Drinking Water Testing Program Link to Test Results
advertisement

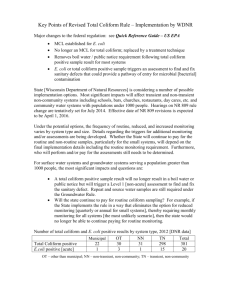
Seattle Public Schools Drinking Water Testing Program Seattle Public Schools is in the midst of a comprehensive water quality testing and remediation program, initiated in January 2004. Information updates are available on the Water Quality Program Web site. This page provides details on testing protocols and standards. The standards noted are included in a water quality policy developed by the School Board's Policy and Legislative Committee and adopted by the School Board. Remediation plans for each school are based on these standards. Link to Test Results For individual school results and action plans, please click here. General Notes on Water Quality Testing Results 1. Testing Criteria The criteria used for various water quality elements are noted below. These standards are included in the School Board's draft water quality policy. Each criterion meets or exceeds federal and state drinking water requirements. Any test results that meet all criteria will not result in either health or aesthetic concerns (such as appearance, taste or odor) at or below this level. Any test results that do not meet these criteria will be investigated further. Health-Related Contaminants: Lead Cadmium Copper Total Coliforms not to exceed 20 ppb not to exceed 5 ppb not to exceed 1.3 ppm 0 cfu/100ml EPA-recommended action level in schools Primary MCL per state water regulations Action Level per federal Lead/Copper Rule MCL goal per federal Total Coliform Rule Aesthetic-Related Constitutents: Iron Zinc Turbidity Color 2. not to exceed 0.3 ppm not to exceed 5 ppm not to exceed 1 ntu not to exceed 15 cu Secondary MCL per state water regulations Secondary MCL per state water regulations Based on typical turbidity levels in SPU-supplied water Secondary MCL per state water regulations Units of Concentration Used in Water Testing Results Parts per million (ppm), used for the concentrations of most constituents in water. Another common way of expressing ppm for constituents in water is milligrams per liter (mg/L). Parts per billion (ppb), used when the concentrations of a contaminant are very small. Ppb is 1000 times smaller than ppm, in other words 1 ppm = 1000 ppb. Most often, lead and cadmium concentrations are shown as ppb. 3. Drinking Water Regulations Primary Drinking Water Regulation: A regulation defined in the federal Safe Drinking Water Act that specifies contaminants that may have an adverse effect on the health of persons. It specifies a maximum contaminant level (MCL) for each contaminant and contains criteria and procedures for compliance. The District criteria meet or exceed these criteria for lead, copper and total colifom. Note: Schools are not required to meet the federal primary drinking water requirements, but the School Board will consider adopting its own standards. Secondary Drinking Water Regulation: A US EPA non-enforceable regulation for a contaminant that may adversely affect the taste, odor, or appearance of water, but not adversely affect health. Secondary maximum contaminant levels (SMCLs) have been established for secondary contaminants. 4. Description of Contaminants / Constituents Being Sampled Turbidity is a measurement of the relative cloudiness of the water caused by the presence of suspended matter or air bubbles, resulting in the scattering and absorption of light. Turbidity is a general indicator of water quality, both from a health and an aesthetic standpoint. The units of measurement for turbidity are "nephelometric turbidity units" (NTU). Low turbidity values indicate very clear water with relatively few particles, while higher turbidity values indicate greater numbers of particles and degraded water quality. At turbidity values less than about 5 NTU, there is no cloudiness perceptible to the human eye - the water looks perfectly clear. For water samples taken from older steel plumbing (as is most common in Seattle schools), the source of turbidity is almost completely attributable to iron oxide (rust) particles that are not harmful to human health. Color is a physical characteristic describing the appearance of water (different from turbidity, which is the cloudiness of water). Color is measured in "color units" (CU), with larger numbers indicating stronger color. Generally, color measured at 5 CU or less has no apparent color to the naked eye. For water samples taken from older steel plumbing (as is most common in Seattle schools), the source of color is almost completely attributable to iron oxide (rust) particles which are not harmful to human health. Coliform Bacteria are organisms that are present in the environment and in the feces of all warm-blooded animals and humans. Coliform bacteria will likely not cause illness. However, the presence of coliform bacteria in drinking water indicates that disease-causing organisms (pathogens) may be present in the water system, so testing for coliform bacteria is a long-standing method for assessing drinking water quality that is accepted by all public health agencies. There are three forms of coliform bacteria that are commonly tested for in drinking water: total coliform, fecal coliform, and E.coli. The total coliform group encompasses a large collection of different types of bacteria that are very commonly found in the environment (e.g., soil and vegetation), and are generally harmless. Fecal coliform bacteria are a sub-group of the total coliform group. They appear in great quantities in the intestines and feces of humans and animals. If only total coliform bacteria are detected, the source is probably environmental. However, if environmental contamination can enter a water system, there may be a pathway for pathogens to enter as well, so it is important to identify the source if possible and correct the problem. The presence of fecal coliform in a drinking water sample often indicates recent fecal contamination and a greater risk of exposure to pathogens. E. coli is a sub-group of the fecal coliform group. Most E. coli are harmless and are also found in the intestines and feces of humans and animals. However, some strains may cause illness, so the presence of E. coli in a drinking water sample almost always indicates recent fecal contamination and a greater risk of pathogens being present. [A note about E. coli: Most E. coli outbreaks have been related to food contamination and are caused by a specific strain of E. coli known as E. coli O157:H7]. When a water sample is reported as "E. coli present", it does not mean that this specific strain is present and in fact it is most likely not present. However, it does indicate fecal contamination and the appropriate protective measures will be made. Drinking water samples collected at various locations in the schools are tested for total coliform. If total coliform is present, the sample is then also tested for E. coli. More information on coliform bacteria is available at the Washington State Department of Health's web site for drinking water: http://www.doh.wa.gov/ehp/dw. Chlorine Residual is the concentration of chlorine compounds that serve as disinfectants in the drinking water supply. Chlorine is added to all water supplied in the City of Seattle. Lead is a metallic element that is commonly found in red brass alloys used in faucet and drinking fountain fixtures of all types, as well as in lead-tin solder that was commonly used for joining copper plumbing pipes until 1992. Lead enters tap water via chemical and physical interactions between the water and the materials. Lead is not normally found in source water supplies; Seattle's water supply has virtually no lead. It is a regulated contaminant for health concerns. Cadmium is a metallic element that is present in trace amounts in zinc (galvanized) coatings. Cadmium enters tap water via chemical and physical interactions between the water and the pipe materials. Cadmium is not normally found in source water supplies; Seattle's water supply has virtually no cadmium. It is a regulated contaminant for health concerns. Copper enters tap water via chemical and physical interactions between the water and copper piping and brass alloys found in fixtures. Copper is generally a concern for aesthetic reasons because it can cause staining of fixtures and hair, but it is also a health concern at higher concentrations for persons with a relatively rare disease called Wilson's Disease. Zinc enters tap water via chemical and physical interactions between the water and galvanized steel pipes. Zinc is not a regulated contaminant for health reasons, but is regulated to limit its concentrations in wastewater. Iron is present in very low levels in Seattle's water supply, but is present in tap water primarily because of chemical and physical interactions between the water and older steel and iron pipes in buildings. Iron is not a regulated contaminant for health reasons, but is regulated because of aesthetic problems like staining of fixtures and discolored water.