiabetic subjects was 0.07% in these two studies. The prevalence of RPN based on autopsy studies may be an underestimate. A prospective study of 76 IDDM individuals showed a prevalence of 23.7% (89). Given that the upper limit of creatinine in the individuals in this study was 200 µmol/l, even this may be an underestimate. The relationship between RPN and diabetes can be traced back to Turner (1888) (90). However, Froboese (1937) (91) and Gunther (1937) (92) are to be given credit for having firmly established the link with diabetes mellitus. In their combined series of 20 subjects, 17 (85%) had diabetes mellitus. Drawing extensively on the German literature, Mandel (1952) (93) identified a total of 160 cases of RPN prior to 1950. Of these, 96 (60%) were diabetic. More recent estimates suggest that the proportion may be nearer to 30%, with obstructive uropathy accounting for the remainder. 91 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS Clinical presentation The presentation of RPN may be acute or chronic. The former may be fulminant with flank pain, fever and septicemia. It is generally unilateral. Papillae may slough, leading to renal colic. The affected kidney may be enlarged (94). The chronic indolent form is more commonly observed. Here changes are often bilateral. Unilateral papillary necrosis suggests the presence of renal artery stenosis or atrophy associated with previous ureteric obstruction. The changes in the kidney may be patchy with the papillae exhibiting differing degrees of necrosis. RPN is most usually seen in the 6th and 7th decades of life (93), women being more often affected than men (3:1) (95). Microscopic hematuria is a common finding, occurring in 44% of diabetic subjects with RPN (89). A previous history of UTI was found in 68% of cases (89). Renal insufficiency develops in 15% (96). The diagnosis should be suspected in a subject with UTI who responds poorly to antibiotics, or develops unexplained renal failure (95). Diagnosis and treatment The diagnosis of RPN depends on the demonstration of papillary/caliceal abnormalities without focal loss of renal tissue (94). Intravenous urography (IVU) is the most sensitive investigation for RPN but it is not frequently used today because of the adverse effect of contrast media on renal function in diabetic subjects. Contrast studies were observed to contribute to worsening of renal function in 50% of diabetic subjects with pre-existing renal impairment (97). However, a more recent assessment in IDDM individuals with creatinine values below 200 µmol/l using newer nonionic contrast agents (Isopaque and Omnipaque) did not demonstrate any adverse effect (89). Clinically apparent RPN is now relatively rare (98). The reasons for this are unclear, but early identification and treatment of UTI, together with a reduced frequency of IVU examinations may contribute to both real and apperant decline. Improved metabolic control cannot be discounted. Treatment includes aggressive antibiotic therapy when infection is demonstrated. Relief of obstruction may also be required. The prognosis for this condition is not well defined. Patients may have well-preserved renal function despite extensive papillary damage (99). In others, progression to renal failure may be inexorable, 92 although this may be due to the underlying parenchymal disease (e.g., diabetic glomerulosclerosis) rather than renal papillary necrosis. EMPHYSEMATOUS RENAL TRACT DISEASE Introduction The presence of gas in the renal tract is relatively uncommon but shows strong association with diabetes mellitus. Pneumaturia was first described as early as 1671 (cited in Taussig, 1907) (100), but this and subsequent observations often failed to specify the origin of gas, which may arise in three ways: 1) prior instrumentation, 2) vesicocolic or vesicovaginal fistula, and 3) spontaneous gas formation in the bladder. Raciborski (1860) (101) is credited for the first description of spontaneous gas formation. An earlier much quoted case described by Brierre de Bosmont (cited in Kelly and MacCallum, 1898) (102) is open to doubt. The link with diabetes mellitus was recognized early by Guiard (1883) (103) who described four cases of pneumaturia in association with glycosuria. The first substantive literature review by Kelly and MacCallum (1898) (102) reports on 9 of 16 cases of pneumaturia to have glycosuria. The validity of some of these cases is questioned by Turman and Rutherford (1971) and Zabbo et al. (1985) (104,105). The classification of emphysematous renal tract disease (ERTD) presents a problem. That of Turman and Rutherford (1971) (104) is most logical and comprehensive (Table 3). However, some of these (e.g., emphysematous ureteritis) may not exist as distinct entities, whilst two or more, such as emphysematous pyelonephritis and perirenal gas may occur in combination. It is proposed to concentrate on the three conditions most commonly seen, i.e. emphysematous pyelonephritis with perirenal gas, emphysematous pyelitis, and emphysematous cystitis. All early descriptions of pneumaturia where a fistula was excluded probably referred to emphysematous cystitis, as emphysematous pyelonephritis rarely gives rise to this symptom. The pathogenesis of ERTD is incompletely understood. Early theories (106) that the gas, usually CO, although N, H, O and CH (methane) are also present, is a product of glucose fermantation, still hold today (107,108). Obstruction, and perhaps diabetic M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS microangiopathy, may facilitate the development of infection. Why occasional cases are seen in nondiabetic individuals is not known. Impaired vascular supply may be important. Schainuck et al. (1968) (109) have proposed that necrotic tissue may act as a substrate for gas-forming organisms. Subramanyam et al. (1980) (110) have described a case of emphysematous pyelonephritis following traumatic renal infarction and tissue necrosis. Table 4. Classification of emphysematous renal tract disease Collecting system Outside of renal tract Tissue Kidney – Emphysematous pyelonephritis Perirenal gas Pelvis Intrapelvis gas Emphysematous pyelitis – Ureter Intraureteral gas Emphysematous ureteritis Periurethral gas Bladder Intracystic gas Emphysematous cystitis Pericystic gas Emphysematous pyelonephritis It is defined as the presence of gas in renal parenchyma and is often associated with perirenal gas. It is a severe life-threatening necrotizing infection with a mortality in excess of 60%. No substantive series of this condition exists, although there are some literature reviews (111-113). Clinical features Ninety percent of cases of emphysematous pyelonephritis are associated with diabetes mellitus (113), although no breakdown of the type of diabetes is given. The mean age reported is 54 (range 19-81) years, and women are affected twice as often as men. In contrast to earlier findings that the left kidney was more commoly affected (112,114), a more recent survey (113) found no difference. Bilateral involvement in association with diabetes has been described in rare cases (105,115,116), and in several cases in transplanted patients (117-119). The clinical presentation is often suggestive of severe acute pyelonephritis, but it may also have an indolent course over several months. In 43 cases the average duration of symptoms was 21 days, ranging from less than a day to eight months (112). Nausea, vomiting (40%) and abdominal pain (55%) are common symptoms. Fever is seen in 80% (113). A palpable mass Diabetologia Croatica 31-2, 2002 is rare as in crepitus, although abdominal tenderness is common. Pneumaturia is not found unless there is coincidental emphysematous cystitis. Laboratory investigations Neutrophil leukocytosis is observed in the majority of cases. Pyuria is found in 96% (113). Pre-existing renal function was abnormal in 82% of 36 subjects (112). Microbiologic investigation shows Escherichia coli to be the most common causative organism. In one series, 68% of cases were due to this organism and 9% due to Klebsiella (120). Multiple organisms were found in 14%. Other organisms are rare and many are reported as single cases. Candida albicans (105), Candida tropicalis (121), cryptococcus (116), and anaerobic streptococcus (43) have been recorded. Clostridium spp. has not been described (112). Pathologic examination of the tissue will often reveal an acute inflammation of the interstitium with multiple micro- and macroabscesses. Diagnosis Renal tract emphysema is a radiologic diagnosis as the symptoms and signs are a little different from other renal infections. The diagnosis should be suspected in any diabetic patient with nausea, vomiting and abdominal pain, particularly where antibiotic therapy fails to improve an ’acute pyelonephritis’ in three to four days. Plain abdominal x-ray or IVU will reveal gas in 50%-80% of cases (112,113). Contrast studies should be used judiciously in diabetic subjects with impaired renal function. Langston and Pfister (1970) (122) describe radiologic features of emphysematous pyelonephritis and highlight three patterns. An initial mottling with gas in the renal parenchyma is followed by the development of a crescent of gas surrounding the parenchyma. Finally, extravasation of gas occurs through Gerota’s fascia into the retroperitoneal space. Such x-ray features are, however, seen in relatively few cases. A simplified classification is suggested by Michaeli et al. (112): stage 1: gas in the renal parenchyma or perirenal tissue stage 2: gas in the kidney and surrounding tissues stage 3: extension of gas through Gerota’s fascia Where there is some doubt, or where delineation is important, the patient should have a CT scan. 93 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS Therapy and prognosis The prognosis for emphysematous pyelonephritis depends on the mode of therapy and the extent of infection. The highest mortality, 80%, is seen in individuals where the infection extends into the perirenal space and who receive medical treatment alone (123,124). Mortality rates of 60% are recorded when infection is confined to the renal parenchyma and is treated with antibiotics plus local drainage. A combination of fluid replacement, intravenous antibiotics and nephrectomy may reduce the mortality rate to 20%. Comparison of these data should allow for improved diagnostic techniques and resuscitation, but it is evident that there has been little change in mortality. Emphysematous pyelitis In this condition, gas is limited to the collecting system. Evanoff et al. (113) found the condition to be more common in women (3:1), with an overall mean age of 51 (range less than one to 79) years. In contrast to emphysematous pyelonephritis, only 59% of subjects had diabetes, presumably due to a higher proportion of patients with obstruction in this group (64% vs. 37%). For reasons that are not clear the left kidney was affected more commonly than the right one (53% vs. 36%), and bilateral gas was rarely observed (125). The total number of cases is not documented. The symptoms are similar to those in emphysematous pyelonephritis. Escherichia coli is again the most common organism. Radiology reveals gas in the collecting system. US or IVU may demonstrate an obstruction. Gas occurs rarely in the bladder in association with emphysematous pyelitis (124). Histopathologic studies in this condition show acute inflammation with submucosal hemorrhage of the renal pelvis and ureter. Renal parenchyma may show an acute interstitial reaction (127). Treatment for this condition includes antibiotic therapy with relief of obstruction where necessary. The reported mortality is 18% (113). emphysematosa (52 cases). Bailey, who considered them to be the same entity, added another 19 cases to the literature. Diabetes mellitus was recorded in 29/46 and 13/19 cases, or in total 51% of the combined series. Why the proportion of diabetes should be so low in the second group is not clear. Women were more common (2:1) in Bailey’s series. Mean age was 54 (range 25-83) years. The mean duration of diabetes was 14 (0-35) years. Escherichia coli is the most common infecting organism. Enterobacter is also relatively common, and there are occasional reports of Proteus species, Staphylococcus aureus, Streptococcus species, and yeasts (128). Gross hematuria is a common finding. Eleven of 19 cases had gas in the lumen and in the bladder wall, two in the wall, and six in the lumen alone. Autopsy studies in two patients showed acute and chronic submucosal inflammation with vesicles and ulcerated areas in the mucosa. X-rays of the bladder are characteristic with a narrow radiolucent line of gas bubbles outlining the bladder wall that may resemble cobblestones (94). IVU or US may demonstrate an obstruction (e.g., prostatic hypertrophy). In contrast to the conditions discussed above, the prognosis for emphysematous cystitis is good and no death as a direct consequence of the infection was seen in Bailey’s series. Conclusion ERTD, although uncommon, may be associated with a high mortality. Many cases are associated with diabetes mellitus. A high index of suspicion, appropriate radiologic assessment, and early surgical intervention offer the best hope for cure in emphysematous pyelonephritis. Emphysematous pyelitis and cystitis are more benign and are treated with antibiotics alone or a combination of antibiotic and relief. Emphysematous cystitis METHODS TO DETECT KIDNEY INFECTIONS IN DIABETES The early descriptions of pneumaturia in the absence of fistulous communication or instrumentation must have referred to this condition. Prior to the review of Bailey (128), gas production was arbitrarily described as primary pneumaturia (46 cases) or cystitis Dip slide culture technique Filter paper method (122,130) Pipette method (122,130) Cup method (122,130) Pad culture method (122,130) 94 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS Alternative methods for detecting urinary leukocytes and bacteria There are a number of rapid techniques for detecting urinary leukocytes and bacteria. Most are highly specific but not particularly sensitive. For these reasons, the tests are recommended primarily for screening purposes in settings where the anticipated prevalence of infection is low and falsely positive results are undesirable (129). * leukocyte esterase test * nitrite test (Greiss test) * automated systems for detection of bacteria Bacteriologic criteria for diagnosis of UTI (8) • 108/ml from two consecutive midstream urine (MSU) specimens • 108/ml from one MSU uncentrifuged specimen, leukocyte count 10/mm • 108/ml from one MSU specimen with symptoms of cystitis or acute pyelonephritis • 107/ml from one MSU specimen and any number from suprapubic aspiration • pure growth of the same bacteria unserotyped Table 5. Radiologic guide to urinary tract infection Patient group IVP - Intravenous pyelography Micturition cystogram Children 4 years After one infection After one infection Recurrent infection Children 4 years Recurrent infection Renal scar or ureter on IVU Women with bacteriuria Recurrence soon after short courses of Abs IVU suggestive of urinary obstruction Women with cystitis Onset in middle age or unassociated with severe cystitis; rarely helpful in young adult Rarely helpful Women with acute pyelonephritis After one infection Rarely helpful Men diabetic After 1-2 episodes of infection whether overt or symptomatic IVU of shows abnormality Elderly men and women Consider early of symptoms of recurrent onset Rarely helpful High risk patients High obstruction urolithiasis, persistent pyuria Rarely helpful Symptomatic women (131): >102 coliform organisms/ml urine plus pyuria or >105 of any pathogenic organism/ml urine or any growth of a pathogenic organism from urine obtained by suprapupic aspiration above the minimal inhibitory concentration of the pathogen (133). There are some agents that do not achieve an adequate serum level to destroy gramnegative bacteria, so these agents must not be included in the treatment of UTI. Therefore, urine culture and sensitivity should be done in each patient (133,138). Symptomatic men (131): >103 pathogenic organisms/ml urine Diabetes control during UTI Asymptomatic patients (132,142): >105 pathogenic organisms/ml urine in two consecutive samples MANAGEMENT OF RENAL INFECTIONS IN DIABETIC PATIENTS The goals of treatment for urinary tract infections in diabetic patients are to relieve acute symptoms and to treat or prevent sepsis, and ultimately to prevent or retard the progression of renal parenchymal damage (35). The general principle of antibiosis is to deliver an adequate concentration of an effective antimicrobial agent to the site of infection. It depends upon whether the pathogen is sensitive or the agent can penetrate or be delivered to the site of infection at a concentration Diabetologia Croatica 31-2, 2002 ¾ Outpatients (134,135): • frequent glycemic control (at 4-hour intervals) • higher insulin dose for correction of hyperglycemia • sufficient liquid intake • consultation with a diabetologist/nephrologist • urgent hospitalization in case of: vomiting development of hyper- or hypoglycemia ¾ Inpatients (134,135): • frequent glycemic control (at 2-hour intervals) • higher insulin dose for correction of hyperglycemia - intravenous rapid acting insulin when required • starting insulin therapy in patients on oral therapy 95 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS • sufficient liquid intake - intravenous liquid intake when required ¾ There are many important principles for the management of UTI infection in diabetic patients: • quantitative urine cultures should be obtained to identify the organism before starting the treatment (136) • antimicrobial sensitivity testing should be used to control infection and significant bacteriuria in diabetic patients (41,137) • good treatment for factors predisposing to infection such as neurogenic bladder, ketoacidosis, indwelling catheter, or any obstruction (28) • good control of blood sugar level and careful treatment of ketoacidosis leads to control of UTI by switching the patient to regular doses of plain insulin 3 or 4 times daily if he was on diabetic diet or oral hypoglycemic agents (28) • regular insulin dose must be increased by 20% of total daily requirement as an average initial dose for supplementary use when there is glycosuria (138) • relief of clinical symptoms does not indicate bacteriologic cure and follow up cultures after therapy should be obtained (138) • bacteriuria must be diagnosed and treated completely to prevent renal damage (139) • lower UTI responds well to low doses and short duration therapy; reinfection indicates lower UTI (139) • upper UTI needs high doses and longer treatment; relapse indicates upper UTI (139) • community acquired infections, especially initial infection, are nearly always due to antibiotic resistant strains There are no antimicrobial agents that are contraindicated for diabetic patients, however, the above principles should be followed to improve antimicrobial therapy in these patients. We must know the types of infection that frequently occur in diabetic patients and the strains causing them. Also, the knowledge of microbial sensitivity will improve the usefulness of antibiotics in diabetic patients and reduce the rate of complications (134). Frequently, diabetics who are most prone to developing infection have some impairment of renal function. Renal dysfunction is the most important host factor for the choice of non-nephrotoxic drugs and antimicrobials (131). If nephrotoxic drugs are to be 96 used in an emergency, they should be switched to a drug that is not nephrotoxic as soon as culture results are available or immediately upon improvement of the patient’s condition. Follow up testing of renal function should be repeated during the treatment period. If renal function becomes worse, the drugs such as aminoglycosides and cephalosporins should be discontinued and a combination of nephrotoxic drugs and non-nephrotoxic drugs such as penicillin and gentamycin should be introduced (131,138). Uncomplicated infection by obstruction or many previous episodes will generally respond to oral therapy with sulfonamides, cotrimoxazole (trimethoprim + sulfamethoxazole, ampicillin, nalidixic acid, cephalexin or nitrofurantoin). Cotrimoxazole and nitrofuration can be used in recurrent cases. Pseudomonas infections respond well to carbencillin. A 10-day duration of treatment for UTI in diabetic patients is enough, sometimes short courses of high doses of parenteral therapy such as ampicillin or gentamycin or their combination or cephlosporin may be required in case of therapeutic failure or poor patient condition (133,140). Bacteria should disappear within 24-48 hours even if pyuria is present. Highly recurrent infections: They may be managed by either very close follow up and treatment of each episode, or by prophylaxis with nitrofurantoin or cotrimoxazole or nalidixic acid and mandelate salt or hippuric acid given as a single bed time dose. Some agents like nitrofurantion and mandelamine, tetracycline, melthicillin, cloxacillin and hippuric acid require acid urine to achieve good effect of pH below 5.5, which can be achieved by high protein diet, ammonium chloride, ascorbic acid, or methionine given at a dose of 10 g/day (13,140,141). Table 6. Drug dosage in renal insufficiency Drug Dose (mg) Nitrofurantoin 50 Trimethoprim 100 Norfloxacin 200 Ciprofloxacin 125 Cephalexin 125 (useful if renal insufficiency) From: Rubin et al., 1992 (142) If infected urine is alkaline and the organisms may be Proteus, cotrimoxazole or ampicillin can be tried, as Proteus is usually resistant to nitrofurantion and tetracycline. Some other agents require alkaline urine to achieve good effect and can be made alkaline by sodium bicarbonate or sodium or potassium citrate, M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS lactate or acetate. These agents are sulfonamides, cotrimoxazole, gentamycin, aminoglycosides and cephalosporins (133,134,138). Other agents are active in neutral urine, e.g., ampicillin, calistin and chloramphenicol (133). Relapse: As relapse is caused by the same species and specific strain of microorganism as before treatment, it is more common with drugs that are effective in urine but not in tissues, e.g., with nitrofurantoin and low dose of sulfonamides relapse implies infection of the upper UTI (143). The treatment should include the use of drugs capable to achieve high tissue levels, e.g., ampicillin and gentamycin (13). Reinfection: Reinfection means that pathogens have appeared during or after treatment due to organisms outside the urinary tract, thus indicating lower UTI, and is treated by continuous prophylactic use of mandelate salt or hippuric acid at doses of 3-6 g to keep the urine sterile (143). ¾ Antimicrobials most widely used in UTI: Ampicillin (133,142,144,145) Cotrimoxazole (138,141,142,146) Nitrofurantoin (141,142,147) Methicillin, amoxicillin, and others (141,142,148) Cephalosporins (141,142,149) Gentamicin and tobramicin (141,142,148,149) Tetracyclines (141,142,149) Amikacin (141,142,148,149) Carbencillin (141,142) Methenamine mandelate and hippuric acid (141,142,149) PREVENTION OF UTI AND KIDNEY INFECTION Good diabetic control is very essential for the control and prevention of urinary tract infection because high hyperglycemia and ketoacidosis may occur, which will lead to reduction in the host mechanisms of defense against infection. This in turn will entail recurrent UTI and bacteriuria (28,151,152). During infection, a number of humoral substances are released, which may complicate the treatment of diabetic patients (153). During infection, there is a decreased caloric intake, which leads to hypoglycemia that stimulates pituitary adrenal axis and sympathetic nervous system to release glucagon and catecholamines. These in turn stimulate hepatic and muscle glycogenolysis and release glucose to the circulation, thus leading to hyperglycemia and glycosuria (13,154). Diabetologia Croatica 31-2, 2002 Also, these hormones cause direct inhibition of pancreatic insulin and increase peripheral insulin resistance (155). Hypoglycemia also stimulates adrenal cortex to release glucocorticoids, which then stimulate glycosuria. Insulin therapy becomes inadequate and blood glucose too high, along with excess levels of free fatty acids in the circulation, so the patient may develop ketoacidosis and even coma (156). The patient becomes more susceptible to infection, so good blood sugar control improves host mechanisms of defense against infection (151). Any diabetic patient requiring an indwelling catheter may get bacteria and infection, so the following advice should be adopted: 1. Careful preselection of criteria for catheterization (157). 2. Use of aseptic close drainage or antimicrobial bladder rinse during prolonged catheterization; the catheter should be removed as soon as it is no longer needed (157). 3. Intermittent self-catheterization may be of great benefit in patients with neurogenic bladders. Each diabetic patient should be taught how to use dip slide method to monitor his urine for bacteria frequently as well as to test urine for sugar acetone (42,157). After proper use and incubation, the urine should be clear from bacteria. The presence of black dots indicates bacteria and the patient should visit the clinic for proper urine culture and appropriate treatment (157). COUNSELING AND EDUCATION An important role is identified for the diabetes specialist nurse in instructing diabetic patients and in briefing the community and practice nurses charged with their care about the following: - renal complications - construction of a data set - health economic analysis - research program RECOMMENDATIONS (25,158) 1. All patients who have had IDDM for more than 1 year and who are above the age of 12 years, and all patients with NIDDM from the time of diagnosis should be screened for albuminuria at least once a year. The monitoring of patients 97 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS with persistently elevated albumin excretion rates should be performed as often as clinically required, along with estimates of glycemic control, blood pressure, serum lipids and serum creatinine, and check up for retinopathy, neuropathy, and coronary and cerebrovascular disease. 2. Persistent microalbuminuria should be treated by improved blood glucose control and antihypertensive therapy when indicated. 3. Counseling and education of diabetic patients and briefing of primary health care personnel should be provided throughout all stages of diabetic kidney disease. 4. Methods of analysis and population studies should be reviewed and updated, and the need of largescale clinical trials should not be overlooked. THERAPEUTIC GOALS These must be appropriate to the individual patient or population. Educational programs have been shown to reduce the frequency of acute metabolic complications such as ketoacidosis and hypoglycemia, thus also reducing hospital admissions (159). Diabetes education has had some impressive results in reducing the frequency of certain diabetic complications such as kidney infections, nephropathy, retinopathy, neuropathy, foot ulcers, and amputations (160). CONCLUSIONS (160,161) Despite great advances in recent decades, diabetic kidney infections and urinary tract infections remain quite poorly treated and disabling diseases in many patients. There is a great need of proper tools for timely detection of kidney infections in diabetics. This is because of our inability to extrapolate the results of basic and clinical research into treatment regimens that are appropriate and acceptable to the individual patient. The main challenge for the immediate future will be to disseminate the message of effective diabetes programs from the relatively few centers where these have been implemented at present to as many patients as possible (162). REFERENCES 1. Mogensen CE, ed. The kidney and hypertension in diabetes mellitus. Boston: Kluwer; 1988. 2. Robbins Sl, Tucker AW. The causes of death in diabetes mellitus: a report of 307 autopsied cases. N Engl J Med 1944;231:868. 3. Vigg B, Rai V. Asymptomatic bacteriuria in diabetics. J Assoc Physicians India 1977;25:57-61. 4. Chow AW, Jewesson PJ. Pharmacokinetics and safety of antimicrobial agents during pregnancy. Rev Infect Dis 1985;7:287. 5. Keane EH, Boyko EJ, Reller LB, Hamman RF. Prevalence of asymptomatic bacteriuria in subjects with NIDDM in San Luis valley of Colorado. Diabetes Care 1988;11:708-712. 9. Murphy DP, Tan JS, File TM. Infectious complications in diabetic patients. Primary Care 1981;8:695-714. 10. Textbook of medicine. Urinary tract infection. Calvin M. Kunin/Shapter 376-1980. što s ovim??? 11. Intravisons text book of medicine – Urinary tract infection, Marvin Turck – 1649-1655, 1983. 12. Ronald AR, Harding GKM. Complicated urinary tract infections. Infect Dis Clin North Am 1997;11:583-592. 13. Nicolle LE. A practical guide to the management of complicated UTI. Drugs 1997;53:583-592. 14. Platt R, Polk BF, Murdock B, Rosner B. Risk factors for nosocomial urinary tract infection. Am J Epidemiol 1986;124:977-985. 6. Wheat LJ. Infection and diabetes mellitus. Diabetes Care 1980;3:187- 197. 7. Kass EH. Asymptomatic infections of the urinary tract. Trans Assoc Am Physicians 1956;69:56-64. 15. Kitabchi AE, Fisher JN, Murphy MB, Rumbak MJ. Diabetic ketoacidosis and the hyperglycemic, hyperosmolar non-ketotic state. In: Kahn CR, Weir GC, eds. Joslin’s Diabetes mellitus, 13th ed. Philadelphia: Lea & Febiger; 1994; p. 738-770. 8. Kunin CM. Detection, prevention and management of UTI, 4th ed. Philadelphia, PA: Lea and Febiger; 1987. 16. Yamada K, Nonaka K. Diabetic ketoacidosis in young obese Japanese men. Diabetes Care 1996;19:671. 98 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS 17. Ellenberg M. Textbook of diabetes. 1994. 18. Feldman EL, Stevens MJ, Greene DA. Clinical management of diabetic neuropathy. In: Veves A, ed. Clinical management of diabetic neuropathy. Totowa: Humana Press, 1998; p. 89-105. 19. Jackson E, Fowler JR. Textbook of UTI and inflammation. 1992. 20. Palsdottir A, Fossdal R, Arnason A. Heterogeneity of human C4 gene size: large intron (6.5 ketone bodies) is present in all C4A genes and some C4b genes. Immunogenetics 1987;25:299-304. 21. Leibovici L, Yehehzkelli Y, Porter A, et al. Influence of diabetes mellitus and glycaemic control on the characteristics and outcome of common infections. Diabet Med 1996;13:457-463. 22. Motani A, Forster L, Tull S, et al. Insulin-like growth factor-I modulates monocyte adhesion to Eahy 926 endothelial cells. Int J Exp Pathol 1996;77: 31-35. 23. Cotran RS, Kumar V, Robbins SL. Robbin’s pathologic basis of disease. 4th ed. Philadelphia: WB Saunders, 1989. 24. Glascock RJ, Brenner BM. The UTI and inflammation. In: Wilson J, et al., eds. Harrison’s Principles of interal medicine. 12th ed. New York: McGraw-Hill, 1991. 25. Rubin RH, Tolkoff-Rubin NE, Cotran RS. UTI, pyelonephritis and reflux neuropathy. In: Brenner BM, Rector FC, eds. The kidney. 3rd ed. Philadelphia: JB Lippincott, 1989. 26. Pickup JC, Williams G, eds. Textbook of diabetes mellitus. 2nd ed. Oxford: Blackwell, 1997. 27. Raptis AE, Viberti G. Pathogenesis of diabetes. Exp Clin Endocrinol Diabetes 2001;109 (Suppl 2):S424-S437. 28. Okada M, Takemura T, Yanagida H, Yoshioka K. Response of mesangial cells to low-density lipoprotein and angiotensin II in diabetic rats. Kidney Int 2002;61:113-124. 31. Warren JW, Abrutyn E, Hebel JR, et al. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Clin Infect Dis 1999;29:745-758. 32. Hostetter MK. Perspectives in diabetes: effects of hyperglycemia on CD and Candida albicans. Diabetes 1990;39:271. 33. Reed BD. Risk factors for candida vulvovaginitis. Obstet Gynecol Surv 1992;47:551-560. 34. Sobel JD, Vazquez JA. UTIs due to Candida species. In: Mobley HLT, Warren JW, eds. UTIs: Molecular pathogenesis and clinical management. Washington, DC: ASM Press, 1996; p. 119. 35. Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med 1999;341:1906-1912. 36. Greifer I. Bacteriuria and urinary tract infection. 1974. 37. Hagberg L, Hull R, Hull S, et al. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun 1983;40:265. 38. Lennette EH. Manual of clinical microbiology. Washington, DC: American Society for Microbiology, 1986. 39. Lipsky BA, Inui TS, Plorde JJ, et al. Is the cleancatch midstrem void procedure necessary for obtaining urine culture specimens from men? Am J Med 1984;76:257. 40. Forland H, Thomas V, Shelko A. Urinary tract infection in patient with DM. Studies on antibody coated bacteria. JAMA 1977;238:1924-1926. 41. Finegold SM, Baron EJ. Diagnostic microbiology. St. Louis: CV Mosby, 1986. 42. Jackson E, Fowler JR. Urinary tract infection and inflammation. 1990. 43. Kass EH. Bacteriuria and the diagnosis of infections of the urinary tract. Arch Int Med 1957;100:709-714. 29. Warren JW. Catheter-associated UTI. Infect Dis Clin North Am 1997;11:609-622. 44. Vigg B, Rai V. Asymptomatic bacteriuria in diabetic. J Assoc Physicians India 1977;25:57-61. 30. Patterson JE, Andriole VT. Bacterial urinary tract infections in diabetes. Infect Dis Clin North Am 1997;11:735-750. 45. Pometta D, Rees SB, Younger D, Kass EH. Asymptomatic bacteriuria in diabetes mellitus. N Engl J Med 1967;276:1118-1121. Diabetologia Croatica 31-2, 2002 99 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS 46. Lindberg U, Bergstrom AL, Carlsson E, Dahlquist G, Hermansson G, Larsson Y, Nilsson KO, Samuelsson G, Sjoblad S, Thalme B. UTI in children with type I diabetes. Acta Paediatr Scand 1985;74:85-88. 47. Schmitt JK, Fawcett CJ, Gullickson G. Asymptomatic bacteriuria and hemoglobin A1. Diabetes Care 1986;9:518-520. 48. Robbins SL, Tucker AW. The cause of death in diabetes. N Engl J Med 1944;231:865-868. 49. Barnard DM, Story RD, Root HF. Urinary tract infection in diabetic women. N Engl J Med 1953;248:136-141. 50. Taft JL, Billson VR, Nankervis A, Kincaid-Smith P, Martin FIR. A clinical – histological study of individuals with diabetes mellitus and proteinuria. Diabet Med 1990;7:215-221. 51. O’Sullivan DJ, Fitzgerald MG, Meynell MJ, Malins JM. Urinary tract infections. Br Med J 1961;1:786788. 52. Williams DN, King H, Harris DM. The microbial flora of the vagina and its relationship to bacteriuria in diabetic and non-diabetic women. Br J Urol 1975;47:453-457. 60. Sawers JSA. Bacteriuria and autonomic nerve function in diabetic women. Diabetes Care 1986;9:460-464. 61. Vjelsgaard R. Studies on urinary tract infection in diabetes. II. Significant bacteriuria in relation to long-term diabetic manifestation. Acta Med Scand 1966b;179:183-188. 62. Obana Y, Shibata K, Nishino T. Adherence of Serratia marcescens in the pathogenesis of urinary tract infections in diabetic mice. J Med Microbiol 1991;35:93-97. 63. Leibovici L, GreenShtain S, Cohen O, Wysenbeek AJ. Toward improved empiric management of moderate to severe urinary tract infections. Arch Intern Med 1992;152:2481-2486. 64. Hooton TM, Stamm WE. Management of acute uncomplicated UTI in adults. Med Clin North Am 1991:339-57. 65. Turck M, Anderson KN, Petersdorf RG. Relapse and reinfection in chronic bacteriuria. N Engl J Med 1966;275:70-73. 66. Ooci BS, Chen B, Yu M. Prevalence and site of bacteriuria in diabetes mellitus. Postgrad Med J 1974;50:497-499. 53. Bahl AL, Chugh RN, Sharma KB. Asymptomatic bacteriuria in diabetics attending a diabetic clinic. Indian J Med Sci 1970;24:1-6. 67. Richet G, Mayaud C. The course of acute renal failure in pyelonephritis and other types of interstitial nephritis. Nephron 1978;22:124-127. 54. MacFarlane IA, Brown RM, Smyth RW, Burdon DW, Fitzgerald MG. Bacteraemia in diabetes. J Infect 1986;12:213-219. 68. Baker LRI, Cattell WR, Fry IKF, Millinson WJW. Acute renal failure due to bacterial pyelonephritis. Q J Med 1979;48:603-612. 55. Leibovici L, Samra Z, Konisberger H, KalterLeibovici O, Pitlik SD, Drucker M. Bacteraemia in adult diabetic patients. Diabetes Care 1991;14:8994. 69. Cattell WR. Urinary tract infection and acute renal failure. In: Raine AEG, ed. Advanced renal medicine. Oxford: Oxford University Press, 1992; p. 302-313. 56. Stamm WE, Martin SM, Bennett JV. Epidemiology of nosocomial infection due to gram negactive bacilli: aspects relevant to development and use of vaccines. J Infect Dis 1977;136 (Suppl): S151S160. 70. Cattell WR, Greenwood RN, Baker LRI. Reversible renal failure due to interstitial infection of the kidney. In: Ishigami J, ed. Recent advances in chemotherapy. Tokyo: University of Tokyo Press, 1985; p. 225-228. 57. Ellenberg M. Diabetic neuropathy: clinical aspects. Metabolism 1976;25:1627-1655. 58. Rayfield EJ, Ault MJ, Keusch GT, Brothers MJ, Nechemias C, Smith H. Infection and diabetes: the case for glucose control. Am J Med 1982;72:439-450. 59. Korzeniowski OM. Urinary tract infection in the impaired host. Med Clin North Am 1991:391-404. 100 71. Parving HH, Kastrup H, Smidt UM, Andersen AR, Feidt-Rasmussen B, Sandahl-Christiansen J. Impaired autoregulation of glomerular filtration rate in type I diabetic patients with nephropathy. Diabetologia 1984;27:547-552. 72. Marks MI, Langston C, Eickhoff TC. Torulopsis glabrata – an opportunistic pathogen in man. N Engl J Med 1970;283:1131-1135. M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS 73. Kauffman CA, Tan JS. Torulopsis glabrata renal infection. Am J Med 1974;57:217-224. 74. Mehnert B, Mehnert H. Yeasts in urine and saliva of diabetic and non-diabetic patients. Diabetes 1958;7:293-297. 89. Groop LC, Laasonen L, Edgren J. Renal papillary necrosis in subjects with IDDM. Diabetes Care 1989;12:198-202. 90. Turner FC. Necrosis of the pyramids of one kidney. Trans Pathol Soc Lond 1888;39:159-161. 75. Wise GJ, Goldberg P, Kozinn PJ. Genitourinary candidiasis: diagnosis and treatment. J Urol 1976;116:778-780. 91. Froboese C. Über sequestrierende Marknekrosen der Nieren bei Diabetes mellitus. Verh Dtsch Ges Pathol 1937;30:431-443. 76. Anonymous. Urinary tract candidosis. Lancet 1988;2:1000-1002. 92. Günther GW. Die Pepillennekrosen der Niere bei Diabetes. Munchen Med Wochenschr 1937;84:1695-1699. 77. Kozinn PJ, Taschdjian CL, Goldberg PK, Wise GJ, Toni EF, Seeling MS. Advances in the diagnosis of renal candidiasis. J Urol 1978;119:184-187. 78. Goldberg PK, Kozinn PJ, Nouri N, Brooks RB. Incidence and significance of candiuria. JAMA 1979;241:582-584. 79. Davies RR, Reeves DS. 5-Fluoro-cystosine and urinary candidiasis. Br Med J 1971;1:577-579. 80. Grant SM, Clissold SP. Fluconazole: a review of its phamacodynamic and pharmacokinetic properties and therapetic potential in superficial and systemic mycoses. Drugs 1990;39:877-916. 81. Francis P, Walsh TJ. Evolving role of flucytosine in immunocompromised patients; new insights into safety, pharmacokinetics, and antifungal therapy. Clin Infect Dis 1992;15:1003-1018. 82. Obrant O. Perirenal abscess. Acta Chir Scand 1949;97:338-353. 83. Salvatierra O, Bucklew WB, Morrow JW. Perinephric abscess: a report of 71 cases. J Urol 1967;98:296-302. 84. Thorley JD, Jones SR, Sandford JY. Perinephric abscess. Medicine 1974;53:441-451. 85. Bevan JS, Griffith GJ, Willams JD, Gibby OM. Bilateral renal cortical abscesses in a young woman with type 1 diabetes. Diabet Med 1989;6:454-457. 86. Atcheson DW. Perinephric abscess with a review of 117 cases. J Urol 1941;46:201-208. 87. Edmondson HA, Martin HE, Evans N. Necrosis of renal papillae and acute pyelonephritis in diabetes mellitus. Arch Intern Med 1947;79:148-175. 88. Robbins SL, Mallory GK, Kimsey TD. Necrotising renal papillitis: a form of acute pyelonephritis. N Engl J Med 1946;235: 885-893. Diabetologia Croatica 31-2, 2002 93. Mandel EE. Renal medullary necrosis. Am J Med 1952;13:322-327. 94. Fry IK. Renal parenchymal disease. In: Grainger RG, Allison DJ, eds. Diagnostic radiology. Edinburgh: Churchill Livingstone, 1986; p. 11151135. 95. Mujais SK. Renal papillary necrosis in diabetes mellitus. Semin Nephrol 1984;4:40-47. 96. Lauler DP, Schreiner GE, David A. Renal medullary necrosis. Am J Med 1960;29:132-156. 97. Harkonen S, Kjellstrand CM. Exacerbation of diabetic renal failure following intravenous pyelography. Am J Med 1977;63:939-946. 98. Freidman EA. Diabetic renal disease. In: Rifkin H, Porte D Jr, eds. Diabetes mellitus. New York: Elsevier, 1990; p. 684-709. 99. Bending JJ. The kidney and renal tract. In: Tattersall RB, Gale EAM, eds. Diabetes clinical management. Edinburgh: Churchill Livingston, 1990; p. 281-292. 100.Taussig AE. Pneumaturia with report of a case. Boston Med Surg J 1907;156:769-774. 101.Raciborski (1860). Exemple de pneumaturia du urine gazeuse chez une malade affecte d’une nevropathie proteiforme avec predomonance des symptomes de hypochondrie. Gaz d’Hop, Paris (cited in Kelly and MacCallum, 1898). 102.Kelly HA, MacCallum WG. Pneumaturia. JAMA 1898;31:375-380. 103.Guiard FP. Du developpment spontane de gaz dans la vessie. Ann Mal Org Genitour 1883;1: 846-848. 104.Turman AE, Rutherford C. Emphysematous pyelonephritis with perinephric gas. J Urol 1971;105:165-170. 101 M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS 105.Zabbo A, Motie JE, Popowniak KL, Weinstein AJ. Bilateral emphysematous pyelonephritis. Urology 1985;25:293-296. 120.Klein FA, Smaith MJ, Vick CW, Schneider V. Emphysematous pyelonephritis; diagnosis and treatment. S Afr Med J 1986;79:41-46. 106.Senator H. Über Pneumaturia in Allgemeinen und bei Diabetes mellitus insbesondere. Int Beitr Wissensch Med 1883;3:317 (cited in Bailey, 1961). 121.Seidenfeild SM, Lemaistre CF, Setiawan H, Munford RS. Emphysematous pyelonephritis caused by Candida tropicalis. J Infect Dis 1982;146:569. 107.Yang W-H, Shen N-C. Gas forming infection of the urinary tract and investigation of fermantation as a mechanism. J Urol 1990;143:960-964. 108.Huang JJ, Chen KW, Ruaan MK. Mixed acid fermantation of glucose as a mechanism of emphysematous urinary tract infection. J Urol 1991;146:148-151. 109.Schainuck LI, Fouty R, Culer RE. Emphysematous pyelonephrtitis: a new case and review of the literature. Am J Med 1968;44:234-239. 110.Subramanyam BR, Lefleur RS, Van Natta FC. Renal emphysema secondary to traumatic renal infection. Urol Radiol 1980;2:53-54. 111.Spagnola AM. Emphysematous pyelonephritis. Am J Med 1978;64:840-844. 112.Michaeli J, Mogle P, Perlberg S, Heiman S, Caine M. Emphysematous pyelonephritis. J Urol 1984;131:203-208. 113.Evanoff GV, Thompson CS, Foley R Weinman EJ. Spectrum of gas within the kidney. Am J Med 1987;83:149-154. 114.Schultz EH, Klorfein EH. Emphysematous pyelonephritis. J Urol 1962;87:762-766. 115.Gillies CL, Flocks R. Spontaneous renal and perirenal emphysema. Am J Roentgenol 1941;46:173-174. 122.Langston CS, Pfister RC. Renal emphysema. A case report and reivew of the literature. Am J Roentgenol 1970;110:778-786. 123.Banks DE, Persky L, Mahoney SA. Renal emphysema. J Urol 1969;102:390-392. 124.Rosenberg JW, Quader A, Browen JS. Renal emphysema. Urology 1973;1:237-239. 125.Soteropoulos C, Kawashima E, Gilmore JH. Cystitis and ureteritis emphysematosa. Radiology 1957;68:866-868. 126.Ohba S, Tsuchiya H, Kohno S, Hamazaki M. Ureteritis and pyelitis emphysematosa in a neonate. Pediatr Radiol 1984;14:116-117. 127.Harrow BR, Slonane JA. Ureteritis emphysematosa; spontaneous ureteral pneumatogram; renal and perirenal emphysema. J Urol 1963;89:43-48. 128.Bailey H. Cystitis emphysematosa. Roentgenol 1961;86:850-862. Am J 129.Jackson E, Fowler JR. UTI and inflammation. 1990; p. 53. 130.Cattell WR. Infections of the kidney and UT. Oxford: Oxford Medical Publications, 1996; p. 2526. 131.Harrison’s Principles of internal medicine. 12th ed. New York: McGraw-Hill, 1991. 116.Kumar D, Rao BR. Case profile: bilateral emphysematous pyelonephritis. Urology 1982;20:96. 132.Zhanel GG, Harding GKM, Guay DRP. Asymptomatic bacteriuria: which patients should be treated? Arch Intern Med 1990;150:1389-1396. 117.Brenbridge ANAG, Buschi AJ, Cochrane JA, Lees RF. Renal emphysema of the transplanted kidney: sonographic apperance. Am J Roentgenol 1979;132:656-658. 133.Clinical pharmacology – Laurence. 286-272 / 1980. 118.Parameswaran R, Feest T. Gas nephrogram: an unusal complication of renal transplantion. Br J Radiol 1977;50:438-440. 135.Consegenase of EDTA 2000. 119.Potter JL, Sullivan BM, Flournoy JC, Gerza C. Emphysema in the renal allograft. Radiology 1985;155:51-52. 137.Lennette EH. Manual of clinical microbiology. Washington, DC: American Society for Microbiology, 1986. 102 134.Ellenberg. Genitourinary infection. Textbook of diabetes mellitus, 1994. 136.Jackson E, Fowler JR. Urinary tract infection and inflammation. 1990, p. 48. M. S. Balachandar, P. Pavkoviæ, @. Metelko / KIDNEY INFECTIONS IN DIABETES MELLITUS 138.Alberti K, Zimmett P, DeFronzo R. International textbook of diabetes mellitus. 2nd ed. Chichester: John Wiley & Sons, 1997. 139.Pozzilli P, Leslie RDG. Infections and diabetes: mechanisms and prospects for prevention. Diabet Med 1994;11:935-941. 153.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type II diabetes (UKPDS 33). Lancet 1998;352: 837-853. 141.Drug dosage in renal insufficiency, 1992. 154.Ling Z, Pipeleers DG. Prolonged exposure of human â cells to elevated glucose levels results in sustained cellular activation leading to a loss of glucose regulation. J Clin Invest 1996;98:28052812. 142.Rubin UH, Shapiro ED, Andriole VT, et al. Evaluation of new antiinfective drugs for the treatment of UTI. Clin Infect Dis 1992;15:S216S227. 155.Davis EA, Jones TW. Hypoglycaemia in children with diabetes: incidence, counterregulation and cognitive dysfunction. J Pediatr Endocrinol Metab 1998;11 (Suppl 1):177-182. 143.Pickup JC. Genitourinary infection. In: Textbook of diabetes mellitus, 1997. 156.Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am 1997;11:609622. 140.Pendergrass M, Graybill J. Infections and diabetes mellitus. In: Current therapy of diabetes mellitus. St Louis: Mosby Year Book, Inc., 1998; p. 218-224. 144.Wright AJ, Wilkowske CJ. The penicillins. Mayo Clin Proc 1987; 62:806. 145.Parry MF. The penicillins. Med Clin North Am 1987;71:1093. 146.Martindale G. The extra pharmacopoeia. 29th ed. London: The Pharmaceutical Press, 1989; p. 328. 147.Boileau MA, Corriere JN, Liss RH. Visualization of bactericidal concentrations of nitrofurantoin macrocrystals on primate and human urinary tract tissue. J Urol 1983;130:1010. 148.Patterson JE, Andriole VT. Bacterial UTI in diabetes. Infect Dis Clin North Am 1997;11:735750. 149.Ronald AR, Harding GKM. Complicated urinary tract infections. Infect Dis Clin North Am 1997;11:583-592. 150.Stamm WE, Hooton TM. Management of UTI in adults. N Engl J Med 1993;329:1328-1334. 151.McMahon MM, Bistrian BR. Host defenses and susceptibility to infection in patients with diabetes mellitus. Infect Dis Clin North Am 1995;9:1-9. 152.Fishbein H, Palumbo PJ. Acute metabolic complications in diabetes. In: National Diabetes Data Group, eds. Diabetes in America, 2nd ed. Bethesda, MD: National Institute of Health, 1995; p. 283-291. (NIH Publications No: 95-1468) Diabetologia Croatica 31-2, 2002 157.Silink M. Hypoglycaemia. In: Silink M, ed. APEG handbook on childhood and adolescent diabetes: the management of insulin dependent DM (IDDM). Sydney: Australian Paediatric Endocrine Gurop, 1996. 158.Jacquemet S, Lacroix A, Perrolini M, et al. Qualitative evaluation of courses intended for patients suffering from chronic diseases. New observation method for the continuous training of the health-care team. Patient Educ Couns 1998;34:201-212. 159.Report of a WHO Working Group on therapeutic patient education. Continuing education programmes for health-care providers in the field of prevention of chronic diseases. Copenhagen, Denmark: WHO-Euro; 1998. 160.Jacquemet S, Grazia-Albano M, Sudre Ph, Assal JPh. Educational methodologies: an analysis of chaos. Diabetologia 1997;40 (Suppl 1):A622 . 161.Freudenberger HJ. La brulure interne: burn-out. Le prix eleve du succes. (burnout: the high price of success). London: Anchor Press Doubleday, 1980. 162.Mitchell P, Pipemeyer J, Glass M, Mazze R. Longterm impact of staged diabetes management (SDM) to improve metabolic control in an American Indian community (abstract). Diabetes 1998;47 (Suppl 1):A179. 103