Department of Earth
advertisement

7KHFRQVHTXHQFHVRIHYROXWLRQDU\DGDSWDWLRQRQWKH
VXOIXULVRWRSHIUDFWLRQDWLRQRIVXOIDWHUHGXFLQJ
PLFURRUJDQLVPV
$QGUp3HOOHULQ
Department of Earth and Planetary Sciences
McGill University
Montréal, Québec
August, 2014
A thesis submitted to McGill University in partial fulfillment of the requirements of the degree
of Doctor of Philosophy
© André Pellerin, 2014
Table of contents
$EVWUDFW
1
5pVXPp
3
$FNQRZOHGJHPHQWV
5
Preface & contributions of authors
7
Introduction
9
Paper 1: 0DVVGHSHQGHQWVXOIXULVRWRSHIUDFWLRQDWLRQGXULQJ
UHR[LGDWLYHVXOIXUF\FOLQJ$FDVHVWXG\IURP0DQJURYH/DNH%HUPXGD
Connection1
49
Paper 2: Evolutionary adaptation of a sulfate reducing bacterium
and its sulfur isotope phenotype
5
Paper 2 Appendix
8
Connection 2
10
Paper 3: Evolutionary response of S isotope fractionation is predicted
by phenotypic plasticity
10
Paper 3 Appendix
12
Conclusion
14
Abstract
Sulfur metabolisms leave behind a record of their activity in the sulfur they utilize. This thesis
seeks to advance our understanding of some of these processes with a particular focus on
dissimilatory sulfate reduction.
The multiple sulfur isotope composition of two porewater sulfate profiles in the anoxic marine
sapropel of Mangrove Lake, Bermuda was investigated. The porewater sulfate profiles exhibit
the distinct isotopic signatures of microbial sulfate reduction and sulfur reoxidation which simple
diagenetic models can reproduce. The reoxidative cycle includes sulfide oxidation to elemental
sulfur followed by the disproportionation of the elemental sulfur to sulfate and sulfide, and this
process turns over from 50 to 80% of the sulfide produced by sulfate reduction. We suggest that
the reoxidative S cycle in any environment can best be identified within two regions of the
multiple sulfur isotope fractionation spectrum. Paper 1 is titled Mass-dependent sulfur isotope
fractionation during reoxidative sulfur cycling: A case study from Mangrove Lake, Bermuda.
The process of evolutionary adaptation has largely been assumed inconsequential on the
sulfur isotopic fractionation produced during dissimilatory sulfate reduction and recorded in the
isotope rock record. Yet, the diversity of sulfur isotope phenotypes displayed by species of
sulfate reducing microorganisms isolated from modern environments amounts to strong evidence
that evolutionary adaptation does matter. If this is the case, important information about the
evolutionary history of DSR may be preserved in the rock record. However, the relationship
between evolutionary adaptation and isotope phenotype is unexplored. To begin addressing this
gap in knowledge, the impact of evolutionary adaptation on the fitness and sulfur isotopic
phenotype of the dissimilatory sulfate reducer Desulfovibrio vulgaris Hildenborough (DvH) was
investigated. The increases in fitness that were observed did not result in a change of the isotope
phenotype. At least in the conditions of the experiment this result indicates that the isotope
phenotype is not very sensitive to evolutionary adaptation on the hundreds of generations
timescale. This suggests that lengthier timescales are necessary for evolutionary-driven
divergence of the isotope phenotype. Paper 2 is titled Evolutionary adaptation of a sulfate
reducing bacterium and its sulfur isotope phenotype.
To address the issues raised in paper 2, pure cultures of Desulfomicrobium baculatm were
evolved in batch culture for 300 generations. A greater than twofold increase in growth rate over
the course of the experiment was measured as well as a change in isotope phenotype (34İ ) from
15 to 12 ‰. The response of 34İ to evolutionary adaptation resembles in some ways the isotopic
response of physiological adaptations to changing environmental conditions. While in the narrow
context of the environment where the evolutionary adaptation took place, the change in isotope
phenotype is incontestable, it remains to be seen if this difference in isotope phenotype is
maintained across different growth environments. Paper 3 is titled Evolutionary response of S
isotope fractionation is predicted by phenotypic plasticity
Résumé
L’évidence des processus biogéochimiques qui utilisent le soufre dans leur métabolisme est
préservée dans les signatures isotopiques du soufre. Cette thèse tente de mieux comprendre
certains de ces processus.
La composition isotopique multiple du soufre dans les sulfates des eaux interstitielles des
sédiments de Mangrove Lake Bermuda sont l’objet de la première section de cette thèse. Le
sulfate dans ces eaux a la signature isotopique diagnostique de l’effet combinée de la sulfato réduction et de la réoxidation. Ces processus peuvent êtres reproduits par de simple modèles
diagénétiques. Le cycle du soufre dans les sédiments de Mangrove Lake comprend un composant
d’oxidation du sulfure en soufre élémentaire suivie par la disproportionation en sulfure et sulfate.
Ces processus vont recycler jusqu’à 50 a 80% du soufre passant par la sulfato réduction. Nous
suggérons que le cycle du soufre peut être facilement identifié dans deux regions du spectrum
des isotopes multiples du soufre. Le premier article est intitulé Mass dependent sulfur isotope
fractionation during reoxidative sulfur cycling: A case study from Mangrove Lake, Bermuda.
Le processus d’évolution a largement été assumé comme sans consequences sur le
fractionnement isotopique produit par la sulfato reduction et preservé dans les sediments. Par
contre, la diversité des phénotypes d’isotopes du soufre des différentes espèces capables de faire
la sulfato réduction semblen sugéré que effectivement, l’évolution a un impact sur le phénotype
isotopique. Si ceci est vrai, de l’information important à propos de l’histoire évolutionnaire de la
sulfato reduction est peut etre preserve dans les sediments. Par contre la relation entre
l’adaptation évolutive et le phenotype isotopique n’est pas documentée. Pour addresser cette
lacune de connaissances, l’impact de l’adaptation évolutive sur le phenotype isotopique de la
bacteria sulfato réductrice Desulfovibrio vulgaris Hildenborough (DvH) a été mesurée. Des
augmentations de l’aptitude évolutive de DvH ont été mesurées mais n’ont pas été corrélés avec
des changements dans le phenotype isotopique. Dans les conditions de croissance de
l’expérience, le phenotype isotopique ne semble pas être très sensible à l’adaptation évolutive sur
l’échelle des centaines de générations. Ceci suggère qu’ il est nécessaire d’avoir de plus longues
échelles de temps pour que la divergence évolutive ait un impact sur le phénotype isotopique. Le
deuxième article est intitulé Evolutionary adaptation of a sulfate reducing bacterium and its
sulfur isotope phenotype.
Pour tenter de documenter la réponse évolutive du phénotype isotopique, une experience
d’évolution avec une espèce (Desulfomicrobium baculatm) ayant un taux de croissance plus lent
fût entrepris pour 300 générations. Un doublement du taux de croissance fût observer pendant
l’expérience ainsi qu’un changement du phenotype isotopique (34İ ) de 15 à 12 ‰. La réponse
évolutive de l’adaptation semble ressembler à la réponse isotopique d’un changement des
conditions environnementales sur une population à court terme. Malgré que dans le context
restraint de l’expérience évolutive, un changement du phénotype isotopique n’est pas
contestable, il reste à voir dans d’autre conditions environnmentales ce changement de
phénotype isotopique serait toujours évident. Le troisième article s’intitule Evolutionary
response of S isotope fractionation is predicted by phenotypic plasticity
Acknowledgements
This thesis is the result of almost five years of research which I undertook at McGill
University in the Department of Earth and Planetary Sciences. My thesis advisor, Boswell
Wing, is the one who got me interested in microbial experimental evolution and who convinced
me that indeed, the questions I attempt to answer in this thesis would be worth four five years of
my life. Boz agreed to support the experimental work and forced me - sometimes kicking and
screaming – to focus on the bigger picture, think outside the box and go wherever was needed in
order to find the solutions to scientific or experimental problems. His insistence on having a
broad perspective but precisely defining questions and ensuring that they are answered in a
natural and logical manner allows his students to tackle high impact scientific concepts in a
successful manner. Boz has played an instrumental role in my development as a scientist and I
thank him for being my mentor over these last few years.
I would also like to acknowledge the co-authors of the multiple manuscripts which
resulted from this thesis. Grant 0Zane, Judy 'Wall, Lyle Whyte, Mikaella Rough, Alfonso
Mucci, Donald E. Canfield, Thi Hao Bui and Luke Anderson-Trocme.
Many people who contributed to this research in some way or another will unfortunately only
be acknowledged here. I am nonetheless grateful for their contributions to this project. Despite
the overwhelming difficulties we were experiencing with method development Rebecca Austin
forced me continue experimenting with our qPCR assay which eventually produced very precise
measurements of fitness we present in this thesis. I also thank Nadia Mykycuk for accepting to
help us with the early training and development on this matter. I am grateful to Emma Wall for
accepting to wash all those bottles in the lab and to Charles Kosman who found that equivalence
in the calculations of fitness which made everything so much simpler. I would like to thank Jesse
Coangelo-Lillis for his critical comments and reviews of my manuscripts without which
reviewers would have shuddered at sentence structure. Sincere thanks are due to Thi Hao Bui for
her positive attitude and willingness to help with laboratory work as well as for having in her
memory all calculus shortcuts. I am grateful to Grant Cox for his camaraderie throughout the
more than three years we overlapped at McGill University and his ability to accept and adapt
stories to constantly changing facts. I thank Galen Halverson for his unfounded faith in my
abilities as a geoscientist and for the passion he so successfully conveys with all things that got
deposited by gravity before he was born. I’d also like to thank Graham Bell, Andrew Hynes and
Gordon Southam for providing support in some way or another during these last five years.
Thank you to the entire PROPS group past and present including Marcus Kunzmann, Emma
Bertran, Kristyn Rodziniak, Matt Hryciuk, Thomas McGuire, Peter Crockford, John Prince and
Clint Scott for providing support in some way or another during these last five years.
All of this would have been much, much more difficult without the administrative staff of
McGill EPS, namely Anne Kosowski, Brigitte Dionne, Nancy Secondo, Angela diNinno,
Brandon Bray and Kristy Thornton.
I would like to thank my parents Doniald and Lorraine Pellerin for their patience,
unconditional support and encouragement throughout these last five years and for teaching me
early on the skills necessary to persevere until objectives are attained.
Finally, a sincere thanks is due to my better half, Elyse Bustros-Lussier who is a loving and
understanding companion without which none of this would have been possible. Elyse
encouraged me to make the leap and undertake this doctorate and remained supportive
throughout these five years despite my nights and weekends in the lab, my constantly open
computer, frequent absences as well all the other demands of academia. She seems to make the
most out of all opportunities and even found a way to get us a small family out of this gig. Thank
you.
This thesis was financially supported by a NSERC CGS grant as well as the NSERC
CREATE Canadian Astrobiology Research Program.
Preface & contributions of authors
The core of this thesis consists of three papers with multiple authors as contributors. Each
manuscript has original contributions to knowledge.
Paper 1 is titled 0DVVGHSHQGHQW VXOIXU LVRWRSH IUDFWLRQDWLRQ GXULQJ UHR[LGDWLYH VXOIXU
F\FOLQJ$FDVHVWXG\IURP0DQJURYH/DNH%HUPXGD and authored by André Pellerin, Thi Hao
Bui, Mikaella Rough, Alfonso Mucci, Donald
Canfield and
Boswell
Wing.
It was
DFFHSWHG LQ the journal Geochimica & Cosmochimica Acta.
The original contributions to knowledge in this manuscript consist of a revised estimate of the
reoxidative cycle in Mangrove Lake based on the multiple sulfur isotopes of porewater sulfates.
By doing so, this manuscript identifies key regions in multiple sulfur isotope space where the
reoxidative cycle can be differentiated from sulfate reduction. Within these boundaries, the
methodology elaborated in Mangrove Lake can be applied to estimate the magnitude of the
reoxidative cycle in sediments and hopefully will be taken up by the community as a tool to do
so.
André Pellerin analyzed and interpreted the data, built the model and wrote the manuscript.
Thi Hao Bui provided assistance in building the model. Mikaella Rough converted the porewater
sulfates to silver sulfide and determined porewater sulfate concentrations. Alfonso Mucci and
Donald Canfield sampled the core at Mangrove Lake and provided input in the manuscript
during the late stages. Boswell Wing contributed equally with André Pellerin in building the
models and provided input in the manuscript throughout its production.
Paper 2 is titled Evolutionary adaptation of a sulfate reducing bacterium and its sulfur isotope
phenotype and authored by André Pellerin, Boswell Wing, Luke Anderson-Trocme, Lyle
Whyte, Judy Wall, and Grant Zane. It DFFHSWHG IRU SXEOLFDWLRQ LQ the journal Applied and
Environmental Microbiology.
This manuscript contains the first experiments investigating the relationship between
evolutionary adaptation and the sulfur isotope phenotype of microorganisms capable of
dissimilatory sulfate reduction. They are significant and original contributions to knowledge
because they provide empirical evidence for a low sensitivity of the isotope phenotype to
evolutionary adaptation at high growth rates. Also, in the methodology developed for these
experiments is a novel, high-sensitivity approach to quantifying fitness in evolution experiments
with anaerobic microorganisms.
André Pellerin performed the laboratory experiments, interpreted the results and wrote the
manuscript. Boswell Wing supervised the project from the design of the experiments to the
interpretation of the results and provided comments on the manuscript. Luke Anderson-Trocme
performed the laboratory work for the 16S contamination assay as well as for the genetic
investigation of respiration genes. Lyle Whyte provided access to facilities for microbiology.
Judy Wall and Grant Zane provided the strain DVU 0600 which was utilized to measure fitness
in this study.
Paper 3 is titled Evolutionary response of S isotope fractionation is predicted by phenotypic
plasticity and authored by André Pellerin, Luke Anderson-Trocme and Boswell Wing. It is to be
submitted to the journal Earth and Planetary Science letters.
This manuscript contains the first empirical evidence demonstrating a relationship between
evolutionary adaptation and the sulfur isotope phenotype. This is an important contribution to
knowledge because it enables the interpretation of sulfur isotope signatures in an evolutionary
context. It establishes the behaviour of the isotope phenotype in the face of evolutionary
adaptation in a constant environment at relatively low growth rate. It also provides a timescale
upon which evolutionary adaptation can affect the isotope phenotype.
André Pellerin performed the laboratory experiments, interpreted the results and wrote the
manuscript. Luke Anderson-Trocme performed the laboratory work for the 16S contamination
assays as well as for the genetic investigation of respiration genes. Boswell Wing supervised the
project from the design of the experiments to the interpretation of the results and provided
comments on the manuscript.
André Pellerin is responsible for the full content of the thesis.
Introduction
7KH UHPLQHUDOL]DWLRQ RI RUJDQLF PDWWHU LQ PRVW DQR[LF HQYLURQPHQWV LV GRPLQDWHG E\
PLFURRUJDQLVPVFDSDEOHRIGLVVLPLODWRU\VXOIDWHUHGXFWLRQ'657KHVHDUHDGLYHUVHJURXSRU
PLFURRUJDQLVPVZKRVHPHWDEROLFFDSDELOLW\LVIHDWXUHGLQWZRGRPDLQVRIOLIHDQGPD\SUHGDWH
WKH FRPPRQ DQFHVWRU RI DOO OLIH RQ (DUWK :DJQHU HW DO ,VRWRSLF HYLGHQFH RI WKH
PHWDEROLVPLVIRXQGLQWKHURFNUHFRUGGDWLQJEDFNWRDWOHDVW*D6KHQHWDO'65
JDLQV WKH HQHUJ\ QHFHVVDU\ WR JURZ DQG UHSURGXFH E\ XWLOL]LQJ VXOIDWH DV D WHUPLQDO HOHFWURQ
DFFHSWRUWRR[LGL]HRUJDQLFPDWWHU,WFRQYHUWVWKHVXOIDWHLQWRVXOILGHZKLFKLVWKHQH[FUHWHGLQWR
WKHHQYLURQPHQWDVDZDVWHSURGXFWRIWKHPHWDEROLVP'XULQJWKLVELRORJLFDOUHDFWLRQWKHOLJKWHU
LVRWRSHVRIVXOIXUZLOOSURJUHVVDWDIDVWHUUDWHWKDQWKHKHDYLHURQHVUHVXOWLQJLQDZDVWHSURGXFW
VXOILGHZKLFKLVLVRWRSLFDOO\HQULFKHGLQWKHOLJKWLVRWRSHVRIVXOIXUUHODWLYHWRWKHVXOIDWH7KH
ZDVWH SURGXFWV RI '65 FDQ EH SUHVHUYHG SHUPDQHQWO\ LQ WKH VHGLPHQWV DV PHWDO VXOILGHV DQG
FRQVWLWXWHV HYLGHQFH RI WKH SUHVHQFH RI WKLV PHWDEROLVP 7KLV HYLGHQFH LV VWDEOH RQ JHRORJLFDO
WLPHVFDOHV +RZHYHU WKH LVRWRSLF VLJQDWXUH LV QRW VROHO\ D UHFRUG RI ELRORJLFDO DFWLYLW\ 7KH
PHWDEROLF DFWLYLW\ RI '65FDSDEOH PLFURRUJDQLVPV LV DIIHFWHG E\ WKH SK\VLFDO HQYLURQPHQW
7KXVVLPXOWDQHRXVO\UHFRUGHGLQWKHVHGLPHQWDU\VXOILGHDUHFOXHVRIWKHFRQGLWLRQVLQZKLFKWKH
PLFURRUJDQLVPVJUHZ
<HW '65 LV QRW WKH RQO\ FXOSULW LQYROYHG LQ WKH IUDFWLRQDWLRQ RI VXOIXU LVRWRSHV 7KH VXOILGH
SURGXFHGE\'65LVRIWHQF\FOHGE\UHR[LGDWLYHSURFHVVHV±ERWKFKHPLFDODQGELRORJLFDOZKLFK
MXVWOLNH'65DIIHFWWKHLVRWRSHVRIVXOIXUDQGRIWHQRYHUSULQWVWKH'65VLJQDOZLWKWKHLURZQ
6XFKSURFHVVHVRIWHQRFFXUVLPXOWDQHRXVO\DQGLQFOXGHFKHPLFDODQGELRORJLFDOVXOILGHR[LGDWLRQ
SURFHVVHVZKLFKFDQSURGXFHLQWHUPHGLDWHVXOIXUFRPSRXQGVDVZHOODVWKHIXOO\R[LGL]HGVXOIDWH
:HUHIHUWR-¡UJHQVHQDQG1HOVRQDQGUHIHUHQFHVWKHUHLQIRUDPRUHWKRURXJKUHYLHZRI
UHR[LGDWLYHSURFHVVHV,QVRPHLQVWDQFHVLQWHUPHGLDWHVXOIXUFRPSRXQGVZKLFKDUHDE\SURGXFW
RIVXOILGHR[LGDWLRQDUHGLVSURSRUWLRQDWHGDSURFHVVVZKLFKSURGXFHVERWKUHGXFHGDQGR[LGL]HG
VSHFLHVVLPXOWDQHRXVO\5HR[LGDWLYHSURFHVVHVFRQWULEXWHWRWKHVXOIXUF\FOHVRPHWLPHVF\FOLQJ
ODUJH SRUWLRQV RI WKH ZDVWH SURGXFW RI '65 DOO WKH ZD\ EDFN WR VXOIDWH -¡UJHQVHQ 7KDPGUXSHWDO7KDPGUXSHWDO=HUNOHHWDO7KH\FDQPDVNWKHSULPDU\
LVRWRSLFVLJQDORI'65E\RYHUSULQWLQJLWZLWKWKHLURZQ7KLVLVHVSHFLDOO\SUREOHPDWLFZKHQWKH
IUDFWLRQDWLRQDVVRFLDWHGUHR[LGDWLYHSURFHVVHVLVODUJHVXFKDVLQWKHFDVHRIGLVSURSRUWLRQDWLRQ
EHFDXVHWKHSULPDU\VLJQDORI'65FDQEHJUHDWO\DOWHUHGDQGHYHQPRUHZRUULVRPHWKDW
VXFKRYHUSULQWHGVLJQDWXUHVFDQEHZURQJO\LQWHUSUHWHGDVDSULPDU\'65VLJQDO&DQILHOGDQG
7KDPGUXS +DELFKW DQG &DQILHOG +DELFKW HW DO +DELFKW HW DO -RKQVWRQHWDO<HWWKHLVRWRSLFEHKDYLRXURIUHGXFWLYHDQGUHR[LGDWLYHSURFHVVHVPD\EH
GLIIHUHQWLDEOHEHFDXVHWKHPLQRUVXOIXULVRWRSHVPD\EHXQLTXHWRHDFKSURFHVV-RKQVWRQHWDO
-RKQVWRQ HW DO ,I WKLV LV WKH FDVH WKH PLQRU LVRWRSHV RI VXOIXU FDQ EH XVHG WR
TXDQWLWDWLYHO\DVVHVVWKHUHODWLYHFRQWULEXWLRQVRIUHGXFWLYHDQGR[LGDWLYHSURFHVVHVHJ=HUNOH
HW DO DQG JDLQ LQVLJKW LQWR WKH G\QDPLFV RI VSHFLILF VXOIXU PHWDEROLVPV SULPRUGLDOO\
'65 ,Q WKH ILUVW FKDSWHU RI WKLV WKHVLV ZH DWWHPSW GHFRUWLFDWH WKH UHGXFWLYH DQG UHR[LGDWLYH
SURFHVHVLQWKHVHGLPHQWVRI0DQJURYH/DNH:HILQGWKDWLQGHHGWKHPXOWLSOHVXOIXULVRWRSHV
WKDWDUHSUHVHUYHGLQSRUHZDWHUVFDQEHXWLOL]HGWRDVVHVVWKHUHODWLYHFRQWULEXWLRQVRIUHGXFWLYH
DQGUHR[LGDWLYHSURFHVVHV
,Q WKH ILUVW FKDSWHU ZH VHH WKDW SULPDU\ PHWDEROLF VLJQDWXUHV RI '65 FDQ EH LVRODWHG IURP
HQYLURQPHQWDOVDPSOHV+RZHYHUWKHLVRWRSLFVLJQDWXUHSURGXFHGE\'65LVDFWXDOO\DUHIOHFWLRQ
RI WKH SK\VLFDO HQYLURQPHQW EXW DOVR RI ELRORJ\ 7KH SDVW GHFDGHV KDYH RIIHUHG DPSOH FDVHV
LQYHVWLJDWLQJ WKH GLIIHUHQW HQYLURQPHQWDO DQG SK\VLRORJLFDO FRQWUROV WKDW LPSDFW WKH LVRWRSLF
VLJQDWXUH OHIW EHKLQG E\ '65 LQ SXUH FXOWXUHV &DQILHOG +DUULVRQ DQG 7KRGH -RKQVWRQHWDO.DSODQDQG5LWWHQEHUJ.OHLNHPSHUHWDO6LPHWDOD
6LP HW DO E 6WRJEDXHU HWDO DQG LQ QDWXUH &DQILHOG &DQILHOG HW DO )DUTXKDUHWDO+DELFKWDQG&DQILHOG+DELFKWDQG&DQILHOG0XOWLSOH
DXWKRUV DWWHPSWHG WR H[SODLQ WKH FKDUDFWHULVWLF IUDFWLRQDWLRQV RI '65 ZLWK PRGHOEDVHG
DSSURDFKHV %UXQQHU DQG %HUQDVFRQL 5HHV :LQJ DQG +DOHY\ 5HFHQWO\
TXDQWLWDWLYH REVHUYDWLRQV LQ FKHPRVWDWV UHODWLQJ WKH SK\VLRORJLFDO UHVSRQVHV RI 6 LVRWRSH
IUDFWLRQDWLRQ GXULQJ '65 WR FKDQJLQJ HQYLURQPHQWDO FRQGLWLRQV KDV DGGHG D QHZ OHYHO RI
SUHGLFWLYHEHKDYLRXUZKLFKFDQEHDSSOLHGWRVHGLPHQWDU\VXOILGHVHQDEOLQJDGHHSHULQWHUSUHWLYH
SRWHQWLDORISDVWVXOIXUF\FOHV/HDYLWWHWDO%XWGHVSLWHWKLVDEXQGDQWLQWHUHVWLQ'65
ZLWK DQ (DUWK¶V KLVWRU\ REMHFWLYH LQ PLQG OLWWOH FRQVLGHUDWLRQ KDV EHHQ JLYHQ WR WKH UROH
HYROXWLRQDU\ DGDSWDWLRQ PD\ KDYH RQ WKH 6 LVRWRSHV WKDW DUH UHFRUGHG LQ VHGLPHQWV <HW
HYROXWLRQDU\KLVWRU\RI'65PD\KDYHDQLPSRUWDQWUROHLQGHWHUPLQLQJWKHLVRWRSLFVLJQDWXUHRI
VXOIDWHUHGXFWLRQ%ROOLJHUHWDO%UXFKHUWHWDO'HWPHUVHWDO.OHLNHPSHUHW
DO 7KLV LV WKH FDVH EHFDXVH WKH LVRWRSLF H[SUHVVLRQ RI D VSHFLILF PLFURRUJDQLVP DW DQ\
JLYHQPRPHQWLVFRQWUROOHGE\WKHLQWHUDFWLRQRIWKHJHQRW\SHDQGWKHHQYLURQPHQWDQGUHVXOWLQ
D XQLTXH SKHQRW\SLF VLJQDWXUH WKH LVRWRSH SKHQRW\SH +RZHYHU HPSLULFDO HYLGHQFH RI WKH
UHODWLRQVKLS EHWZHHQ HYROXWLRQDU\ DGDSWDWLRQ DQG WKH LVRWRSH SKHQRW\SH KDV VR IDU HYDGHG WKH
OLWHUDWXUH
([SHULPHQWDO HYROXWLRQ SURYLGHV D PHDQ WR OLQN HYROXWLRQDU\ DGDSWDWLRQ ZLWK WKH LVRWRSH
SKHQRW\SH%DFWHULDKDYHEHHQXVHGIRUVRPHWLPHDVDPRGHOV\VWHPIRUHYROXWLRQDU\SURFHVVHV
$WZRRG HW DO 'DOOLQJHU /XULD DQG 'HOEUXFN 7ZR SULPDU\ UHDVRQV PDNH
WKHPDQDGYDQWDJHRXVFKRLFHODUJHSRSXODWLRQVL]HVPHDQWKDWDGYDQWDJHRXVPXWDWLRQVDUH
H[SHFWHGHDFKJHQHUDWLRQ'\NKXL]HQDQGWKHVKRUWJHQHUDWLRQWLPHVHQDEOHWKHHIIHFWV
RIPXWDWLRQVWRVSUHDGWKURXJKWKHSRSXODWLRQLQUHODWLYHO\VKRUWKXPDQWLPHVFDOH/HQVNL
7KH V\VWHPDWLF GRFXPHQWDWLRQ RI VORZ ILWQHVV LQFUHDVHV RYHU WLPH DV D SRSXODWLRQ DGDSWV WR D
QHZHQYLURQPHQW/HQVNLHWDODQGWKHGUDVWLFHYROXWLRQVWHPPLQJIURPPXWDWLRQVZLWKD
KLJK HQYLURQPHQWDO LPSDFW %ORXQW HW DO VXJJHVW WKDW WKH WLPHVFDOH RI ODERUDWRU\
H[SHULPHQWV RYHUODSV ZLWK PDQ\ HYROXWLRQDU\ SURFHVVHV ,Q WKH VHFRQG DQG WKLUG FKDSWHU ZH
DWWHPSW WR SURGXFH D ILUVW RUGHU FRQVWUDLQW RQ WKH VWDELOLW\ RI WKH LVRWRSH SKHQRW\SH RI '65 WR
HYROXWLRQDU\ DGDSWDWLRQ ,Q FKDSWHU ZH XWLOL]HG WKH PRGHO RUJDQLVPV Desulfovibrio vulgaris
+LOGHQERURXJK'Y+DIDVWJURZLQJEDFWHULDLQWKHFRQGLWLRQVZHSURYLGHGIRUWKHH[SHULPHQW
7KLV HQDEOHG WKH HYROXWLRQ H[SHULPHQW WR SURJUHVV DW D IDVW UDWH EHFDXVH D ODUJH QXPEHU RI
JHQHUDWLRQV FRXOG EH DFFXPXODWHG LQ D VKRUW DPRXQW RI WLPH ,Q FKDSWHU WKUHH ZH XWLOL]H
Desulfomicrobium baculatum'EDFDVDPRGHORUJDQLVP,QWKHH[DFWVDPHJURZWKFRQGLWLRQV
DV'Y+'EDFKDVDVLJQLILFDQWO\VORZHUJURZWKUDWHDQGDZKROO\GLIIHUHQWLVRWRSHSKHQRW\SH
References
$WZRRG .& 6FKQHLGHU /. 5\DQ )- 3HULRGLF 6HOHFWLRQ LQ (VFKHULFKLD &ROL
3URFHHGLQJVRIWKH1DWLRQDO$FDGHP\RI6FLHQFHVRIWKH8QLWHG6WDWHVRI$PHULFD
%ORXQW =' %RUODQG &= /HQVNL 5( +LVWRULFDO FRQWLQJHQF\ DQG WKH HYROXWLRQ RI D
NH\ LQQRYDWLRQ LQ DQ H[SHULPHQWDO SRSXODWLRQ RI (VFKHULFKLD FROL 3URFHHGLQJV RI WKH 1DWLRQDO
$FDGHP\RI6FLHQFHVRIWKH8QLWHG6WDWHVRI$PHULFD
%ROOLJHU & 6FKURWK 0+ %HUQDVFRQL 60 .OHLNHPSHU - =H\HU - 6XOIXU LVRWRSH
IUDFWLRQDWLRQ GXULQJ PLFURELDO VXOIDWH UHGXFWLRQ E\ WROXHQHGHJUDGLQJ EDFWHULD *HRFKLPLFD HW
&RVPRFKLPLFD$FWD
%UXFKHUW 9 .QREODXFK & -RUJHQVHQ %% &RQWUROV RQ VWDEOH VXOIXU LVRWRSH
IUDFWLRQDWLRQ GXULQJ EDFWHULDO VXOIDWH UHGXFWLRQ LQ $UFWLF VHGLPHQWV *HRFKLPLFD (W
&RVPRFKLPLFD$FWD
%UXQQHU % %HUQDVFRQL 60 $ UHYLVHG LVRWRSH IUDFWLRQDWLRQ PRGHO IRU GLVVLPLODWRU\
VXOIDWHUHGXFWLRQLQVXOIDWHUHGXFLQJEDFWHULD*HRFKLPLFD(W&RVPRFKLPLFD$FWD
&DQILHOG ' 7KDPGUXS % 7KH SURGXFWLRQ RI 6GHSOHWHG VXOILGH GXULQJ EDFWHULDO
GLVSURSRUWLRQDWLRQRIHOHPHQWDOVXOIXU6FLHQFH
&DQILHOG '( ,VRWRSH IUDFWLRQDWLRQ E\ QDWXUDO SRSXODWLRQV RI VXOIDWHUHGXFLQJ EDFWHULD
*HRFKLPLFDHW&RVPRFKLPLFD$FWD
&DQILHOG '( )DUTXKDU - =HUNOH $/ +LJK LVRWRSH IUDFWLRQDWLRQV GXULQJ VXOIDWH
UHGXFWLRQLQDORZVXOIDWHHX[LQLFRFHDQDQDORJ*HRORJ\
'DOOLQJHU:+7KHSUHVLGHQW
VDGGUHVV-50LFURVF6RF
'HWPHUV - %UFKHUW 9 +DELFKW .6 .XHYHU - 'LYHUVLW\ RI VXOIXU LVRWRSH
IUDFWLRQDWLRQV E\ VXOIDWHUHGXFLQJ SURNDU\RWHV $SSOLHG DQG (QYLURQPHQWDO 0LFURELRORJ\ '\NKXL]HQ'(([SHULPHQWDO6WXGLHVRI1DWXUDO6HOHFWLRQLQ%DFWHULD$QQXDO5HYLHZ
RI(FRORJ\DQG6\VWHPDWLFV
)DUTXKDU - &DQILHOG '( 0DVWHUVRQ $ %DR + -RKQVWRQ ' 6XOIXU DQG R[\JHQ
LVRWRSH VWXG\ RI VXOIDWH UHGXFWLRQ LQ H[SHULPHQWV ZLWK QDWXUDO SRSXODWLRQV IURP ) OOHVWUDQG
'HQPDUN*HRFKLPLFDHW&RVPRFKLPLFD$FWD
+DELFKW.6&DQILHOG'(6XOSKXULVRWRSHIUDFWLRQDWLRQLQPRGHUQPLFURELDOPDWVDQG
WKHHYROXWLRQRIWKHVXOSKXUF\FOH1DWXUH
+DELFKW .6 &DQILHOG '( 6XOIXU LVRWRSH IUDFWLRQDWLRQ GXULQJ EDFWHULDO VXOIDWH
UHGXFWLRQLQRUJDQLFULFKVHGLPHQWV*HRFKLPLFDHW&RVPRFKLPLFD$FWD
+DELFKW.6&DQILHOG'(,VRWRSHIUDFWLRQDWLRQE\VXOIDWHUHGXFLQJQDWXUDOSRSXODWLRQV
DQGWKHLVRWRSLFFRPSRVLWLRQRIVXOILGHLQPDULQHVHGLPHQWV*HRORJ\
+DELFKW.6&DQILHOG'(5HWKPHLHU-6XOIXULVRWRSHIUDFWLRQDWLRQGXULQJEDFWHULDO
UHGXFWLRQ DQG GLVSURSRUWLRQDWLRQ RI WKLRVXOIDWH DQG VXOILWH *HRFKLPLFD HW &RVPRFKLPLFD $FWD
+DELFKW .6 *DGH 0 7KDPGUXS % %HUJ 3 &DQILHOG '( &DOLEUDWLRQ RI 6XOIDWH
/HYHOVLQWKH$UFKHDQ2FHDQ6FLHQFH
+DUULVRQ $* 7KRGH +* 0HFKDQLVP RI WKH EDFWHULDO UHGXFWLRQ RI VXOSKDWH IURP
LVRWRSHIUDFWLRQDWLRQVWXGLHV7UDQVDFWLRQVRIWKH)DUDGD\6RFLHW\
-RKQVWRQ'7)DUTXKDU-&DQILHOG'(6XOIXULVRWRSHLQVLJKWVLQWRPLFURELDOVXOIDWH
UHGXFWLRQ:KHQPLFUREHVPHHWPRGHOV*HRFKLPLFD(W&RVPRFKLPLFD$FWD
-RKQVWRQ '7 )DUTXKDU - :LQJ %$ .DXIPDQ $- &DQILHOG '( +DELFKW .6 0XOWLSOH VXOIXU LVRWRSH IUDFWLRQDWLRQV LQ ELRORJLFDOV\VWHPV$FDVHVWXG\ZLWKVXOIDWHUHGXFHUV
DQGVXOIXUGLVSURSRUWLRQDWRUV$P-6FL
-¡UJHQVHQ %% $ 7KLRVXOIDWH 6KXQW LQ WKH 6XOIXU &\FOH RI 0DULQH 6HGLPHQWV 6FLHQFH
-¡UJHQVHQ%%1HOVRQ'&6XOILGHR[LGDWLRQLQPDULQHVHGLPHQWV*HRFKHPLVWU\PHHWV
PLFURELRORJ\*HRORJLFDO6RFLHW\RI$PHULFD6SHFLDO3DSHUV
.DSODQ ,5 5LWWHQEHUJ 6& 0LFURELRORJLFDO IUDFWLRQDWLRQ RI VXOSKXU LVRWRSHV - *HQ
0LFURELRO
.OHLNHPSHU - 6FKURWK 0+ %HUQDVFRQL 60 %UXQQHU % =H\HU - 6XOIXU LVRWRSH
IUDFWLRQDWLRQGXULQJJURZWKRIVXOIDWHUHGXFLQJEDFWHULDRQYDULRXVFDUERQVRXUFHV*HRFKLPLFD
HW&RVPRFKLPLFD$FWD
/HDYLWW :' +DOHY\ , %UDGOH\ $6 -RKQVWRQ '7 ,QIOXHQFH RI VXOIDWH UHGXFWLRQ
UDWHV RQ WKH 3KDQHUR]RLF VXOIXU LVRWRSH UHFRUG 3URFHHGLQJV RI WKH 1DWLRQDO $FDGHP\ RI
6FLHQFHV
/HQVNL5(3KHQRW\SLFDQG*HQRPLF(YROXWLRQGXULQJD*HQHUDWLRQ([SHULPHQW
ZLWKWKH%DFWHULXPEscherichia coli3ODQW%UHHGLQJ5HYLHZV
/HQVNL 5( 5RVH 05 6LPSVRQ 6& 7DGOHU 6& /RQJ7HUP ([SHULPHQWDO
(YROXWLRQ LQ (VFKHULFKLD FROL , $GDSWDWLRQ DQG 'LYHUJHQFH 'XULQJ *HQHUDWLRQV 7KH
$PHULFDQ1DWXUDOLVW
/XULD6('HOEUXFN00XWDWLRQVRIEDFWHULDIURPYLUXVVHQVLWLYLW\WRYLUXVUHVLVWDQFH
*HQHWLFV
5HHV &( $ VWHDG\VWDWH PRGHO IRU VXOSKXU LVRWRSH IUDFWLRQDWLRQ LQ EDFWHULDO UHGXFWLRQ
SURFHVVHV*HRFKLPLFDHW&RVPRFKLPLFD$FWD
6KHQ<%XLFN5&DQILHOG'(,VRWRSLFHYLGHQFHIRUPLFURELDOVXOSKDWHUHGXFWLRQLQ
WKHHDUO\$UFKDHDQHUD1DWXUH
6LP 06 %RVDN 7 2QR 6 D /DUJH 6XOIXU ,VRWRSH )UDFWLRQDWLRQ 'RHV 1RW 5HTXLUH
'LVSURSRUWLRQDWLRQ6FLHQFH
6LP062QR6'RQRYDQ.7HPSOHU63%RVDN7E(IIHFWRIHOHFWURQGRQRUVRQ
WKHIUDFWLRQDWLRQRIVXOIXULVRWRSHVE\DPDULQH'HVXOIRYLEULRVS*HRFKLPLFDHW&RVPRFKLPLFD
$FWD
6WRJEDXHU$.R\GRQ6%HUQHU=:LQWHU-6WXEHQ'(IIHFWRIPRO\EGDWHDQGFHOO
JURZWK RQ 6LVRWRSH IUDFWLRQDWLRQ GXULQJ EDFWHULDO VXOIDWH UHGXFWLRQ *HRPLFURELRORJ\ -RXUQDO
7KDPGUXS % )LQVWHU . +DQVHQ -: %DN ) %DFWHULDO GLVSURSRUWLRQDWLRQ RI
HOHPHQWDO VXOIXU FRXSOHG WR FKHPLFDO UHGXFWLRQ RI LURQ RU PDQJDQHVH $SSOLHG DQG
(QYLURQPHQWDO0LFURELRORJ\
7KDPGUXS % )RVVLQJ + -¡UJHQVHQ %% 0DQJDQHVH LURQ DQG VXOIXU F\FOLQJ LQ D
FRDVWDO PDULQH VHGLPHQW $DUKXV ED\ 'HQPDUN *HRFKLPLFD HW &RVPRFKLPLFD $FWD :DJQHU 0 5RJHU $- )OD[ -/ %UXVVHDX *$ 6WDKO '$ 3K\ORJHQ\ RI
'LVVLPLODWRU\ 6XOILWH 5HGXFWDVHV 6XSSRUWV DQ (DUO\ 2ULJLQ RI 6XOIDWH 5HVSLUDWLRQ -RXUQDO RI
%DFWHULRORJ\
:LQJ %$ +DOHY\ , $ EOXHSULQW IRU WKH VXOIXU LVRWRSH SKHQRW\SHV RI VXIDWHUHVSLULQJ
PLFUREHV*ROGVFKPLGW6DFUDPHQWR&$
=HUNOH$/.DP\VKQ\-U$.XPS/5)DUTXKDU-2GXUR+$UWKXU0$6XOIXU
F\FOLQJ LQ D VWUDWLILHG HX[LQLF ODNH ZLWK PRGHUDWHO\ KLJK VXOIDWH &RQVWUDLQWV IURP TXDGUXSOH 6
LVRWRSHV*HRFKLPLFDHW&RVPRFKLPLFD$FWD
Paper 1 : 0DVVGHSHQGHQWVXOIXU
LVRWRSHIUDFWLRQDWLRQGXULQJ
UHR[LGDWLYHVXOIXUF\FOLQJ$FDVH
VWXG\IURP0DQJURYH/DNH%HUPXGD
Mass-dependent sulfur isotope fractionation during reoxidative
sulfur cycling: A case study from Mangrove Lake, Bermuda
André Pellerin1, Thi Hao Bui1, Mikaella Rough1, Alfonso Mucci1, Donald E. Canfield2 and Boswell A. Wing1
[1] Department of Earth and Planetary Sciences and GEOTOP, McGill University, 3450 University Street,
Montréal, Canada, H3A 2A7
[2] Institute of Biological Sciences, Odense University, Campusvej 55, 5230 Odense M, Denmark
Correspondance to: André Pellerin (andrepellerin@gmail.com)
Abstract
The multiple sulfur isotope composition of porewater sulfate from the anoxic marine sapropel of
Mangrove Lake, Bermuda was measured in order to establish how multiple sulfur isotopes are
fractionated during reoxidative sulfur cycling. The porewater-sulfate G34S and ѐ33S dataset exhibits the
distinct isotopic signatures of microbial sulfate reduction and sulfur reoxidation. We reproduced the
measurements with a simple diagenetic model that yielded fractionation factors for net sulfate removal
of between -29.2 and -32.5‰. A new approach to isotopic modelling of the sulfate profiles, informed by
the chemistry of sulfur intermediate compounds in Mangrove Lake, reveals that sulfate reduction
produces a relatively small intrinsic fractionation and that an active reoxidative sulfur cycle increases the
fractionation of the measured values. Based on the model results, the reoxidative cycle of Mangrove
Lake appears to include sulfide oxidation to elemental sulfur followed by the disproportionation of the
elemental sulfur to sulfate and sulfide. This model also indicates that the reoxidative sulfur cycle of
Mangrove Lake turns over from 50 to 80% of the sulfide produced by microbial sulfate reduction. The
Mangrove Lake case study shows how sulfur isotope fractionations can be separated into three different
“domains” in '33S-G34S space based on their ability to resolve reductive and reoxidative sulfur
transformations. The first domain that differentiates reductive and reoxidative sulfur cycling is well
illustrated by previous studies and requires 34S-32S fractionations more negative than у-70‰, beyond the
fractionation limit of microbial sulfate reduction at earth surface temperatures. The second domain that
distinguishes reductive and reoxidative processes is between 34S-32S fractionations of -40 ‰ and 0‰,
where the 33S-32S fractionations of sulfate reduction and reoxidation are significantly different. In the
remaining domain (between 34S-32S fractionations -70‰ and -40‰), the similarity of the multiple sulfur
isotope signals from microbial sulfate reduction and disproportionation means that the two processes
cannot be discriminated from each other.
1. Introduction
Reoxidative sulfur (S) cycling converts the waste product of microbial sulfate reduction – aqueous sulfide
– to S compounds of higher oxidation states. With the possible exception of reactive Fe-rich sediments
(e.g., (Gagnon et al., 1995; Lefort et al., 2012), sulfide oxidation is an important control on the electron
budget of marine sediments since a small fraction of the aqueous sulfide produced by sulfate reduction
is trapped as metal sulfides and buried, while the rest is ultimately recycled back to the porewater
sulfate pool (80-95%, (Canfield and Teske, 1996; Diaz et al., 2012; Jørgensen and Nelson, 2004).
Although the biogeochemical reactions responsible for sulfide oxidation are complex, rapid, difficult to
observe, and often interrelated (Aller et al., 2010; Canfield and Thamdrup, 1994; Canfield, 2001;
Canfield et al., 1993; Howarth and Jørgensen, 1984; Kühl and Jørgensen, 1992; Werne et al., 2008), they
can broadly be grouped into overall pathways in which disproportionation plays a major role, and those
where sulfide is more completely oxidized to sulfate (Jørgensen and Nelson, 2004).
Microbial sulfate reduction, sulfide oxidation, and S disproportionation all produce characteristic
relationships between 33S-32S and 34S-32S ratios of their respective products and reactants (Farquhar et
al., 2007; Johnston et al., 2005; Zerkle et al., 2009). As a result, multiple S isotopes can be used to
differentiate among a variety of biogeochemical S pathways, even if the associated 34S-32S fractionations
alone are not diagnostic. Within this framework, for example, multiple S-isotope measurements in
ancient sulfates seem to reflect the early initiation of global microbial S disproportionation over 1.8
billion years ago (Johnston et al., 2005). Likewise, a multiple S isotope approach suggests that microbial
sulfate reduction alone accounts for the S-isotope systematics in the water column of euxinic Lake
Cadagno, Switzerland (Canfield et al., 2010) whereas the relative roles of reduction and reoxidation on
the isotopic signature of dissolved S compounds are ambiguous in Baltic Sea sediments (Strauss et al.,
2012). Importantly, multiple S-isotope measurements can also provide quantitative constraints on the
relative influence of reduction and reoxidation; reaction-diffusion modeling of 33S-32S and 34S-32S ratios in
water column sulfide of euxinic Green Lake, NY suggests disproportionation rates that are roughly 3050% of the sulfate reduction rates (Zerkle et al., 2010).
In Mangrove Lake, a thin layer of oxic marine surface waters overlies many meters of organic carbonand sulfide-rich sediments that are iron- and manganese-poor (Boudreau et al., 1992; Canfield et al.,
1998a; Knicker and Hatcher, 2001). Given the dearth of alternate electron acceptors, the marine
sapropels of Mangrove Lake distinguish themselves from typical marine sediments in which a multi-step
redox ladder separates oxygen-bearing from sulfidic porewaters. Biogeochemical studies have broadly
constrained the S cycle in the upper sediments at Mangrove Lake and infer that it is controlled primarily
by microbial sulfate reduction in the sulfidic porewaters (Boudreau et al., 1992; Canfield et al., 1998a).
Stoichiometric mass balance suggests that the majority of the sulfide so produced diffuses to the
sediment surface (Boudreau et al., 1992), where it can locally support sulfide-oxidizing mats of Beggiota.
The distribution of 34S-32S ratios among various S pools indicates that a small fraction of the sulfide is
also sequestered as organic S (Boudreau et al., 1992; Canfield et al., 1998a). Whereas budgets based on
34
S-32S ratios and total S imply that a reductive pathway dominates S cycling in the sulfidic sediments of
Mangrove Lake, minor S isotopes offer an independent way to validate this conclusion and to identify
where and how sulfide reoxidation might take place. This study presents a new dataset of the multiple S
isotope composition of sulfate in porewaters from Mangrove Lake sediments. When interpreted with a
model developed here for multiple S-isotope fractionation during S reoxidation, this dataset constrains
both the intrinsic S-isotope fractionation associated with microbial sulfate reduction at Mangrove Lake,
as well as the relative magnitude of the reoxidative S flux. These results highlight how multiple Sisotope measurements of porewater sulfate can provide a discriminating record of S reoxidation that
complements those based on
34
S-32S measurements of porewater sulfide organic and mineral
sedimentary sulfur fractions. A potentially powerful characteristic of the approach described here is that
it can be applied to samples obtained from porewater profiles, independent of experimental
manipulation to stimulate or monitor S reoxidation. The method can also be applied to archived
samples, enabling historical records to be developed for processes – like the relative rate of S oxidation
– that are typically only accessible in the present day through innovative reoxidation experiments. It can
be used to study S cycling in other sedimentary systems, keeping in mind that it only provides diagnostic
results in specific domains of multiple S-isotope space.
2. Methods
2.1 Site description and sampling
Mangrove Lake, Bermuda is an approximately 300m by 400m interdune waterbody, connected to the
ocean through fissures and fractures in the calcareous bedrock. The sediment-water interface is found
50 to 100 cm below the water surface (Boudreau et al., 1992). The lake is filled with a stratified sapropel
capped by a 0-20cm layer in which the sediment is well mixed (Boudreau et al., 1992; Canfield et al.,
1998a). Within this mixed layer, 210Pb activity (Boudreau et al., 1992), porewater sulfate concentrations,
and the sulfate S-isotope compositions are invariant and the latter two are typical of seawater (Canfield
et al., 1998a). Below this layer, sulfate concentrations decrease and porewaters eventually become
sulfidic with maximum measured sulfide concentrations of 12-17 mM (Boudreau et al., 1992; Canfield et
al., 1998a). The mixed layer and upper stratified layer host abundant purple and green phototrophic
sulfur bacteria that persist down to 30cm below sediment-water interface (Stolz, 1991). The organic
carbon content of the sediments ranges between 33% and 47% dry weight with little variation with
depth over the sampling interval (Boudreau et al., 1992). In contrast, iron concentrations in the solid
sedimentsare very low and register a slight increase of 0.34% to 0.55% dry weight from 5 cm to 45 cm
depth (Boudreau et al., 1992). Porewater Fe and Mn concentrations do not exceed, respectively, 10 PM
and 0.5 PM (Boudreau et al., 1992).
Two sediment cores of approximately 60cm in length were collected in 5.5 cm inner diameter Plexiglas
tubes at nearly the same location on August 2nd and August 4th, 1992 during the sampling campaign
reported in (Boudreau et al., 1992). We refer to these as core 1 (2/08/92) and core 2 (4/08/92). Holes
were predrilled in the tubes at 2.5 cm intervals and covered with electrical tape. Sediment was
withdrawn sequentially from the surface into 60 mL plastic syringes after puncturing the tape. The tape
acted as a gasket so that no air was drawn into the syringe with the sediment. The filled syringes were
then taken to the Bermuda Biological Station where their content was transferred to Reeburgh-type
squeezers (Reeburgh, 1967) in a N 2 –filled glove bag. Porewaters were extracted under 275 kPa of
nitrogen pressure and filtered simultaneously through a glass-fiber filter and a 0.45µm Millipore filter
(type MA) as they passed directly into a pre-washed 60 mL plastic syringe without atmospheric contact.
The filtered porewaters were transferred to a plastic screw-top test tube, purged with a stream of N 2
and acidified with a few drops of concentrated hydrochloric acid to exsolve the dissolved sulfide. These
acidic, H 2 S-free porewaters were stored in a refrigerator until the dissolved sulfate was precipitated as
BaSO 4 .
2.2 Sulfate concentrations
A barium chloride solution (10% w/w) was added to the H 2 S-free porewater samples. They were then
gently heated to ~50 °C overnight to quantitatively precipitate dissolved sulfate as barium sulfate. The
barium sulfate precipitate was filtered from the solution, dried, and weighed to determine porewater
sulfate concentrations gravimetrically. International Association for the Physical Sciences of the Ocean
(IAPSO) standard seawater ([SO 4 2-] = 28 mM) was used as a control and repeated measurements of this
standard yielded a relative reproducibility of 5%.
2.3 Sulfur isotope analyses
Sulfate was reduced to sulfide by reacting ~10 mg of BaSO 4 with 15 mL of Thode solution (mixture of
(32:15:53) HI, H 3 PO 2 and HCl) at 100 oC under a stream of pure N 2 for 90 minutes (Thode et al., 1961).
The N 2 carried the generated H 2 S through a zinc acetate solution, which quantitatively precipitated the
H 2 S as ZnS. A few drops of silver nitrate (0.1 N) were added to the zinc acetate solution to convert the
ZnS to Ag 2 S. This reaction was carried out overnight in the dark. The Ag 2 S was then separated from the
solution by filtration on a 0.2µm membrane filter, rinsed with a few millilitres of ammonium hydroxide
and three times with Milli-Q water, scraped from the filter, and dried for > 24 hrs at 50 oC. The samples
were then weighed to evaluate the efficiency of the recovery process relative to the starting amounts of
BaSO 4 . Typical recoveries were >90% S. We view these as acceptable recoveries given the potential
losses in manipulating such small quantities of solids.
The Ag 2 S was reacted in the presence of excess fluorine gas for 12 hours in a Ni reaction vessel heated
to 250 oC. The SF 6 generated by the reaction was first purified by removing non-condensable byproducts of the reaction by cryo-separation at -120 °C. A second purification was carried out by passing
the SF 6 through two GC columns (~2m Haysep Q and ~2m Molsieve 5A) with ultrapure He as the carrier
gas at a rate of 20 ml/min. The SF 6 peak was isolated from residual contaminants and the carrier gas by
trapping the SF 6 on a cold finger at -192 oC as the carrier gas was pumped out. The isotopic composition
of the purified SF 6 was then determined on a ThermoElectron MAT 253 dual Inlet isotope ratio mass
spectrometer in the Stable Isotope Laboratory of the Earth and Planetary Sciences Department at McGill
University.
2.4 Isotope notation
Isotopic compositions are reported using the delta notation:
(1)
ଷ
ܴ ௦
ߜ ଷܵ = ቆ ଷ
െ 1ቇ × 1000
ܴ ି்
where 3iR = 3iS/32S, i is 3 or 4 and V-CDT refers to the Vienna-Cañon Diablo Troilite international
reference scale. On the V-CDT scale, the ɷ34S value of the Ag 2 S reference material, IAEA-S-1, is defined
as -0.3‰ (Ding et al., 2001). The uncertainty on the measured G34S values is less than ±0.2‰.
The capital delta notation is used to report deviations among the fractionation relationships of 33S-32S
and 34S-32S ratios.
(2)
.ହଵହ
ߜ ଷସܵ
ο ܵ = ߜ S െ 1000 × ൭ቆ1 +
ቇ
1000
ଷଷ
ଷଷ
െ 1൱
(Farquhar et al., 2000; Hulston and Thode, 1965)͘tĞƚĂŬĞƚŚĞȴ33S value of IAEA-S-1 to be 0.094‰ VCDT. dŚĞƵŶĐĞƌƚĂŝŶƚLJŽŶƚŚĞŵĞĂƐƵƌĞĚȴ33S values is less than ±0.01‰.
3. Results
3.1 Porewater sulfate concentration profile
Both cores show similar exponential decreases in sulfate concentrations with depth below the surface
mixed layer (around 10 cm) of the sediment (Figure 1). At depth, sulfate concentrations decreased to
<1.5 mM, corresponding to the detection limit of our gravimetric technique. The only significant
difference between the two profiles was how rapidly the sulfate concentration decreased with depth.
Core 1 reached [SO 4 2-] <1.5 mM at 45 cm depth whereas, in core 2, it reached this concentration at a
depth of 64 cm (Figure 1). The results are comparable to porewater sulfate profiles reported in previous
studies on Mangrove Lake (Boudreau et al., 1992; Canfield et al., 1998a), indicating that long-term
storage and handling of our samples did not affect the results.
3.2 Porewater sulfate ɷ34S profile
The surface mixed layer of the sediment is characterized by ɷ34S values near that of seawater sulfate
(20‰). Core 1 shows a largely linear enrichment in ɷ34S values from 23.7‰ at 7.0cm to 61.8‰ at
28.0cm, at which point isotopic measurements were impossible to perform due to low sulfate
concentrations (Figure 2). Similarly, for core 2, ɷ34S values increased linearly from 23.3‰ at 7.5 cm to
66.0‰ at 47.5 cm. These results are similar to those reported in Canfield et al. (1998).
3.3 Porewater sulfate ѐ33S-G34S patterns
In the surface mixed layer of the sediment, ѐ33S values for cores 1 and 2 (Figure 3) are close to published
measurements of seawater sulfate from the open ocean most recently measured at 0.05 ±0.014к;ϮʍͿ
(Tostevin et al., 2014). At the mixed layer – stratified layer transition, ѐ33S values for both cores appear
to converge on a values between 0.03 and 0.04. These values are followed, at depth, by ѐ33S values that
correlate positively with the increasing G34S values in both cores (Figure 3). In core 1, this trend was
shallow, with '33S values increasing up to 0.06 ‰ but the correlation slope was not significantly
different from 0 at the 95% confidence interval (p=0.15). In core 2, ѐ33S values increase from 0.04‰ at
the bottom of the stratified layer to 0.13‰ in the deepest and most
34
S-enriched sample along the
profile (Figure 3). The correlation slope is much steeper than for core 1 and significantly different from 0
at the 95% confidence interval (p<0.01).
4. Discussion
4.1 Isotopic constraints on sulfur cycling in Mangrove Lake sediments
The sulfur cycle in Mangrove Lake sediments was constrained through a stepwise analysis. First, the
measured sulfate and sulfur isotope profiles were fit to the predictions of a model of net sulfate removal
(as described in section 4.2). The net fractionations estimated from this exercise were then broken
down into component fractionations associated with the major pathways of sulfur flow in the sediments
(sulfate reduction, sulfide reoxidation, and sulfide sequestration; Figure 5). By decomposing the wholecore isotopic fractionations in this way, the reoxidative signal that is preserved in the porewater sulfate
was quantified.
4.2 Estimation of net isotopic fractionation accompanying sulfate removal
In order to isotopically characterize the net sulfate removal process, the measured profiles were split
into two zones: (1) a mixed layer, in which sulfate concentrations and isotopic compositions were
treated as constant boundary conditions; and (2) a stratified layer, beneath the mixed layer, in which
decreasing sulfate concentrations and increasing G34S and '33S values were modelled (cf.Boudreau et al.
(1992)). This geochemical division has been recognized in previous studies (Boudreau et al., 1992;
Canfield et al., 1998a).
Steady-state mass conservation for sulfate can be described with the following simplified diagenetic
equation (Boudreau et al., 1992):
(3)
ܦ௦ௗ
݀ଶ ܥ
= ݎ௧
݀ ݖଶ
where ܦ௦ௗ is the diffusion coefficient of sulfate in the sediment porewaters, ݎ௧ is the net local
removal rate of sulfate, and C is the porewater sulfate concentration. Equivalent expressions hold for
each of the sulfate isotopologues (32SO 4 2-, 33SO 4 2-, and 34SO 4 2-). The sulfur isotope fractionation
associated with net sulfate removal can be expressed as:
ଷ
(4)
ଷ
ߙ௧ = ቆ
ݎ௧
ଷ
ܥ
ଷଶ
ቇ/ቆ
ݎ௧
ቇ
ܥ
ଷଶ
where ଷߙ is the fractionation factor for the heavy isotopologues of sulfate relative to 32S, and i is either
3 or 4. From the steady-state diagenetic equation for each isotopologue, this can also expressed as
ଷ
(5)
ߙ௧
݀ଶ [ ଷଶ]ܥ൘
݀ଶ [ ଷ]ܥ൘
ଶ
݀ ݖଶ
݀ۇ ۊ ݖ
ۇ
ۊ
/
=ۈ
ۋ
ۈ
ۋ
ଷଶ
ଷ
ۋ
ۈ
ۈ ۋ
ܥ
ܥ
ۉ ی
ۉ
ی
such that profiles of each sulfate isotopologue can be used to constrain the isotopic fractionation
accompanying net sulfate removal (Donahue et al., 2008; Goldhaber and Kaplan, 1980; Jørgensen,
1979). This expression assumes that the sulfate diffusion coefficient in the sediment porewaters is
negligibly sensitive to isotopic substitution. The fractionation factors for the heavy isotopologues are
related by:
݈݊ ଷଷߙ
ߣ = ଷସ
݈݊ ߙ
ଷଷ
(6)
where
ratios.
ଷଷ
ߙ is the fractionation factor for
33
S-32S ratios and
ଷସ
ߙ is the fractionation factor for
34
S-32S
Measurements in the stratified layer were interpolated with an exponential decay equation:
(7)
where i is 2, 3, or 4,
ଷ
ଷ
= )ݖ( ܥ
ଷ
ܥ ݁ ି
య
௭
ܥ is the concentration of the various sulfate isotopologues at the top of the
stratified layer and 3ik is a fitting parameter that governs the rate of change of concentration of each
sulfate isotopologue with depth. Although this equation is strictly the solution to the diagenetic
equation for sulfate under the assumption of complete consumption of sulfate at infinite depth (Berner,
1964), solutions that more accurately describe sulfate variations at low sulfate levels (Boudreau et al.,
1992; Boudreau and Westrich, 1984) produced similar quality fits over the depth interval of our
measurements. The exponential expression was used for simplicity.
The total sulfate concentration ɷ34S and '33S profiles were reproduced for each core by treating
ଷ
ܥ
and 3ik, as free parameters in equations (4-7) (Table 1). The fits to the data are visualized in Figures 1, 2
and 3. The parameters were estimated by minimizing the total least squares distance weighted by the
variance of each measurement between the model predictions and the measured values at every
sampled depth below the mixed layer (i.e., a weighted reduced chi-squared value, ߯ఔଶ , where v is the
number of degrees of freedom in the fit and equals the number of measurements minus the number of
parameters in the fit; (Press et al., 1992)). The estimated fractionation factors are expressed as:
(8)
Confidence regions for each
ଷସ
ଷସ
ߝ௧ -
ଷସ
ଷଷ
ߝ௧ (‰) = ( ଷସߙ௧ െ 1) × 1000
ߣ௧ pair were computed by finding the correlated changes in
ߝ௧ and ଷଷߣ௧ values that led to prescribed increases in the ߯ఔଶ values associated with each fit (Press
et al., 1992). The ߯ఔଶ increases were selected so that there was a 95% probability of finding the true
ଷସ
fit
ߝ௧ and ଷଷߣ௧ values within the ellipse around each ଷସߝ௧ - ଷଷߣ௧ pair (Press et al., 1992). The bestଷସ
ߝ௧ -
ଷଷ
ߣ௧ pairs from both cores plot outside the empirical field (shown in grey, Figure 4)
characteristic of pure cultures of sulfate-reducing microorganisms, but the bottom of the 95%
confidence ellipse from core 1 overlaps with this field.
In both cores, the majority of the misfit between model and measurements was taken up by the
differences between the modelled and measured G34S values rather than sulfate concentrations or ѐ33S.
In core 2, the G34S misfit was distributed randomly with depth. Nevertheless, in core 1, the G34S misfit
was greatest at shallow and deep depths (Figure 2A), suggesting that a model with depth-dependent ଷସߝ
and ଷଷߣ values might better fit the data. This possibility was explored by letting ଷସߝ௧ values in core 1
change at the depth of the inflection point in the G34S profile (7.5 cm below the mixed layer; Figure 2A).
In this case, a smaller net fractionation characterized the upper part of the core compared to the net
fractionation in the lower part of the core (Table 1).
In both cores, the majority of the misfit between model and measurements was taken up by the
differences between the modelled and measured G34S values rather than sulfate concentrations or ѐ33S.
In core 2, the G34S misfit was distributed randomly with depth. Nevertheless, in core 1, the G34S misfit
was greatest at shallow and deep depths (Figure 2A), suggesting that a model with depth-dependent ଷସߝ
and ଷଷߣ values might better fit the data. This possibility was explored by letting ଷସߝ௧ values in core 1
change at the depth of the inflection point in the G34S profile (7.5 cm below the mixed layer; Figure 2A).
In this case, a smaller net fractionation characterized the upper part of the core compared to the net
fractionation in the lower part of the core (Table 1).
Based on the
ଷସ
ߙ௧ values that were calculated from cores 1 and 2, the expected ɷ34S H2S of the first
sulfide accumulated in the core can be estimated from:
ଷସ
ߙ௧
ߜ ଷସ ܵுଶௌ
+1
= 1000
ଷସ
ߜ ܵௌைସ
1000 + 1
where ɷ34S H2S is derived from the porewater sulfide of the shallowest sample that has been measured
(Canfield et al., 1998a) and ɷ34S SO4 is the depth-equivalent measure of the porewater sulfate. The
estimates of ɷ34S H2S from modelling based on porewater sulfates ranged from -9 to -7.4‰ (Table 1),
whereas the shallowest porewater sulfides measured at Mangrove Lake are slightly less depleted in 34S
(ɷ34S H2S = -4.8‰; (Canfield et al., 1998a). Taking into consideration that the measured porewater
sulfides were collected from different cores than the ones studied here, the ɷ34S H2S predictions are in
reasonable agreement with the previously measured values. This exercise provides an independent test
of our modelling procedure and illustrates that multiple S-isotope measurements of porewater sulfate
complement techniques based on 34S-32S measurements of porewater sulfide and organic and mineral
sedimentary sulfur fractions (Böttcher et al., 2000). Combined with a similar calculation for 33S-32S, this
method predicts the ѐ33S H2S values of the first-formed sulfide (Table 1). Although a future investigation
of porewater sulfide multiple sulfur isotopes would be required to evaluate the accuracy of this
prediction for Mangrove Lake, we note that these ѐ33S H2S -ߜ ଷସ ܵுଶௌ pairs are outside the range of those
observed for pure cultures of sulfate reducing microbes, but similar to those of environments were S
reoxidation and disproportionation have been hypothesized (Zerkle et al., 2010).
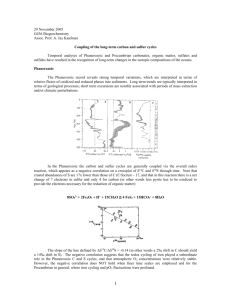
4.3 Isotopic discrimination of the reoxidative sulfur cycle
The net isotopic fractionations calculated in section 4.1 were dissected by expanding the net sulfur
transformation pathway into three component fluxes (Figure 5): (1) a flux of sulfate to sulfide by
microbial sulfate reduction (߮ெௌோ ), (2) a flux of sulfide to sulfate through a reoxidative pathway (߮௫ ),
and (3) a permanent flux of sulfide to sequestered sulfur (߮௦ ). The sequestered sulfur pool includes all
sulfur that is isolated from the porewater system. In the stratified layer at Mangrove Lake, the
sequestered sulfur will largely be organic sulfur produced during diagenesis plus any sulfide fixed as iron
sulfides (Canfield et al., 1998a). Sulfide that escapes into the mixed layer would also be part of this pool.
Although most of the organic sulfur is isotopically static, a small portion [<15%, (Canfield et al., 1998a)]
will still exchange S isotopes with the dissolved sulfide pool. In the representation shown in Figure 5,
this labile organic S would be included in the porewater sulfide pool.
The net fractionation factor accompanying the local removal of porewater sulfate is a function of these
fluxes and their associated fractionation factors. Assuming that the local fluxes are at steady state, the
following elemental mass balance applies:
where ƒ௫ =
ఝೝೣ
ఝಾೄೃ
ఝ
and ƒ௦ = ఝ ೞ .
ಾೄೃ
1 = ƒ௫ + ƒ௦
Assuming no isotopic fractionation associated with sulfur
sequestration, ( 34ߝ = ݍ݁ݏ0), the net local fractionation factor can be expressed as:
ଷ
య
where ଷߙெௌோ = ቆ య
ோಹమ ೄ,ಾೄೃ
ோೄೀ మష,ೢ
ర
ߙ௧ =
ଷ
ଷ
ߙெௌோ
ቀ ߙ௫ ƒ௫ + (1 െ ƒ௫ )ቁ
ቇ and represents the fractionation between the sulfide produced by
microbial sulfate reduction (MSR) and porewater sulfate, whereas ଷߙ௫ = ൭
య
ோೄೀ మష
య
ర
,ೝೣ
ோಹమ ೄ,ೢ
൱ represents
the fractionation between the sulfate ultimately produced by the reoxidation of porewater sulfide and
the porewater sulfide. In turn, this fractionation factor can be expressed as:
ଷ
ఝ
ߙ௫ =
ଷ
ߙ௫ ଷߙ௦
ቀ ଷߙ ƒ + ଷߙ௦ (1 െ ƒ )ቁ
where ƒ = ఝ is the fraction of the sulfide oxidation flux that is returned to the porewater sulfide
pool,
ଷ
ೣ
య
ߙ௫ = ൬య
ோೄ,ೣ
ோಹమೄ,ೢ
൰ represents the fractionation between the S compounds of intermediate
oxidation state (e.g, elemental sulfur, sulfite, thiosulfate) produced by sulfide oxidation and porewater
sulfide,
ଷ
య
ோಹమ ೄ,
ߙ = ቆ య
ோೄ,ೢ
ቇ represents the fractionation between sulfide produced from the S
ଷ
intermediate compounds and the S intermediates in the porewater, and ߙ௦ = ൭
య
ோೄೀ మష
య
ర
,ೞ
ோೄ,ೢ
൱ represents
the fractionation between the sulfate produced from the S intermediate compounds and the S
intermediates in the porewater. For convenience, we recast these fractionation factors as
in the discussion that follows.
4.4 Constraints on individual fractionation factors
ଷସ
ߝ values
ଷସ
ߝெௌோ and ଷଷߣெௌோ : Experiments with pure cultures of sulfate-reducing microbes have produced ଷସߝெௌோ
values that range from near zero to nearly -70‰, close to the limit defined by S-isotope equilibrium
between aqueous sulfate and sulfide (Detmers et al., 2001; Harrison and Thode, 1958; Kaplan and
Rittenberg, 1964; Kemp and Thode, 1968; Leavitt et al., 2013; Sim et al., 2011a; Sim et al., 2011b).
Furthermore, there is an intrinsic linear correlation between
ଷସ
ߝெௌோ and
ଷଷ
ߣெௌோ for pure cultures of
sulfate-reducing microbes (Figures 4 and 6) (Leavitt et al., 2013; Sim et al., 2011a; Sim et al., 2012; Sim
ଷସ
ߝெௌோ and
et al., 2011b). We used a linear fit to published
fixes a value for
ଷଷ
ɉெௌோ once a value for
ଷସ
ଷଷ
ߣெௌோ pairs (cf. Ono et al. (2012)), which
ɂெௌோ is chosen. Although it is clear that there is variability
around such a linear relationship (Wing and Halevy, 2014; Wu and Farquhar, 2013) this approach
captures the first-order impact of MSR on S-isotope fractionation. For the present calculations, we
varied
ଷସ
ɂெௌோ between -10 and -50‰ because values lower and higher did not appear to fit the S cycle
in Mangrove Lake.
ଷସ
ߝ௫ and
ଷଷ
ߣ௫ : Sulfide oxidation experiments on pure cultures of phototrophic sulfide oxidizers have
produced ଷସߝ௫ values that range from 0 to +3‰ (Chambers and Trudinger, 1979; Fry et al., 1984; Fry et
ଷସ
ߝ௫ values associated with abiotic sulfide
al., 1988; Ivanov et al., 1977; Zerkle et al., 2009). The
oxidation by oxygen can extend from -7.5 to -4.1‰ (Fry et al., 1988). Sulfide oxidation by metal oxides
such as MnO 2 , has been assumed to produce the same fractionation as sulfide oxidation with oxygen
(Böttcher and Thamdrup, 2001). Sulfide oxidation by chemoautotrophic sulfide oxidation is not e
isotopically constrained but we assume it has fractionations resembling abiotic sulfide oxidation with
oxygen. The small fractionation factors of these processes are characteristic of sulfide oxidation (Zerkle
et al., 2009), but some secondary side reactions can produce larger fractionations in less common sulfur
species (like thiosulfate), and this may potentially
Rittenberg, 1964). Our current knowledge of
characterized by
ଷଷ
produce higher net fractionation (Kaplan and
ଷଷ
ߣ௫ values is limited. Phototrophic sulfide oxidation is
ߣ௫ = 0.529 at ଷସߝ௫ = +1.5‰, whereas S-isotope fractionation during abiotic sulfide
oxidation has been estimated to be
ଷଷ
ߣ௫ = 0.5145 at
ଷସ
ߝ௫ = -5‰ (Zerkle et al., 2009). Fractionation
values within this range are used in sensitivity tests of the impact of sulfide oxidation on our model
calculations.
ଷସ
ߝ௦ and
ଷଷ
ߣ௦ ,
ଷସ
ߝ and
ଷଷ
ߣ : Two end-member situations are considered for the fate of S
compounds of intermediate oxidation state, specifically elemental sulfur, sulfite and thiosulfate. In the
first case, all intermediate S compounds produced by sulfide oxidation are ultimately oxidized to sulfate.
In this case, ƒ௦ = 1 (ƒ = 0) and the fractionation associated with the transformation of intermediate S
compounds to sulfate is not expressed (i.e.,
ଷ
ߙ௫ =
ଷ
ߙ௫ ). In the second case, the transformation
of intermediate S compounds occurs by microbial disproportionation of elemental sulfur (S0), sulfite, or
thiosulfate. Experiments with pure cultures and natural communities of sulfur disproportionating
ଷସ
ߝ௦ has a positive value while
microbes show that
ଷସ
ߝ takes on a complementary negative value,
R
with the overall isotopic separation largely depending on the compound undergoing disproportionation
(Böttcher and Thamdrup, 2001; Canfield and Thamdrup, 1994; Canfield and Thamdrup, 1996; Canfield et
al., 1998b; Cypionka et al., 1998).
Here, we consider two disproportionation reactants for which multiple sulfur isotope data are available:
sulfite and elemental sulfur (Johnston et al., 2005). The stoichiometry of sulfite disproportionation leads
to the production of three sulfate molecules for every sulfide molecule (Bak and Cypionka, 1987). Given
this stoichiometry, the fractionation parameters for disproportionation of sulfite to sulfate are
10‰ and
ଷଷ
ߣ௦ = 0.528 whereas for sulfite to sulfide they are
ଷସ
ߝ = -45‰ and
ଷଷ
ଷସ
ߝ௦ =
ߣ = 0.5115.
Elemental sulfur disproportionation occurs with a product stoichiometry of one sulfate molecule for
every three sulfide molecules (Thamdrup et al., 1993). In this case,
ଷସ
ߝ௦ = 18.5 ‰ and
ଷଷ
ߣ௦ = 0.5195
whereas ଷସߝ = -6 ‰ and ଷଷߣ = 0.5165 (Zerkle et al., 2009). As there is only a single porewater sulfide
pool in our steady-state model, the stoichiometry of the disproportionation reactions sets ƒ௦ = 0.75 (ƒ
= 0.25) when sulfite is the reactant and ƒ௦ = 0.25 (ƒ = 0.75) when it is elemental sulfur.
Controls on net isotopic fractionation
Results of our calculations are presented as contour plots of ଷସߝ௧ and ଷଷߣ௧ , with variable ଷସߝெௌோ and
ƒ௫ where bold lines indicate
ଷସ
ߝெௌோ and thin lines are ƒ௫ (Figure 6). When net sulfate loss is only
R
due to microbial sulfate reduction such that it is the sole process affecting ଷସߝ௧ and ଷଷߣ௧ - shown by
the contour where ƒ௫ = 0 ଷସ
ଷଷ
ߣ௧ climbs from 0.5094 at
ଷସ
ߝ௧ = 0‰ to approximately 0.5148 at
ߝ௧ = -50‰ (Figure 6). As the compilation in Figure 4 illustrates, there are environmental and possibly
microbial species-specific controls on this ideal relationship that lead to ଷଷߣ௧ variations on the order of
0.002 for a given
ଷସ
ߝெௌோ . When viewed in light of the small fractionations associated with sulfide
oxidation, the variability associated with sulfate reduction masks the isotopic signatures of sulfide
oxidation.
These speculations are supported by calculations that demonstrate the small scale of sulfide
reoxidation. For example, a large fraction of the sulfide produced by microbial sulfate reduction needs
to be reoxidized to produce a sizeable change in the net isotopic fractionation.
In Figure 6A,
phototrophic sulfide oxidation produces a ଷସߝ௧ that is slightly more negative (and ଷଷߣ௧ slightly more
positive) than it would have been if sulfide oxidation was absent from the system. Yet, the variations in
the empirical field of
ଷସ
ଷଷ
ߝெௌோ and
ߣெௌோ alone cover nearly the entire range of model predictions.
Similar conclusions are reached if abiotic sulfide oxidation is considered (Figure 6B). In these
calculations, the model predictions essentially track the contour of pure microbial sulfate reduction
except when
ଷସ
ߝ௧ is less than -10 ‰. At more positive
ଷସ
ߝ௧ , predicted
ଷଷ
ߣ௧ values produced by a
combination of sulfate reduction and abiotic sulfide oxidation fall outside the empirical field of microbial
sulfate reduction, reaching a ଷଷߣ௧ as low as 0.505 when a ଷସߝெௌோ of -5‰ is used.
Although the disproportionation metabolisms are dependent on the presence of sulfur intermediates,
and therefore sulfide oxidation,
ଷସ
ߝ௫ is set to 0 in our next calculations in order to isolate the isotopic
impact of the disproportionation metabolisms. Sulfite disproportionation produces large changes in
ଷଷ
ߣ௧ when ଷସߝெௌோ is small, but these get progressively smaller as ଷସߝெௌோ increases (Figure 6C). In fact,
beyond
ଷସ
ߝெௌோ у -40‰,
ଷଷ
ߣ௧ approaches the value originally imprinted by the fractionation
R
associated with microbial sulfate reduction. Despite a different stoichiometry, elemental sulfur
disproportionation produces similar, although somewhat larger, signatures (Figure 6D). Here again, the
signal in
ଷଷ
ଷସ
ߣ௧ is large when
ߝெௌோ is smaller but collapses to
about -40‰.
R
ଷଷ
ߣ௦ as
ଷସ
ߝெௌோ increases beyond
R
Microbial sulfate reduction alone (Figure 6 A-D when ƒ௫ =0) will not explain the large
33
ߣ݊݁ ݐvalues
observed in Mangrove Lake. Furthermore, fractionations added by sulfide oxidation alone are too small
to account for these large
33
ߣ݊݁ ݐvalues (Figure 6A, 6B). These results appear to rule out a S cycle that
consists only of microbial sulfate reduction or one in which sulfate reduction is accompanied by the
complete reoxidation of sulfide to sulfate without disproportionation. On the other hand, when
disproportionation is considered, the modelled field expands to include the results from both cores
because of the slightly larger
33
ߣ value associated with this process (Figure 6C, 6D).
4.5 Analysis of the sulfur cycle in Mangrove Lake
Here, we highlight the important issues identified in the previous section that impact the overall
understanding of the S cycle in Mangrove Lake. These results are discussed in the context of the
scenario that best reflects both the new isotopic constraints as well as the existing body of knowledge
(Figure 6D).
The sensitivity modelling in Figure 6 shows that both cores require
34
ߝ ܴܵܯbetween -15 and -30‰.
These values are comparable to the lower end of sulfur isotope fractionations during sulfate reduction,
as estimated from other marine environments (Canfield et al., 2010). The small
34
ߝ ܴܵܯvalues may
R
reflect the physical and chemical conditions present in Mangrove Lake. The average temperature of the
marine ƐĂƉƌŽƉĞůŝƐƌĞůĂƚŝǀĞůLJŚŝŐŚ;уϮϴ °C) and organic matter is extremely abundant > 60% dry weight),
resulting in high sulfate reduction rates in Mangrove Lake sediments (Boudreau et al., 1992; Canfield et
al., 1998a). Elevated temperatures can lead to lower
34
ߝ ܴܵܯvalues (Hoek et al., 2006) as can the
presence of readily metabolizable organic matter because both factors promote high sulfate reduction
rates which, in turn, lead to smaller
ଷସ
ߝெௌோ values (e.g. (Habicht and Canfield, 1996; Harrison and
R
Thode, 1958; Kaplan and Rittenberg, 1964; Leavitt et al., 2013; Sim et al., 2011a; Sim et al., 2011b).
Net sulfate removal rates decrease with depth, dropping by nearly one order of magnitude over ~30 cm
(Boudreau et al., 1992), and the data gathered from core 1 are potentially consistent with a rate control
on fractionation. The isotopic profile from this core would suggest that
34
ߝ݊݁ ݐand
ଷଷ
ߣ௧ increase with
depth (Table 1). The rate control on fractionation, a behavior which has been recorded since the earliest
studies of the dissimilatory sulfate reducing metabolism (Harrison and Thode, 1958; Kaplan and
Rittenberg, 1964), has recently been shown to display a consistent minor (33S) isotope trajectory (Leavitt
et al., 2013; Sim et al., 2011a; Sim et al., 2011b). Whether or not the fractionation estimated from core 1
results from a rate control on fractionation during sulfate reduction, however, hinges on whether the
isotopic fractionation is completely set by microbial sulfate reduction, or whether re-oxidation plays a
role.
Mangrove Lake sediments lack the metal oxides (Fe(III)O x , Mn(III,IV)O x ) that normally serve as electron
acceptors during sulfide oxidation in marine sediments (Jørgensen and Nelson, 2004). Nevertheless, the
presence of photolithotrophic green and purple sulfur bacteria within the upper sediments of Mangrove
Lake (Stolz, 1991) suggests that anoxygenic photosynthesis might drive sulfide oxidation. The benthic
cyanobacterial community that is also present (Stolz, 1991) leads to daytime O 2 maxima (>150% air
saturation) at the sediment-water interface and O 2 penetration depths of ~0.5 cm (Canfield et al.,
1998a). Wind-induced mixing of the surface-sediment mixed layer could intermittently inject oxygen
deeper into the sediment, enabling direct abiotic oxidation of sulfide as well as chemoautotrophic
sulfide oxidation by Beggiatoa (Nelson and Jannasch, 1983). Thus, sulfur intermediates are likely
produced in-situ within the sediment mixed layer and the upper parts of the underlying stratified layer.
This suggestion is supported by process-rate measurements as well as direct radiotracer incubation
experiments that showed net sulfate removal (or evidence for sulfide production) is highest in the
surface-sediment mixed layer (Boudreau et al., 1992). In-situ porewater sulfide accumulation, on the
other hand, is only observed the upper stratified layer (Boudreau et al., 1992; Canfield et al., 1998a;
Hatcher et al., 1982). This imbalance sustains a strong S0 concentration gradient in the sediment of the
mixed layer and the upper stratified layer of the sediment where concentrations of up to roughly 300
µmol g-1 of wet? sediment in the mixed layer gradually decrease to zero at a depth of approximately
20cm (Canfield et al., 1998a).
Although S0 is a potential substrate for disproportionation-based
metabolisms, there are two competing demands that must be satisfied if S-disproportionation is the
source of the S isotopic signatures identified here.
First, only the S0 that is mixed deeper into the sediment from overlying sediments or synthesized in-situ
via sulfide oxidation in the stratified layer, can be a substrate for disproportionation and generate the
isotopic signal. Second, disproportionation is favored when sulfide concentrations remain low (<1mM or
lower) since this keeps the process thermodynamically viable (Canfield and Thamdrup, 1996; Finster et
al., 1998; Thamdrup et al., 1993). These two conditions (low [H 2 S] and a significant standing stock of S0)
are only met in ĂƐŚŽƌƚĚĞƉƚŚŝŶƚĞƌǀĂů;уϭϬĐŵͿbordered by the base of the mixed layer and the sulfidic
porewaters below (Canfield et al., 1998a). Although this region is thin, the interplay between the high
net rates of sulfate removal and the sizeable S0 pool enables biogeochemical processes that occur within
this region to set the S-isotope signature of sulfate measured in the deeper parts of the profile.
In sulfidic sediments of the Black Sea and Weser Estuary, S0 is the main product of sulfide oxidation with
Fe(III) or Mn(IV) oxides, with S0 concentrations orders of magnitude higher than sulfite (where
considered on cm3 of total sediment basis) (Zopfi et al., 2004). Sulfite rarely accumulates to high
concentrations in porewaters because it is quickly removed by reaction with S0 to form S 2 O 3 2-. For
instance, micron-resolution concentrations profiles in highly active microbial mats consisting of
cyanobacteria and purple sulfur bacteria show that a peak in sulfite is present only in the first 2mm
(Wieland et al., 2001). This being said, although we suspect S0 disproportionation to be a major
component of Mangrove Lake’s S cycle, the influence of sulfite (and thiosulfate) disproportionation
cannot be ruled out since neither sulfite nor its immediate reaction products (e.g., thiosulfate) have
been measured in the sediments of Mangrove Lake.
4.6 Estimate of the reoxidative S flux
The geochemical and isotopic constraints on the S cycle in Mangrove Lake appear to suggest that sulfide
oxidation followed by S0 disproportionation is the main reoxidative pathway. S0 in the Mangrove Lake
sediments (Canfield et al., 1998a) is likely sourced by phototrophic, chemoautotrophic, direct abiotic
sulfide oxidation or a combination of the three. In metal-poor environments, rates of chemical sulfide
oxidation in the presence of oxygen are orders of magnitude slower than microbial oxidative processes
(Luther III et al., 2011). The geochemical conditions in Mangrove Lake may therefore favor sulfide
oxidation via a microbial pathway. Regardless of the dominant sulfide oxidation process in Mangrove
Lake sediments, the isotope effects associated with oxidation barely expands the
34
ߝ݊݁ ݐand
33
ߣ݊݁ ݐfield
relative to S0 disproportionation alone (results not shown). As discussed above, the fractionation due to
microbial sulfate reduction is limited, and elemental S disproportionation extends these initial
fractionations to produce the observed ଷସߝ௧ values (Figure 6D).
Following contours of constant 34ߝ ( ܴܵܯ34ߝ ܴܵܯу-ϮϱкĨŽƌĐŽƌĞϭĂŶĚу-15‰ for core 2) to the estimated
values of
34
ߝ݊݁ ݐand
ଷଷ
ߣ௧ shows that that ƒ௫ may be up to у0.5 in core 1 and у0.8 in core 2 (Figure
6D). This means that for each mole of SO 4 2- reduced to H 2 S in Mangrove Lake, у0.5 to 0.8 moles will be
reoxidized back to SO 4 2-. Of the remaining sulfide, roughly 5% will be sequestered in the sediments as
organic sulfides in the sediments (Canfield et al., 1998a) while the rest (15-45%) will be “lost” from the
system by diffusing out of the stratified layer into the mixed layer. This lost sulfur likely also gets
reoxidized back to sulfate but reoxidation likely occurs within the sediment surface mixed layer where
physical mixing leads to exchange and dilution with sulfate in the overlying water column. The '33S
values for sulfate in the mixed layer of core 2 may be providing a record of this process. They are
slightly larger than the '33S values from the sediments below, but have similar G34S values. Phototrophic
sulfide oxidation produces this type of effect (Figure 6A).
4.7 Implications for isotopic discrimination of the reoxidative sulfur cycle
In order to explore how well reoxidative sulfur cycling can be discriminated on the basis of S isotope
measurements, we compare the 34ߝ݊݁ ݐ- 33ߣ݊݁ ݐfield produced by pure cultures of sulfate-reducing
bacteria to the results of our modelling (Figure 6). This exercise reveals that there are three regions in
ߝ݊݁ ݐ- 33ߣ݊݁ ݐspace that enable reoxidative sulfur cycling to be isotopically distinguished from microbial
34
sulfate reduction.
First, isotopic fractionation of multiple sulfur isotopes can be diagnostic of reoxidative processes when
ଷସ
ߝெௌோ is very close to zero (between 34ߝ݊݁ = ݐ0‰ and -20‰). In such a situation, abiotic and biological
sulfide oxidation could be identified because they produce large or small ଷଷߣ௧ values (Figure 6A, 6B)
that migrate outside of the field associated with MSR. In an environment displaying these fractionations,
minimal disproportionation reactions would mask the sulfide oxidation signal because of its larger 34ɸ͘
Environments with high sulfide concentrations e or lacking a bioavailable S-substrate of intermediate
oxidation states will prevent disproportionation and may even promote a direct oxidation pathway
(Habicht and Canfield, 2001). In such instances, the minor S isotopic signature of sulfide oxidation might
be distinguished from sulfate reduction alone.
Second, an ଷସߝோ் ƚŚĂƚŝƐŵŽƌĞŶĞŐĂƚŝǀĞƚŚĂŶу-66‰ – the largest fractionation yet measured in a pure
culture of sulfate reducers and very similar to the maximum observed in natural populations (Canfield et
al., 2010; Sim et al., 2011a) - likely indicates the presence of reoxidative sulfur cycling. Only the
compounding effect of multiple large fractionation factors can explain these signatures. For example,
some measurements in sulfidic Black Sea sediments (Figure 4) yield ଷସߝ௧ values that are more negative
than -66‰. Apart from the isotopic evidence, the presence of intermediate oxidation products which
can serve as substrate for disproportionation (Konovalov et al., 2007) coupled with low sulfate reduction
rates have been argued to be responsible for the large fractionations observed in sediment porewaters
(Neretin et al., 2003).
R
R
Finally, when ଷସߝ௧ is less negative than ~ -66‰, ଷଷߣ௧ values above the field of MSR outlined in
Figure 4 indicate an active reoxdative cycle. For example, the sediments in Wedderwarden (Figure 4), a
marine tidal flat in northern Germany, are characterized by relatively low 34ߝ݊݁ ݐcoupled with high 33ߣ݊݁ݐ
(Johnston et al., 2008). Elemental sulfur disproportionation was documented as a major microbial
metabolism in the Wedderwarden tidal flats in the thin interface between the overlying oxic layer and
the region of H 2 S accumulation (Canfield and Thamdrup, 1996). Although the sediment at
Wedderwarden has higher abundances of Fe and Mn oxides compared to Mangrove Lake, the sulfidic
conditions at the interface between the oxic layer and the H 2 S accumulation layer appear similar,
favouring S0 disproportionating microorganisms. This likely contributes to the high
both systems.
33
ߣ݊݁ ݐobserved in
Nevertheless, there is a diagnostic limitation to large ଷଷߣ௧ values. As ଷସߝெௌோ gets more negative, the
isotopic effect of some reoxidative processes will impart a smaller change in ଷଷߣ௧ , producing ଷଷߣ௧ ଷସ
ߝ௧ trajectories that parallel the MSR field (Figures 6C, 6D). For these cases,
33
ߣ݊݁ ݐshould fall within
the field of MSR when 34ߝ ܴܵܯis more negative than -40‰ despite the presence of reoxidative cycling.
This behavior renders the reoxidative cycle invisible to the ଷଷߣ௧ isotopic diagnostic. For example, the
isotopic fractionation in Aarhus Bay (Figure 4) appears only as a MSR signature despite biogeochemical
conditions being conducive to active reoxidative cycling in the sediments (Habicht and Canfield, 2001;
Moeslund et al., 1994). In such instances, other techniques might be used to trace the S cycle. For
instance, in the same example as above, the likely presence of disproportionation was determined by
comparing natural population fractionations with sediment values (Habicht and Canfield, 2001).
5. Conclusions
New porewater sulfate multiple S isotope profiles from Mangrove Lake Bermuda show a linear increase
in '33S with increasing G34S. A simple diagenetic model accounts for whole-core abundance and isotopic
variability of porewater sulfate with a single set of 34ߝ݊݁ ݐand
R
33
ߣ݊݁ ݐvalues that characterize the net
fractionation in this system. Relatively small 34ߝ݊݁ ݐvalues are coupled to large 33ߣ݊݁ ݐ. This behavior is
R
inconsistent with a S cycle driven solely by microbial sulfate reduction. We developed a new model of
reoxidative S-isotope fractionation that constrains the contribution of reoxidation in Mangrove Lake to
between 50 and 80% of the microbial sulfate reduction flux. This model implies that sulfide oxidation to
S0, followed by the disproportionation of S0 to sulfide and sulfate, is critical in generating the isotopic
signature. Although they are applied here specifically to Mangrove Lake, the model predictions (i.e..,
Figure 6) can be directly applied to other sedimentary systems where reoxidative cycle is thought to be
important. The new understanding developed from the Mangrove Lake case study highlights the
diagnostic capabilities of the multiple sulfur isotope approach in identifying the importance of the
reoxidative S cycle in sedimentary porewaters.
6. Acknowledgements
This work was supported by NSERC through a Canada Graduate Fellowship to AP and a Discovery grant
to BAW. AM acknowledges NSERC for grants that allowed sample acquisition and participation in this
work. DEC acknowledges the Danish National Research Foundation (grant DNRF53) and the “Oxygen”
grant from the ERC. The Stable Isotope Laboratory at McGill is supported by FQRNT through the
GEOTOP research center. We thank two anonymous reviewers for their constructive comments that
improved our understanding and presentation of the study described here.
7. List of Tables and Figures
Table 1: Diagenetic parameters for the two cores described in this study and previous cores taken at
Mangrove Lake
Figure 1: Sulfate concentration profiles taken from sampled cores in Mangrove Lake (ML). A) Sulfate
concentrations from core 1. Filled symbols are samples from the sediment mixed layer. Unfilled symbols
are from the stratified layer. The black line corresponds to the model fit to all samples in the stratified
layer that have both sulfate concentration and isotope composition measurements. The dashed line
corresponds to the upper core model fit. The dotted line corresponds to the lower core model fit. B)
Sulfate concentrations from core 2. Filled symbols are samples from the sediment mixed layer. Unfilled
symbols are from the stratified layer. The black line corresponds to the model fit to all samples in the
stratified layer that have both sulfate concentration and isotope composition measurements.
Figure 2: ɷ34S isotopic profile of sulfate in ML. A) Sulfate ɷ34S values from core 1. Filled symbols are
samples from the sediment mixed layer. Unfilled symbols are from the stratified layer. The black line
corresponds to the model fit to all samples in the stratified layer that have both sulfate concentration
and isotope composition measurements. The dashed line corresponds to the upper core model fit. The
dotted line corresponds to the lower core model fit. B) Sulfate ɷ34S values from core 2. Filled symbols
are samples from the sediment mixed layer. Unfilled symbols are from the stratified layer. The black line
corresponds to the model fit to all samples in the stratified layer that have both sulfate concentration
and isotope composition measurements.
Figure 3͗ŽŵƉĂƌŝƐŽŶŽĨѐ33S and ɷ34S measurements. A) Sulfate ѐ33S and ɷ34S values from core 1.
Unfilled circles are samples taken from the stratified layer. Filled circles indicate samples taken from the
mixed layer. The grey triangle is the measured seawater value of Tostevin et al. (2014). Black bars are
2ʍ measurement uncertainties. The black line indicates the full core model fit for samples from the
stratified layer and the shaded grey area corresponds to the 95% confidence interval on this fit. The
dashed line corresponds to the upper core model fit. The dotted line corresponds to the lower core
model fit. B) Sulfate ѐ33S, ɷ34S values and model fit from core 2. Unfilled squares are samples taken
from the stratified layer. Filled squares indicate samples taken from the mixed layer. The grey triangle is
the seawater value. Black bars are 2ʍ measurement uncertainties. The black line indicates the full core
model fit for samples from the stratified layer and the shaded grey area corresponds to the 95%
confidence interval on this fit.
Figure 4: Comparison of
ߝ݊݁ ݐand ଷଷߣ௧ of the Mangrove Lake modeled cores with results of previous
34
studies of the sulfur cycle in modern lakes or sediments. Circle is Core 1, square is Core 2. Elliptical
outlines indicate the modelled 2ʍ uncertainty. The grey field outlines fractionations produced by pure
cultures of sulfate reducers (Johnston et al., 2005; Leavitt et al., 2013; Sim et al., 2011a; Sim et al.,
2011b). Data displayed in panel A are environmental samples and reflect S-isotope fractionation
associated with S cycling (including sulfate reduction and sulfide reoxidation) in natural microbial
populations (Johnston et al., 2008; Strauss et al., 2012) whereas data in panel B are from incubation or
diagenetic models that were designed to isolate the fractionations associated with microbial sulfate
reduction in natural populations (Canfield et al., 2010; Farquhar et al., 2008; Zerkle et al., 2010).
Figure 5: Conceptual model of sulfur cycling in Mangrove Lake. The four boxes correspond to the sulfate,
sulfide, intermediate oxidation-state S compounds (e.g., elemental sulfur, thiosulfate, sulfite), and
sequestered pools of sulfur (e.g., metal sulfides, organic sulfides). The arrows between the boxes
correspond to sulfur fluxes . Each arrow also has a specific 34ɲ and 33ʄ associated with it. Sulfate entering
the system is reduced to sulfide by microbial sulfate reduction. The generated sulfide is either reoxidized
to sulfate via intermediate S compounds or is taken out of the system by sequestration.
Figure 6: Modeled reoxidative processes potentially affecting the sulfur cycle in Mangrove Lake. The
circle is Core 1, the square is Core 2 and correspond to the calculated 34ߝ݊݁ ݐand ଷଷߣ௧ . The cores are
underlain with contour lines as a function of changing 34ɸ msr as well as the fraction of reoxidation.
Arrows indicate the direction of increasing 34ߝ݉ ݎݏand f reox . The fractionations associated with microbial
sulfate reduction alone (bold lines, 34ߝ݉ ݎݏvaried between -10 and -50‰) are displayed as the set of
contours lines where the fraction of reoxidation (f reox ) equals zero. The other set of contours traces the
fraction of the sulfate reduction flux that is reoxidized. A) Corresponds to a model where only the
phototrophic sulfide oxidation route has assigned fractionation factors, 34ߝ = ݔ1.54, ଷଷߣ୭୶ = 0.5290. B)
Corresponds to a model where the fractionation resembles abiotic sulfide oxidation with oxygen: 34ߝ= ݔ
-5, ଷଷߣ୭୶ = 0.5149. C) Corresponds to a model with sulfite disproportionation alone: 34ߝ݄݅ = -45.24, 34ߝ݅ܽ =
9.97, ଷଷߣ = 0.5115 and ଷଷߣ = 0.5281. D) Corresponds to the field of elemental sulfur
disproportionation alone: 34ߝ݄݅ = -6.18, 34ߝ݅ܽ = 18.53, ଷଷߣ = 0.5165 and ଷଷߣ = 0.5195.
8. References
Aller, R.C., Madrid, V., Chistoserdov, A., Aller, J.Y., Heilbrun, C., 2010. Unsteady diagenetic processes and
sulfur biogeochemistry in tropical deltaic muds: Implications for oceanic isotope cycles and the
sedimentary record. Geochimica et Cosmochimica Acta 74, 4671-4692.
Bak, F., Cypionka, H., 1987. A novel type of energy metabolism involving fermentation of inorganic
sulphur compounds. Nature 326, 891-892.
Berner, R.A., 1964. An idealized model of dissolved sulfate distribution in recent sediments. Geochimica
et Cosmochimica Acta 28, 1497-1503.
Böttcher, M.E., Hespenheide, B., Llobet-Brossa, E., Beardsley, C., Larsen, O., Schramm, A., Wieland, A.,
Böttcher, G., Berninger, U.-G., Amann, R., 2000. The biogeochemistry, stable isotope geochemistry, and
microbial community structure of a temperate intertidal mudflat: an integrated study. Cont Shelf Res 20,
1749-1769.
Böttcher, M.E., Thamdrup, B., 2001. Anaerobic sulfide oxidation and stable isotope fractionation
associated with bacterial sulfur disproportionation in the presence of MnO 2 . Geochimica et
Cosmochimica Acta 65, 1573-1581.
Boudreau, B.P., Canfield, D.E., Mucci, A., 1992. Early diagenesis in a marine sapropel, Mangrove Lake,
Bermuda. Limnology & Oceanography 37, 1738-1753.
Boudreau, B.P., Westrich, J.T., 1984. The Dependence of Bacterial Sulfate Reduction on Sulfate
Concentration in Marine-Sediments. Geochimica et Cosmochimica Acta 48, 2503-2516.
Canfield, D., Thamdrup, B., 1994. The production of 34S-depleted sulfide during bacterial
disproportionation of elemental sulfur. Science 266, 1973-1975.
Canfield, D.E., 2001. Biogeochemistry of Sulfur Isotopes. Reviews in Mineralogy and Geochemistry 43,
607-636.
Canfield, D.E., Boudreau, B.P., Mucci, A., Gundersen, J.K., 1998a. The early diagenetic formation of
organic sulfur in the sediments of Mangrove Lake, Bermuda. Geochimica et Cosmochimica Acta 62, 767781.
Canfield, D.E., Farquhar, J., Zerkle, A.L., 2010. High isotope fractionations during sulfate reduction in a
low-sulfate euxinic ocean analog. Geology 38, 415-418.
Canfield, D.E., Jørgensen, B.B., Fossing, H., Glud, R., Gundersen, J., Ramsing, N.B., Thamdrup, B., Hansen,
J.W., Nielsen, L.P., Hall, P.O.J., 1993. Pathways of organic carbon oxidation in three continental margin
sediments. Marine Geology 113, 27-40.
Canfield, D.E., Teske, A., 1996. Late proterozoic rise in atmospheric oxygen concentration inferred from
phylogenetic and sulphur-isotope studies. Nature 382, 127-132.
Canfield, D.E., Thamdrup, B., 1996. Fate of elemental sulfur in an intertidal sediment. FEMS
Microbiology Ecology 19, 95-103.
Canfield, D.E., Thamdrup, B., Fleischer, S., 1998b. Isotope fractionation and sulfur metabolism by pure
and enrichment cultures of elemental sulfur-disproportionating bacteria. Limnology and Oceanography
43, 253-264.
Chambers, L.A., Trudinger, P.A., 1979. Microbiological fractionation of stable sulfur isotopes: A review
and critique. Geomicrobiology Journal 1, 249-293.
Cypionka, H., Smock, A.M., ƂƩĐŚĞƌ, M.E., 1998. A combined pathway of sulfur compound
disproportionation in Desulfovibrio desulfuricans. FEMS Microbiology Letters 166, 181-186.
Detmers, J., Brüchert, V., Habicht, K.S., Kuever, J., 2001. Diversity of sulfur isotope fractionations by
sulfate-reducing prokaryotes. Applied and Environmental Microbiology 67, 888-894.
Diaz, R., Moreira, M., Mendoza, U., Machado, W., Böttcher, M.E., Santos, H., Belém, A., Capilla, R.,
Escher, P., Albuquerque, A.L., 2012. Early diagenesis of sulfur in a tropical upwelling system, Cabo Frio,
southeastern Brazil. Geology 40, 879-882.
Ding, T., Valkiers, S., Kipphardt, H., De Bievre, P., Taylor, P.D.P., Gonfiantini, R., Krouse, R., 2001.
Calibrated sulfur isotope abundance ratios of three IAEA sulfur isotope reference materials and V-CDT
with a reassessment of the atomic weight of sulfur. Geochimica Et Cosmochimica Acta 65, 2433-2437.
Donahue, M.A., Werne, J.P., Meile, C., Lyons, T.W., 2008. Modeling sulfur isotope fractionation and
differential diffusion during sulfate reduction in sediments of the Cariaco Basin. Geochimica Et
Cosmochimica Acta 72, 2287-2297.
Farquhar, J., Bao, H., Thiemens, M., 2000. Atmospheric Influence of Earth's Earliest Sulfur Cycle. Science
289, 756-758.
Farquhar, J., Canfield, D.E., Masterson, A., Bao, H., Johnston, D., 2008. Sulfur and oxygen isotope study
of sulfate reduction in experiments with natural populations from Fællestrand, Denmark. Geochimica et
Cosmochimica Acta 72, 2805-2821.
Farquhar, J., Johnston, D.T., Wing, B.A., 2007. Implications of conservation of mass effects on massdependent isotope fractionations: Influence of network structure on sulfur isotope phase space of
dissimilatory sulfate reduction. Geochimica et Cosmochimica Acta 71, 5862-5875.
Finster, K., Liesack, W., Thamdrup, B., 1998. Elemental sulfur and thiosulfate disproportionation by
Desulfocapsa sulfoexigens sp. nov., a new anaerobic bacterium isolated from marine surface sediment.
Applied and Environmental Microbiology 64, 119-125.
Fry, B., Gest, H., Hayes, J.M., 1984. Isotope effects associated with the anaerobic oxidation of sulfide by
the purple photosynthetic bacterium, Chromatium vinosum. FEMS Microbiology Letters 22, 283-287.
Fry, B., Ruf, W., Gest, H., Hayes, J.M., 1988. Sulfur isotope effects associated with oxidation of sulfide by
O2 in aqueous solution. Chemical Geology: Isotope Geoscience Section 73, 205-210.
Gagnon, C., Mucci, A., Pelletier, É., 1995. Anomalous accumulation of acid-volatile sulphides (AVS) in a
coastal marine sediment, Saguenay Fjord, Canada. Geochimica et Cosmochimica Acta 59, 2663-2675.
Goldhaber, M.B., Kaplan, I.R., 1980. Mechanisms of sulfur incorporation and isotope fractionation during
early diagenesis in sediments of the gulf of California. Marine Chemistry 9, 95-143.
Habicht, K.S., Canfield, D.E., 1996. Sulphur isotope fractionation in modern microbial mats and the
evolution of the sulphur cycle. Nature 382, 342-343.
Habicht, K.S., Canfield, D.E., 2001. Isotope fractionation by sulfate-reducing natural populations and the
isotopic composition of sulfide in marine sediments. Geology 29, 555-558.
Harrison, A.G., Thode, H.G., 1958. Mechanism of the bacterial reduction of sulphate from isotope
fractionation studies. Transactions of the Faraday Society 54, 84-92.
Hatcher, P.G., Simoneit, B.R.T., Mackenzie, F.T., Neumann, A.C., Thorstenson, D.C., Gerchakov, S.M.,
1982. Organic geochemistry and pore water chemistry of sediments from Mangrove Lake, Bermuda.
Organic Geochemistry 4, 93-112.
Hoek, J., Reysenbach, A.-L., Habicht, K.S., Canfield, D.E., 2006. Effect of hydrogen limitation and
temperature on the fractionation of sulfur isotopes by a deep-sea hydrothermal vent sulfate-reducing
bacterium. Geochimica et Cosmochimica Acta 70, 5831-5841.
Howarth, R.W., Jørgensen, B.B., 1984. Formation of 35S-labelled elemental sulfur and pyrite in coastal
marine sediments (Limfjorden and Kysing Fjord, Denmark) during short-term 35SO 4 Ϯо reduction
measurements. Geochimica et Cosmochimica Acta 48, 1807-1818.
Hulston, J.R., Thode, H.G., 1965. Cosmic-ray-produced 36S and 33S in metallic phase of iron meteorites. J
Geophys Res 70, 4435-&.
Ivanov, M.V., G.I. Gogotova, A.G. Matrosov, Zyakun, A.M., 1977. Fractionation of sulfur isotopes by
phototrophic sulfur bacteria Ectothiorhodospira shaposhnikovii
Microbiology (Engl. Transl. Mikrobiologiya) 45, 655-659.
Johnston, D.T., Farquhar, J., Habicht, K.S., Canfield, D.E., 2008. Sulphur isotopes and the search for life:
strategies for identifying sulphur metabolisms in the rock record and beyond. Geobiology 6, 425-435.
Johnston, D.T., Farquhar, J., Wing, B.A., Kaufman, A.J., Canfield, D.E., Habicht, K.S., 2005. Multiple sulfur
isotope fractionations in biological systems: A case study with sulfate reducers and sulfur
disproportionators. Am J Sci 305, 645-660.
Jørgensen, B.B., 1979. A theoretical model of the stable sulfur isotope distribution in marine sediments.
Geochimica et Cosmochimica Acta 43, 363-374.
Jørgensen, B.B., Nelson, D.C., 2004. Sulfide oxidation in marine sediments: Geochemistry meets
microbiology. Geological Society of America Special Papers 379, 63-81.
Kaplan, I.R., Rittenberg, S.C., 1964. Microbiological fractionation of sulphur isotopes. J. Gen. Microbiol.
34, 195-212.
Kemp, A.L.W., Thode, H.G., 1968. The mechanism of the bacterial reduction of sulphate and of sulphite
from isotope fractionation studies. Geochimica et Cosmochimica Acta 32, 71-91.
Knicker, H., Hatcher, P.G., 2001. Sequestration of organic nitrogen in the sapropel from Mangrove Lake,
Bermuda. Organic Geochemistry 32, 733-744.
Konovalov, S.K., Luther III, G.W., Yücel, M., 2007. Porewater redox species and processes in the Black
Sea sediments. Chemical Geology 245, 254-274.
Kühl, M., Jørgensen, B.B., 1992. Microsensor Measurements of Sulfate Reduction and Sulfide Oxidation
in Compact Microbial Communities of Aerobic Biofilms. Applied and Environmental Microbiology 58,
1164-1174.
Leavitt, W.D., Halevy, I., Bradley, A.S., Johnston, D.T., 2013. Influence of sulfate reduction rates on the
Phanerozoic sulfur isotope record. Proceedings of the National Academy of Sciences 110, 11244-11249.
Lefort, S., Mucci, A., Sundby, B., 2012. Sediment response to 25 years of persistent hypoxia. Aquat
Geochem 18, 461-474.
Luther III, G.W., Findlay, A.J., MacDonald, D.J., Owings, S.M., Hanson, T.E., Beinart, R.A., Girguis, P.R.,
2011. Thermodynamics and Kinetics of Sulfide Oxidation by Oxygen: A Look at Inorganically Controlled
Reactions and Biologically Mediated Processes in the Environment. Frontiers in Microbiology 2.
Moeslund, L., Thamdrup, B., Jørgensen, B.B., 1994. Sulfur and iron cycling in a coastal sediment:
Radiotracer studies and seasonal dynamics. Biogeochemistry 27, 129-152.
Nelson, D., Jannasch, H., 1983. Chemoautotrophic growth of a marine Beggiatoa in sulfide-gradient
cultures. Archives of Microbiology 136, 262-269.
Neretin, L.N., Böttcher, M.E., Grinenko, V.A., 2003. Sulfur isotope geochemistry of the Black Sea water
column. Chemical Geology 200, 59-69.
Ono, S., Keller, N.S., Rouxel, O., Alt, J.C., 2012. Sulfur-33 constraints on the origin of secondary pyrite in
altered oceanic basement. Geochimica et Cosmochimica Acta 87, 323-340.
Press, W.H., Teukolsky, S.A., Vetterling, W.T., Flannery, B.P., 1992. Numerical Recipes in Fortran 77:
Second edition. Cambridge University Press.
Reeburgh, W.S., 1967. An improved interstitial water sampler. Limnology and Oceanography 12, 163-&.
Sim, M.S., Bosak, T., Ono, S., 2011a. Large Sulfur Isotope Fractionation Does Not Require
Disproportionation. Science 333, 74-77.
Sim, M.S., Ono, S., Bosak, T., 2012. Effects of iron and nitrogen limitation on sulfur isotope fractionation
during microbial sulfate reduction. Appl Environ Microbiol 78, 8368-8376.
Sim, M.S., Ono, S., Donovan, K., Templer, S.P., Bosak, T., 2011b. Effect of electron donors on the
fractionation of sulfur isotopes by a marine Desulfovibrio sp. Geochimica et Cosmochimica Acta 75,
4244-4259.
Stolz, J.F., 1991. The ecology of phototrophic bacteria, in: Stolz, J.F. (Ed.), Structure of Phototrophic
Prokaryotes. CRC Press, pp. 106-123.
Strauss, H., Bast, R., Cording, A., Diekrup, D., Fugmann, A., Garbe-Schonberg, D., Lutter, A., Oeser, M.,
Rabe, K., Reinke, D., Teichert, B.M.A., Westernstroer, U., 2012. Sulphur diagenesis in the sediments of
the Kiel Bight, SW Baltic Sea, as reflected by multiple stable sulphur isotopes. Isotopes in Environmental
and Health Studies 48, 166-179.
Thamdrup, B., Finster, K., Hansen, J.W., Bak, F., 1993. Bacterial disproportionation of elemental sulfur
coupled to chemical reduction of iron or manganese. Applied and Environmental Microbiology 59, 101108.
Thode, H.G., Monster, J., Dunford, H.B., 1961. Sulphur isotope geochemistry. Geochimica et
Cosmochimica Acta 25, 159-174.
Tostevin, R., Turchyn, A.V., Farquhar, J., Johnston, D.T., Eldridge, D.L., Bishop, J.K.B., McIlvin, M., 2014.
Multiple sulfur isotope constraints on the modern sulfur cycle. Earth and Planetary Science Letters 396,
14-21.
Werne, J.P., Lyons, T.W., Hollander, D.J., Schouten, S., Hopmans, E.C., Sinninghe Damsté, J.S., 2008.
Investigating pathways of diagenetic organic matter sulfurization using compound-specific sulfur isotope
analysis. Geochimica et Cosmochimica Acta 72, 3489-3502.
Wieland, A., De Beer, D., Damgaard, L.R., Kühl, M., Van Dusschoten, D., Van As, H., 2001. Fine-scale
measurement of diffusivity in a microbial mat with nuclear magnetic resonance imaging. Limnology and
Oceanography 46, 248-259.
Wing, B.A., Halevy, I., 2014. Intracellular metabolite levels shape sulfur isotope fractionation during
microbial sulfate respiration. Proc. Nat. Acad. Sci. (2014) http://dx.doi.org/10.1073/pnas.1407502111
[Epub ahead of print].
Wu, N., Farquhar, J., 2013. Metabolic rates and sulfur cycling in the geologic record. Proceedings of the
National Academy of Sciences 110, 11217-11218.
Zerkle, A.L., Farquhar, J., Johnston, D.T., Cox, R.P., Canfield, D.E., 2009. Fractionation of multiple sulfur
isotopes during phototrophic oxidation of sulfide and elemental sulfur by a green sulfur bacterium.
Geochimica et Cosmochimica Acta 73, 291-306.
Zerkle, A.L., Kamyshny Jr, A., Kump, L.R., Farquhar, J., Oduro, H., Arthur, M.A., 2010. Sulfur cycling in a
stratified euxinic lake with moderately high sulfate: Constraints from quadruple S isotopes. Geochimica
et Cosmochimica Acta 74, 4953-4970.
Zopfi, J., Ferdelman, T.G., Fossing, H., 2004. Distribution and fate of sulfur intermediates—sulfite,
tetrathionate, thiosulfate, and elemental sulfur—in marine sediments. Geological Society of America
Special Papers 379, 97-116.
Table 1: Sulfur cycle parameters for the two cores described in this study and previous cores
taken at Mangrove Lake.
Core/study
decay
depth
C0
į34S 0
constant
of C 0
(mM)
(‰)
ଷସ
ଷଷ
ߝ௧
(‰)
ߣ௧
į34S H2S
¨33S H2S
(‰)
(‰)
-1
(cm )
(cm)
Core 1
0.12
8.25
26.0
20.2
-32.5
0.514
-9.6d
0.08d
Core 1 (upper)
0.08
8.25
26.0
23.7
-31.2
0.513
-8.3d
0.11d
Core 1 (lower)
0.13
15.75
12.6
31.9
-35.5
0.514
n/a
n/a
d
0.04d
Core 2
0.06
6.25
27.1
22.4
-29.2
0.515
-7.5
Porewatersa,b
0.05
16.0
27.3
21.7
-34.1c
n/a
-4.8
n/a
Incubationsa
0.07
n/a
n/a
n/a
n/a
n/a
n/a
n/a
a
Boudreau et al. (1992)
Canfield et al. (1998)
c
calculated from measured į34S H2S and į34S SO42- (29.1‰) when H 2 S first appears in the profile
d
predicted value based on porewater sulfate model
b
Figure 1
A
[SO4-2] (mM)
0
0
5
10
15
20
25
30
35
Mixed layer
Depth (cm)
10
Stratified layer
20
30
40
Core 1 (stratified layer)
Core 1 (mixed layer)
50
Full core model
Upper core model
Lower core model
60
B
[SO4-2] (mM)
0
0
5
10
15
20
25
30
Mixed layer
Depth (cm)
10
Stratified layer
20
30
40
50
Core 2 (stratified layer)
Core 2 (mixed layer)
Full core model fit
60
35
Figure 2
A
0
0
20
δ34S (‰)
40
60
80
100
Mixed layer
Depth (cm)
10
Stratified layer
20
30
40
Core 1 (stratified layer)
Core 1 (mixed layer)
50
Full core model
Upper core model
Lower core model
60
B
0
0
20
δ34S (‰)
40
60
80
Mixed layer
Depth (cm)
10
Stratified layer
20
30
40
50
Core 2 (stratified layer)
Core 2 (mixed layer)
Full core model fit
60
100
Figure 3
A
0.15
Core 1 (stratified layer)
Core 1 (mixed layer)
∆33S (‰)
0.12
Full core model
Upper core model
Lower core model
0.09
Seawater
0.06
0.03
0.00
20
30
40
50
60
70
60
70
δ34S (‰)
B
0.15
Core 2 (stratified layer)
Core 2 (mixed layer)
∆33S (‰)
0.12
Full core model fit
Seawater
0.09
0.06
0.03
0.00
20
30
40
50
δ S (‰)
34
Figure 4
Core 1 (this study)
A
Lago di Cadagno
Core 2 (this study)
0.520
Black Sea
Wedderwarden
Aarhus Bay
Solar Lake
Baltic sea sediments
0.516
33
λ
0.512
-70
-60
-50
-40 -30
34
εnet(‰)
-20
0.508
0
-10
B
Core 1 (this study)
Core 2 (this study)
0.520
Lago di Cadagno - accumulation experiments
Natural populations of MSR
Modeled MSR
0.516
33
λ
0.512
-70
-60
-50
-40 -30
34
εnet(‰)
-20
-10
0.508
0
Figure 5
SO
ijmsr Įmsr
H 2S
ijox Įox
ijih Įih
-2
4
ijis Įis
Si
ijseq Įseq
Figure 6
Phototrophic sulfide oxidation
0.520
A
freox
0.516
34
εmsr
-50‰
1.0
33
λ
33
λ
33
λ
33
λ
0.512
-40‰
-30‰
-20‰
-10‰
Core 1 (Mangrove Lake)
0.0
Core 2 (Mangrove Lake)
-70
-60
-50
0.508
-40
34
-30
-20
-10
0
εnet(‰)
0.520
B
Abiotic sulfide oxidation
34
εmsr
freox
0.516
-50‰
-40‰
0.512
-30‰
-20‰
-10‰
0.0
Core 1 (Mangrove Lake)
Core 2 (Mangrove Lake)
-70
-60
-50
0.508
-40
34
-30
-20
-10
0
εnet(‰)
0.520
C
Sulfite disproportionation
freox
0.516
34
εmsr
1.0
0.8
0.6
0.4
-50‰
-40‰
0.512
0.2
-30‰
-20‰
0.0
-10‰
Core 1 (Mangrove Lake)
Core 2 (Mangrove Lake)
-70
-60
-50
0.508
-40
34
-30
-20
-10
0
εnet(‰)
Elemental Sulfur disproportionation
0.520
D
freox
1.0
0.516
34
εmsr
0.8
0.6
0.4
-50‰
0.512
0.2
-40‰
-30‰
-20‰
Core 1 (Mangrove Lake)
-10‰
0.0
Core 2 (Mangrove Lake)
0.508
-70
-60
-50
-40
34
-30
εnet(‰)
-20
-10
0
Connection 1: Mangrove Lake to Experimental evolution
In the previous chapter we presented evidence for a previously unreported S cycle in
Mangrove Lake. A simple S cycling scheme was elaborated to measure the contributions of
reductive and reoxidative processes and a significant portion of the S cycle was found to occur
in-situ. An important question which arose during our investigation of Mangrove Lake
porewaters was whether the isotopic signatures that are produced by the metabolisms involved in
the S cycle are constant or have continually changed over the course of the Lake’s history. Since
all life on Earth is involved in a perpetual evolutionary race, it seemed reasonable to assume that
the microorganisms living in Mangrove Lake evolved over the past 10 000 years of its existence.
It was however unclear whether the isotopic signatures would reflect the evolutionary race
despite the knowledge that evolution can affect metabolism. An extensive literature search on the
subject only yielded suggestions of the role evolutionary adaptation might play on isotopic
signatures, no empirical evidence. It would remain so if this question was tackled on an
experimental system such as Mangrove Lake. The study site was simply not up to the task of
answering these first-principle questions given its heterogenous nature, the complex
biogeochemical processes which take place within it and the large diversity of microorganisms it
supports. Too many variables were at play. In order to investigate the isotopic phenotype as an
evolutionary signature, it was necessary to make a switch to pure cultures in chemically defined
media. In the next chapter we introduce the concept of the isotope phenotype and test its
sensitivity to evolutionary adaptation. We utilize DvH as a model organism.
50
Paper 2 : Evolutionary adaptation of a
sulfate reducing bacterium and its sulfur
isotope phenotype
Sulfur isotope fractionation during the evolutionary adaptation of a
sulfate reducing bacterium
André Pellerin1, Luke Anderson-Trocme1, Lyle G. Whyte2, Grant M. Zane3, Judy D. Wall3
and Boswell A. Wing1
[1] Department of Earth and Planetary Sciences and GEOTOP, McGill University, 3450
University Street, Montréal, Canada, H3A 0E8
[2] Department of Natural Resource Science, Macdonald-Stewart Building, 21111 Lakeshore
Road, Ste-Anne de Bellevue, Canada, H9X 3V9
[3] Biochemistry 117 Schweitzer Hall, University of Missouri Columbia, MO 65211
Correspondance to: André Pellerin (andrepellerin@gmail.com)
ABSTRACT
Dissimilatory sulfate reduction is a microbial catabolic pathway that preferentially processes
less massive sulfur isotopes relative to their heavier counterparts.
This sulfur isotope
fractionation is recorded in ancient sedimentary rocks and is thought to reflect a phenotypic
response to environmental variations rather than to evolutionary adaptation. Modern sulfatereducing microorganisms isolated from similar environments exhibit a wide range of sulfur
isotope fractionations suggesting that adaptive processes may influence the sulfur isotope
phenotypes. To date, the relationship between evolutionary adaptation and isotopic phenotypes
has not been explored. We addressed this by studying the covariation of fitness and the sulfur
isotope phenotype of in Desulfovibrio vulgaris Hildenborough with experimental evolution.
After 560 generations, the mean fitness of the evolved lineages relative to the starting isogenic
population had increased by ~17 %. After 927 generations, the mean fitness relative to the intial
ancestor had increased to ~20 %. Growth rate in exponential phase experienced increases during
the course of the experiment, suggesting that this was a primary influence behind the fitness
increases. Consistent changes were observed within selection intervals between fractionation and
fitness. Fitness changes were associated with changes in exponential growth rate but changes in
fractionation were not and appeared to be a response to changes in the parameters that govern the
overall growth rate: yield and cell-specific sulfate respiration rate. We hypothesize that these act
together through the direct influence of cell-specific sulfate respiration rate on fractionation, such
that higher yields at a constant growth rate lead to larger fractionation.
1. Introduction
Dissimilatory sulfate reduction (DSR) is a microbial metabolism that consumes sulfate and
utilizes this sulfur as terminal electron acceptor, excreting sulfide. This process creates
characteristic enrichments and depletions in the stable isotopes of sulfur that are preserved in
sediments and sedimentary rocks as a legacy of the metabolic processing (1). In this way, sulfur
isotope fractionation can be thought of as a phenotypic trait of the specific microbes that perform
DSR. When the rock record is examined like this, the S isotope phenotype has been interpreted
to be continually present in ancient sediments back to at least 3.47 billion years ago (2).
However, the interpretation of S isotope fractionation as a phenotypic trait that can be preserved
in ancient rocks opens up a basic question: does evolutionary adaptation influence the S isotope
phenotype?
Evolutionary-driven modifications to lineages of sulfate reducers may be capable of
influencing the isotope phenotype by modifying the relative processing rates within the DSR
pathway. If growth, and in turn the energy supplied by sulfate respiration, influence survival,
then the controls on sulfate uptake, the internal regulation of concentrations of metabolites and
the structure of enzymes involved in the sulfate reducing pathway (3, 4) could be key selective
targets that influence the isotope phenotype.
Previous work has emphasized exclusively
physiological and environmental controls on the S isotope phenotype (including temperature,
sulfate concentrations, and the nature and supply rate of the electron donor (1, 5-13). Among
these controls, cell-specific sulfate respiration rate (csSRR) has emerged as a sort of ‘master
variable’ that sets the level of S isotope fractionation. However, there are examples where large
changes in fractionation are not correlated with variations in csSRR, or any environmental
parameters (14-17).
This suggests that there may be an evolutionary influence preserved in the S isotope
fractionation expressed by extant sulfate-reducing microorganisms. For example, sulfate
reducers metabolizing at similar near maximal rates can exhibit diverse S isotope fractionations
(quantified as 34İYDOXHVwhich are equal to the difference in molar 34S-32S ratios between sulfate
and sulfide, relative to the molar
34
S-32S ratio in sulfide; Figure 1). Although environmental
variation may be responsible for some of the fractionation diversity, the genetic differences
among these strains is likely to be influential as well. In this light, although environments have
unquestionably changed throughout Earth history, the specific metabolic variant of DSR that was
active in contemporaneous microorganisms will also influence the preserved S isotopic
signature. The sulfur isotope variations in ancient rocks may therefore be, in part, a ’fossil’
record how the DSR metabolism has changed through time.
As a step towards addressing this possibility, we performed selection experiments that
examined the response of the isotope phenotype to evolutionary adaptation. Pure cultures of a
sulfate reducing bacterium were propagated through daily serial transfer in batch cultures,
maintaining constant environmental challenges to JURZWK IRU § generations. Since
evolutionary adaptation typically occurs in a hyperbolically decreasing manner (18), major
enhancement in fitness - the ability of an organism to directly outcompete its ancestors and the
primary indicator of evolutionary adaptation - occur early on, within experimentally obtainable
timeframes of hundreds of generations. In the same environment as the adaptive evolution
experiment, the populations were monitored for changes in fitness, 34İ, exponential growth rate,
cell-specific respiration rate and cell yield. By archiving the sample populations, these
experiments produced a short span of evolutionary history, where selective pressures were
tightly controlled and the impact of evolutionary adaptation on the S isotope phenotype could be
directly compared with more evolved lineages.
2. Methods
2.1 Choice of model organism
We used a wild-type sulfate reducing G-proteobacterium, Desulfovibrio vulgaris
Hildenborough (DVH), in our study. DVH has a sequenced genome (19) and is commonly used
as a model organism to investigate the evolutionary, physiological, enzymatic, genetic and
growth characteristics of sulfate-reducing bacteria e.g. (19-24). Importantly, experiments have
shown that populations of DVH can express a wide range of S isotope fractionations that vary
predictably with the rate of sulfate respiration (7, 9, 10, 25). This plasticity in the S isotope
phenotype provides a well-defined framework against which to compare any adaptive effects on
S isotope fractionation.
2.2 Design of microbial evolution experiment
The evolution experiments were designed to select for increased growth rate in a simple and
reproducible manner, rather than to investigate selective responses to novel environmental,
physiological, or genetic challenges. Twelve replicate lines of DVH were propagated in a
chemically defined growth medium optimized for DVH (Figure 2). Most of the cells divisions in
this media occur during a “scramble” for resources. This type of serial selection experiment in
batch culture has been shown to lead to changes in fitness as well as net growth rate (18, 24). Six
of the lines were taken from an isogenic wild-type population whereas six were taken from a
reference mutant strain (DVU0600; http://www.microbesonline.org/ ) constructed from the wildtype DVH.
The mutant strain contains unique oligonucleotides (a ‘barcode’) flanking an
antibiotic-resistance cassette for kanamycin that replaced a gene encoding a putative lactate
dehydrogenase. This genetic manipulation was neutral with respect to fitness of the mutant strain
relative to the wild-type lines (Table 1). We refer to lineages arising from the wild-type ancestral
population as DVH-wt and those arising from the mutant ancestral population as DVH-mut.
Every 24 h, each of the replicate lines was propagated in batch culture by inoculating 0.6 mL
of the previous culture into 10 mL of fresh, defined medium in 20-mL serum bottles capped with
blue butyl rubber stoppers (Figure 2). The headspace was 100% N 2 gas (99.995% purity).
Cultures were incubated at 33°C and shaken at 110 rpm. Subculturing was alternated between
wild type and mutant lineages because cross contamination between DVH-wt and DVH-mut can
be monitored (see section 2.4). Approximately every 10th transfer, the twelve replicate lines were
preserved in a glycerol stock solution at -80°C to obtain a “fossil” record of the evolution
experiment (Figure 2). These frozen stocks were revived at later times for isotopic, growth and
fitness measurements. All inoculations, sampling, and transfers were performed under strictly
anaerobic conditions.
2.3 Growth media
All experiments were performed in a Tris-buffered chemically defined medium (MOLS4) that
consists of 30 mM sodium sulfate, 60 mM sodium lactate, 8 mM MgCl 2 , 20 mM NH 4 Cl, 2 mM
K 2 HPO 4 -NaH 2 PO 4 , 30 mM Tris-HCl as well as solutions of trace elements, Thauer’s vitamins
and rezasurin as an oxygen indicator (26). The pH was adjusted to 7.2 with hydrochloric acid.
For the solid medium, 1.5% w/v of agar was added. For the evolution experiments, 10 mL of
MOLS4 was placed into 20mL serum bottles, while 80 mL of MOLS4 was placed into 120 mL
serum bottles for the fitness and isotope assays. Bottles were crimp sealed with butyl rubber
stoppers, and the headspace was purged of oxygen by flushing with pure N 2 gas. After gassing,
individual crimp-sealed medium bottles were sterilized in an autoclave. Except for the culture
volume, all our experiments (including evolution experiments and individual assays of growth
and fitness) were performed in exactly the same medium under the same environmental
conditions. Because sulfur isotope fractionation has a strong physiological control (27), we
consistently used the same culture configurations to isolate changes in phenotypic characters that
were associated with the adaptive process.
2.4 Contamination checks
We performed two types of contamination checks.
The first tracked potential cross-
contamination between different selection lines and the second looked for contamination by
foreign microbes. Subculturing was alternated between wild-type and mutant lines during the
evolution experiment. As a result, cross contamination was more likely to occur between DVHwt and DVH-mut lines than between DVH-wt lines. In order to determine if such crosscontamination occurred, samples of the wild-type cultures were screened for growth on plates
made with an antibiotic (400µg G418 antibiotic per mL of MOLS4 medium) that selects for the
kanamycin cassette defining the DVH-mut. These contamination checks were performed
approximately every 100 generations, and were always negative.
We also PCR amplified and sequenced the 16S rRNA gene in order to ascertain that the
cultivated lineages were composed of DVH. A detailed procedure is available in the
supplemental material. In all twelve evolved lineages and the ancestor, the 16S sequences were
identical matches to the 16S rRNA gene from the DVH reference genome(19). We interpret this
similarity to show that exogenous strains with higher fitness did not take over any of the
populations of the evolution experiment.
2.5 Fitness assay
We quantified fitness of the evolved populations by direct competition experiments with
ancestral populations (Figure 2). Direct competition experiments between two strains account for
environmental or demographic variations that may not be detectable when measuring culture
growth independently. Detailed procedures are available in the supplemental material.
Cultures maintained at -80oC were thawed and inoculated into MOLS4. Growing cultures
were transferred three times at 24 h intervals prior to performing the fitness assay. Fitness was
assayed after serially transferring mixed cultures of wild-type lineages with the ancestral
UHIHUHQFH PXWDQW RYHU § 0 generations.
Cultures were inoculated with equal numbers of
exponentially growing cells of each strain. At each transfer, we tracked the relative frequency of
the genetic barcode of the reference mutant relative to a gene shared by both strains (dsrA ;
supplemental material) with real-time polymerase chain reaction (RT-PCR). Since the evolved
lineages ended up having higher fitness than the ancestral population, this technique was only
able to monitor the fitness of the evolved wild-type strains. A similar method has been used for
monitoring the fitness of RNA viruses (28) and for quantifying the relative survival of algae
under predation by rotifer (29). We compared the fitness of the experimental lineages over two
selection intervals. The first selection interval lasted from the start of the experiment (i.e., with
the ancestral population at generation 0) to generation 560. The second interval spanned from
generation 560 to generation 927.
2.6 Measurement of exponential growth characteristics
Cultures used for exponential growth determinations (Figure 2) were grown in the same way
as cells prepared for fitness assays (above). Specific growth rates (k in day-1) of exponentially
growing cells were calculated as
݇=
ln(ܥଵ ) െ ln(ܥ )
ܶଵ െ ܶ
where T 0 is the time of the initiation of the experiment (in days), T 1 is the first sampling time
(in days) and C 0 and C 1 are the cell concentrations (in cells mL-1) at these times. We estimated
cell concentrations by measuring the optical density (OD) of an actively growing culture at 600
nm. These OD 600 measurements were converted to cell concentrations via a single constant
conversion factor (11.4 x 108) obtained by counting individual cells in dilute, DAPI-stained
aliquots of actively growing ancestral and evolved lines under an epifluoresence microscope.
Our cell concentrations were comparable to previously published estimates of exponentially
growing DVH in identical growth conditions (30). Uncertainty estimates on growth rate were
propagated from the uncertainty on measurements of OD (Table S1).
Determinations of yield (Y, in 106 cells per µmol SO 4 -2 consumed) and cell specific sulfate
reduction rate (csSRR, in femtomoles SO 4 -2 consumed cell-1 day-1) were based on concentrations
of hydrogen sulfide produced by exponentially growing cultures. Concentrations were measured
with a commercial sulfide kit based on the colorimetric method of Cline (1969). Absorbance was
measured on a Genesys 10S UV-VIS spectrophotometer at 670nm. The spectrophotometer was
calibrated with mixed standards of dissolved sodium sulfide and zinc chloride that were
reproducible to ± 0.1mM.
Once H 2 S concentrations and cell numbers were measured, we estimated yield during
exponential growth as
ܻ=
1
ܥ௫ െ ܥ
×
[ܪଶ ܵ]௫ െ [ܪଶ ܵ] 10
where [H 2 S] 0 is the concentration of H 2 S (in mM) at the initiation of the experiment, [H 2 S] x
concentration of H 2 S (in mM) at later sampling times, and the factor of 106 adjusts units to 106
cells/micromole SO 4 2-.
This expression assumes a 1-to-1 stoichiometry between SO 4 -2
consumed and H 2 S produced. The cell specific sulfate reduction rate during exponential growth
was calculated from estimates of growth rate and the yield as
ܿ= ܴܴܵݏ
݇
× 10ଷ
ܻ
where the factor of 103 adjusts units to femtomole cell-1 day-1.
2.7 Characterization of sulfur isotope fractionation
We measured S isotope fractionation by ancestral DVH wild-type and mutant populations six
times each (Figure 2). We also measured fractionation of all lineages over the same intervals at
which fitness was assayed (560 and 927 generations). A detailed methodology is available in
supplemental material. Cultures used for sulfur isotope fractionation measurements were grown
in the same way as cells prepared for fitness assays (above). At the start of the assay, 5 mL of a
mid-exponential phase culture (OD 600 ~ 0.2) was inoculated into gassed and sterile assay bottles
containing 80 mL of MOLS4 and a magnetic stir bar. The assay bottles were vigorously stirred
while being simultaneously purged with pure N 2 gas for two to three hours to remove any sulfide
that was carried-over with the inoculum. Repeated tests showed that the sulfide blank in the
assay medium after purging was <5 ppm. Immediately after purging, we took a sample (labeled
T 0 ) to characterize the initial S isotope composition of the sulfate (and sulfide, see above) in the
medium. Assay cultures were incubated at 33oC and shaken at 110 rpm. We halted cell growth
and sulfate respiration once enough sulfide was produced for a reliable isotope measurement,
typically when <10% of the initial sulfate had been consumed (Table 2). The assay was stopped
by adding 10 mL of an acidic, 4% (wt/vol) zinc acetate solution. This also preserved the sulfide
that had been produced since T 0 as ZnS. This sample (labelled T 1 ) provided the S isotope
composition of the product sulfide as well as the residual sulfate.
The
34
S-32S and
33
S-32S ratios of sulfate at T 0 and T 1 , and sulfide at T 1 provided six data
points that could be used to constrain the three parameters influencing the S isotope phenotype at
the assay conditions, as well as their uncertainty (supplemental material). These are the fraction
of sulfate left unconsumed during the assay (f), the intrinsic discrimination of 34S from 32S during
sulfate respiration (34İ), and the characteristic discrimination of
respiration (33O). We use the following definitions for 34İ and 33O
ଷସ
ߝ =
ଷସ
ଷସ
ܵ
ܵ
ቈ ଷଶ
െ ቈ ଷଶ
ܵ sulfate
ܵ sulfide
ଷସ
ܵ
ቈ ଷଶ
ܵ sulfide
33
S from
32
S during sulfate
ଷଷ
ߝ =
ଷଷ
ଷଷ
ܵ
ܵ
ቈ ଷଶ
െ ቈ ଷଶ
ܵ sulfate
ܵ sulfide
ଷସ
ܵ
ቈ ଷଶ
ܵ sulfide
ଷଷ
ߣ
In the text and in the figures,
=
݈݊൫ ଷଷߝ + 1൯
݈݊൫ ଷସߝ + 1൯
İ values are multiplied by a factor of 1000 in order to be
34
expressed as parts per thousand (‰).
3. Results & discussion
3.1 Fitness trajectories reflect an optimization regime of evolutionary adaptation
The mean fitness of the DVH wild-type ancestor relative to the mutant reference strain was of
1.002 ± 0.003 (uncertainty is reported as the sample standard deviation unless otherwise noted;
Figure 3A; Table 1), justifying our use of the mutant ancestor as a reference strain for the RTPCR fitness assays. With this assay, the DVH-wt lineages showed fitness increases over the first
560 generations that were between 14.8% and 18.5%. This corresponds to a mean fitness
increase across these lines of 17.2 ± 1.2% over the first selection interval, which is an average
change in mean fitness of 0.031 ± 0.002% per generation (Figure 3A; Table 1). Between
generation 560 and generation 927, the fitness of individual lineages increased between 2.0%
and 3.6% relative to the fitness at generation 560, with a mean fitness increase across lineages of
2.4% +/- 1.3%. The average change in mean fitness for this selection interval is 0.007 ± 0.004%
per generation, which shows that the rate of increase in mean fitness slowed as the experiment
progressed (Figure 3).
We interpret the competition experiments as evidence for a difference in fitness between the
ancestral and evolved populations at generation 560 as well as between the evolved populations
at generation 560 and generation 927. This inference is supported statistically as the null
hypothesis,of no change can be rejected at a > 99% significance level (p<0.01 for no difference
in means via two sided Student’s t-test assuming equal variance). It is also clear graphically
from plots of the difference in fitness for each lineage from the start to the end of a selection
interval (‘paired’ fitness differences) against the average fitness for the lineage over that interval
(Figure 3B). This behavior attests to the precision and reliability of estimating fitness with the
RT-PCR assay developed here.
The fitness dynamics of the DVH-wt lines resemble those seen in other serial transfer
experiments with microbial populations over similar timescales. For example, rates of mean
fitness increases measured in evolving glucose-limited populations of the J-proteobacterium,
Escherichia coli, increase quickly over the first 600 generations (approximately 0.0375% per
generation) but then decrease to approximately 0.008% per generation over the next 400
generations (18).
Similarly, in selection experiments with the methylotrophic D-
proteobacterium, Methylobacterium extorquens AM1, growing on a single-carbon substrate,
mean fitness increased at a rate of 0.054% per generation over the initial 300 generations of
growth and decrease to 0.009% per generation between generations 900 and 1500 (31). In five
hundred generations of evolution on nutrient-rich medium, 640 separate lines of the model
eukaryotic microbe, Saccharomyces cerevisiae, exhibited a mean fitness that increased at a rate
of 0.013 % per generation (32). With a wide range of microorganisms and in a variety of
selective environments, it seems that experimental evolutionary adaptation produces a common
pattern of generally decreasing rates of fitness increase, often with strikingly similar magnitudes
in the deceleration of the actual rates. This behavior is characteristic of adaptive evolution in a
regime of ‘optimization’ rather than ‘innovation’ (33). In this regime, beneficial mutations tend
to modify the extent, rather than the kind, of existing metabolic and genetic networks leading to,
for example, changes in the levels of gene expression and magnitudes of metabolic fluxes (33).
The overall fitness trajectories of the evolved DVH-wt lineages appear to be similar, but this
does not necessarily translate into reproducibility of the underlying population or genetic
dynamics in each lineage. It is possible that the close correlation in fitness among lineages at
generation 560 and 927 (Figure 3) might be because high fitness mutants that evolve in a defined
environment tend to be phenotypically similar and, thus, might have accumulated similar
beneficial mutations (34). However, fitness responses of decreasing sensitivity can be a more
general consequence of the hierarchal fixation of beneficial mutations (35-38), affecting the
dynamics of ultimate mutation incorporation in individuals and populations (39-44). Clonal
interference, for example, is a population-level process that leads to a slowing down of overall
adaptive rates because of the competition between multiple subpopulations, each with mutations
of similar benefit (39). Diminishing-returns epistasis, on the other hand, is an individual-level
effect where mutations confer smaller fitness benefits in combination than individually, leading
to deceleration of adaptation (45). Both of these processes may play a role in setting the powerlaw trajectory of fitness as adapting microbial populations sample a larger and larger number of
mutations (46). However, both are apparently underlain by an inherent unpredictability regarding
which groups of beneficial mutations actually become fixed (32, 47). Detailed examinations of
whole genomes are required to determine whether the parallel fitness trajectories of the evolved
DVH-wt lineages reflect these more stochastic events or the deterministic fixation of shared
beneficial mutations. In either case, however, the patterns of fitness changes seen here indicate
that the populations of DVH-wt genotypes at generations 560 and 927 are distinct from the
original isogenic ancestral population and from each other.
3.2 S isotope fractionation changes consistently during evolutionary adaptation
Ancestral populations had
İ values between 5.14 and 10.06‰, and
34
Ȝ YDOXHV EHWZHHQ
33
0.5044 and 0.5141 (Table 2; Figure 4A). These values are consistent with previous
characterizations of the S isotope phenotype of sulfate-reducing microbes at high rates of
respiration (7, 8, 12). Ancestral populations of DVH-wt and DVH-mut were assayed six times
each and no significant differences in the underlying distribution of 34İ (p=0.17 for no difference
in means via two sided Student’s t-test assuming equal variance) or 33Ȝp=0.23) were observed.
The grand mean of all twelve biological replicates of the ancestral
34
İZDV 6.96 ± 1.34‰ while
Ȝ was on average 0.5077 ± 0.0023.
33
The range, grand means and standard deviations of 34İDQG33ȜYDOXHVZHUHVLPLODUWKURXJKRXW
the course of the adaptive evolution experiment (Table 2; Figure 4A). At generation 560,
34
İ
ranged from 5.90 to 10.08 across the DVH-wt and DVH-mut lines, with a grand mean of 8.06 ±
1.38‰ (average
33
Ȝ = 0.5105 ± 0.0024). At generation 927,
34
İ ranged from 5.05 to 8.05‰
across the DVH-wt and DVH-mut lines, with a grand mean of 6.59 ± 0.51 ‰ (average
Ȝ =
33
0.5094 ± 0.0013). Across lines, variability in 33Ȝ values was always similar to the magnitude of
analytical uncertainty (Figure S1). However, variability in
34
than the uncertainty with which we were able assay the
İ values was 5 to 10 times greater
34
İ associated with an individual
population (Table 2). As a result, we looked to see if there were consistent signals in 34İ for each
lineage from the start to the end of a selection interval, as the fitness of that population increased.
This was only possible for the DVH-wt lines. Our fitness assay relies on the ancestral DVH-mut
as a reference strain and was not able to track increasing fitness in the DVH-mut lines.
When differences in
İ for each lineage from the start to the end of a selection interval
34
(paired differences in İ) are plotted against the paired average fitness value for the lineage over
34
that interval (Figure 4B), it is clear that the S isotope phenotype of these lineages changed
consistently during evolutionary adaption. If
İ values at later generations were unrelated to
34
those at earlier generations, the paired differences in 34İ should be centered on 0, and show no
systematic variation. Instead, paired differences in
34
İ are uniformly positive over the first
selection interval, and dominantly negative over the second (Figure 4B).
Although these
phenotypic changes were consistent over each selection interval, their association with increasing
fitness was not monotonic (Figure 4B). Increased variability in a phenotypic trait has been seen
in microbial evolution experiments when that trait itself was not under direct selection (48).
Selection is unlikely to act on the isotopic phenotype itself. Sulfur isotope exchange between
sulfate and sulfide – the initial reactant and final product of sulfate respiration - is ~0.1 kJ mol-1,
much less than the minimum free energy required to sustain anaerobic metabolisms (~10 kJ mol1
; (49)). Accordingly, we looked at the DVH-wt lineages for variations in phenotypic traits that
may have affected both fitness and 34İ.
3.3 Increases in exponential growth rate accompany increased fitness, but not increased
34ɂ
Replicate experiments with the ancestral population established exponential growth rates (k)
from 3.6 to 4.6 day-1, clustered around a mean of 4.2 ± 0.4 day-1 (Figure 5A; Table 1). Across all
DVH-wt lines, the average exponential growth rate increased to 4.8 ± 0.7 day-1 at generation 560
and to 5.5 ± 0.5 day-1 at generation 927 (Table 1). The consistency of these changes across each
lineage is striking, as shown by a plot of the exponential growth rate differences for each lineage
from the start to the end of a selection interval (paired growth rate differences) against the paired
fitness differences for that lineage across the same interval (Figure 6A). While fitness increases
showed a nonlinear deceleration over the course of the adaptive evolution experiment, increases
in exponential growth rate did not appear to follow a similar trajectory.
In principle, overall fitness could be enhanced by adaption to any of the four phases of
density-regulated growth that accompany serial transfer in batch culture (50). The competition
assays accounted for fitness in both the low density, nutrient-rich conditions that characterize the
early phases of growth, as well as the crowded, nutrient poor conditions that came later.
Adaptive effects at high population density may have been more important during the first
selection interval than during the second, accounting for the lack of proportionality between
exponential growth rate and fitness (Figure 6A). However, as was the case for DVH adapting to
salt-stress (23), or a mutualistic, non-sulfate-reducing lifestyle with the archaeon Methanococcus
maripaludis (24), exponential growth rate appears to be a primary selective target for adaption
during serial transfer of batch cultures of DVH.
Although exponential growth rates increased throughout the course of the experiment,
İ
34
values did not. The same relative changes in growth rate § 0.5 to 0.6 day-1) over each selection
interval were associated with opposite, and near equal, effects in 34İ (Figure 7A). Although there
may be a direct association of growth rate increases with enhanced fitness during evolutionary
adaptation, changes in the S isotope phenotype do not appear to reflect these increases.
3.4 Exponential yield and respiration rates co-vary with fitness increases and 34İ changes
Across the DVH-wt lines, cell yields during exponential growth increased slightly after the
first selection and fell by a similar amount after the second (Figure 5B). Cell-specific sulfate
respiration rates during exponential growth (csSRR), on the other hand, exhibited the opposite
behavior (Figure 5C). The signal-to-noise ratio for determinations of yield is hampered by the
small accumulation of H 2 S during the assay interval. Extending these estimates over longer
times only changes these results in detail (Table S1). Since yield estimates over longer times
consider a portion of the growth cycle where growth rate is not monitored, we thus utilized the
initial estimates of yield for calculating csSRR and further analysis.
Paired differences in yield show a consistent co-variation with fitness, with the large fitness
gains seen in each lineage over the first selection interval accompanied by increases in yield, and
the smaller fitness gains of the second interval associated with yield decreases (Figure 6B). As
expected from the trade-off implicit in the definition of csSRR, decreased respiration rates
appear with the initial fitness increases, while larger increases in respiration accompany the
smaller fitness gains that came later (Figure 6C). One lineage (A) was a clear exception to these
patterns, largely as a result of the atypically low exponential growth rates estimated for this
population at generations 560 and 927 (Table 1). However, lineage A had a fitness trajectory that
is consistent with other lines, and
34
İ YDOXHV WKDW DUH HTXLYDOHQW DV ZHOO :H GR QRW KDYH DQ
explanation for the atypical growth rate behavior that it exhibited.
Like fitness changes, paired differences in
İ VKRZ D V\VWHPDWLF YDULDWLRQ ZLWK H[SRQHQWLDO
34
yield and csSRR (Figure 7B,C). When yield changes were positive, 34İFKDQJHVZHUHpositive;
when yield decreased,
İ decreased (Figure 7B). Since exponential growth rates increased
34
monotonically throughout the course of the adaptive evolution experiment (Figure 5A),
variations in exponential csSRR are a reflection of those seen in yield (Figure 5B,C). For the
first selection interval, this led to decreased csSRR associated with positive changes in 34İZKLOH
the negative 34İFKDQJHVRYHUWKHVHFRQGVHOHFWLRQLQWHUYDODFFRPpanied csSRR increases (Figure
7C). Again, lineage A showed behavior that did not fit these patterns.
3.5 Consequences of adaptive evolution for the S isotope phenotype
Over the last 60 years great efforts have been made to understand the controls on isotope
fractionation during dissimilatory sulfate reduction e.g. (25, 51, 52) because a working
knowledge of how environmental conditions affect S isotopes during DSR allows the
reconstruction of past environmental conditions (6, 53). Temperature, pH, sulfate concentrations
all impose constraints on portions of the DSR metabolism which in turn affects the S isotope
signature that is produced (1, 5, 6). At high concentrations of sulfate, it is the rate of respiration
of sulfate within a microbial cell that appears to explain a large portion of the isotopic selectivity.
An increase in csSRR, caused by the changing nature or supply of electron donors, affects the
magnitude of S isotope fractionation in a hyperbolically decreasing manner (7-10, 12, 13, 54) .
With this physiological response as a guide, our results suggest that the adaptive
consequences on
İ might recapitulate physiological ones, within the ‘optimization’ adaptive
34
regime in which we conducted our experiments. If a common mechanism is shared by the
physiological and adaptive influences of csSRR, this implies that the lineages in the evolution
experiment likely saw beneficial mutations that changed protein expression levels rather than
protein structure. This inference is consistent with experiments that directly test how adaptive
optimization works at a molecular level, by first tuning protein expression (55) to remove
pathways that are not necessary and to enhance flux through of essential metabolic pathways
(56). We might expect such a similarity between evolutionary adaptation and physiological
acclimation given the rapidity of these regulatory changes during adaptation and the languid
occurrence of ones that affect enzyme structure.
However, the interaction between evolutionary adaptation and the isotope phenotype is sure to
be more complex than a simple resemblance to physiological acclimation. Some evolutionarydriven adaptations might influence genes that epistatically interact with genes affecting the
isotope phenotype while others may not. If this is the case, then isotope phenotype and
evolutionary adaptation may not be correlated in a broad sense but might be more contingent
upon the development and optimization of particular functional traits (e.g., sulfate uptake,
enzyme activity, csSRR). If the isotope phenotype is correlated with specific traits, the expected
trajectory of the isotope phenotype during instances of evolutionary adaptation may be able to be
anticipated. A microbial evolution experiment that starts with a strain further from the fitness
‘peak’ than the DVH-wt used here might be able to answer these questions. It may also point
towards a way to reconcile evolutionary adaptation with the isotope phenotype, and its preserved
variation in the rock record.
4. Conclusion
This investigation shows that short-term evolutionary adaptation can affect the S isotope
phenotype
of
sulfate-reducing
microorganisms. The dissimilatory
sulfate
reducing
bacteria Desulfovibrio vulgaris Hildenborough increased its growth rate during a simple
microbial evolution experiment. Our experimental design led to mean fitness that always
increased with time but at a decreasing rate, reDFKLQJLPSURYHPHQWVRIaDIWHUQHDUO\§
generations of selection. At the high growth rates of the experiment, fitness changes were
directly correlated with changes in exponential growth rate. In contrast, growth rate did not
appear to play a direct role in shaping the isotope phenotype.
Changes in 34İ ZHUH FRQVLVWHQW ZLWKLQ D JLYHQ VHOHFWLRQ LQWHUYDO DOWKRXJK WKH\ GLIIHUHG LQ
sign, from slightly positive over the first interval to slightly negative over the second. These
changes appeared to vary in concert with changes in cell-specific sulfate reduction rate and
yield. A physiological control of cell-specific sulfate reduction rate on34İ LV ZHOO NQRZQ ZLWK
higher rates leading to lower 34İ We observed a similar inverse association between 34İDQGFHOOspecific sulfate reduction rate, leading to the possibility that adaptive effects on the sulfur isotope
phenotype recapitulate physiological ones, at least at the high growth rates encountered here.
Because the initial growth rates of the ancestral DvH are high, evolutionary adaptation may
have a weaker impact on the genes responsible for the expression of 34İ than in other conditions
conducive to slower initial growth rates. Since a common mechanism behind physiological and
evolutionary change is gene expression, we hypothesize that evolutionary situations where
fitness increases are directly hinged on increases in the physiology of sulfate reducers would
promote higher respiration and lower 34İ
5. Acknowledgments
We thank Jesse Colangelo-Lillis for assistance with counting cells and thoughtful comments
on this paper, and Nadia Mykytczuk and Rebecca Austin for their efforts in the development of
the RT-PCR fitness assay. This work was supported by NSERC through a Canada Graduate
Fellowship to AP, Discovery and RTI grants to BAW and LGW, and a USRA to LAT. The
Stable Isotope Laboratory in the Earth and Planetary Sciences department at McGill is supported
by the FQRNT through the GEOTOP research center.
Figure captions
Figure 1: 34İvalues from pure cultures of sulfate-reducing microbes with metabolic rates in a
similar range to this study (50 – 125 femtomole cell-1 day-1). Larger datasets are displayed as
boxplots whereas smaller datasets are displayed as individual points. Data labelled a) from (12),
b) from (7), c) from (11), d) from (14), e) from (15), and f) from (8).
Figure 2: Illustration of the experimental workflow. 'DLO\ VHULDO WUDQVIHUV SURGXFH § generations of growth per day. The resulting evolved lineages are DUFKLYHGDWLQWHUYDOVRI§100
generations. Archived .ancestral and evolved lineages are revived simultaneously to measure the
phenotypic differences over two selection intervals (generation 0 to 560 and generation 560 to
927).
Figure 3: Magnitudes of fitness improvements decrease with increasing generations. A)
Fitness of DVH-wt ancestor and evolved lineages as determined by direct competition
experiments as a function of number of generations. Data from specific generations is jittered
along the x-axis for easier visualization. Symbols indicate individual lineages while colors
indicate different generations. Error bars indicate 1V uncertainty based on triplicate competition
experiments. B) Difference in fitness for each lineage from the start to the end of a selection
interval (‘paired’ fitness difference) against the average fitness for the lineage over that interval.
Symbols indicate individual lineages while colors indicate different selection intervals. Error
bars indicate propagated 1V uncertainties.
Figure 4: Isotope fractionation (34İ) over the course of the evolution experiment. A) 34İ values
as a function of number of generations. Data from specific generations is jittered along the x-axis
for easier visualization. Symbols indicate individual lineages while contrasting colors indicate
different generations. Like colors group lineages by their common ancestor (wt or mut). Error
bars indicate 1V uncertainty estimates based on a Monte Carlo simulation (supplemental
material). B) Difference in
(‘paired’ difference in
34
İ for each lineage from the start to the end of a selection interval
34
İ) against the average
34
İ for the lineage over that interval. Symbols
indicate individual lineages while colors indicate different selection intervals. Error bars indicate
propagated 1V uncertainties.
Figure 5: Growth characteristics of individual lines during exponential growth. Data from
specific generations is jittered along the x-axis for easier visualization. Symbols indicate
individual lineages while colors indicate different generations.. Error bars indicate 1V
uncertainty propagated from measurements of optical density and [H 2 S]. (A) Growth rate (k).
(B) Growth yield (Y). (C) Cell-specific sulfate reduction (csSRR).
Figure 6: Difference in growth characteristics for each lineage from the start to the end of a
selection interval relative to paired differences in fitness. Symbols indicate individual lineages
while colors indicate different selection intervals. Error bars indicate propagated 1V
uncertainties. A) Paired growth rate differences relative to paired fitness differences. B) Paired
yield differences relative to paired fitness differences. C) Paired csSRR differences relative to
paired fitness differences.
Figure 7: Difference in
İ for each lineage from the start to the end of a selection interval
34
relative to the paired differences in growth rate, yield and csSRR. Symbols indicate individual
lineages while colors indicate different selection intervals. Error bars indicate propagated 1V
uncertainties. A) Paired differences in
differences in
34
İ relative to paired growth rate differences. B) Paired
İ relative to paired yield differences. C) Paired differences in
34
İ relative to
34
paired csSRR differences.
Table 1: Fitness and growth characteristics of individual lines during the evolution
experiment.
Table 2: Mass balance and isotopic characteristics of individual lines during the evolution
experiment.
References
1.
Habicht KS, Canfield DE. 1997. Sulfur isotope fractionation during bacterial sulfate
reduction in organic-rich sediments. Geochimica et Cosmochimica Acta 61:5351-5361.
2.
Shen Y, Buick R, Canfield DE. 2001. Isotopic evidence for microbial sulphate reduction
in the early Archaean era. Nature 410:77-81.
3.
Minz D, Fishbain S, Green SJ, Muyzer G, Cohen Y, Rittmann BE, Stahl DA. 1999.
Unexpected population distribution in a microbial mat community: Sulfate-reducing
bacteria localized to the highly oxic chemocline in contrast to a eukaryotic preference for
anoxia. Applied and Environmental Microbiology 65:4659-4665.
4.
Wagner M, Roger AJ, Flax JL, Brusseau GA, Stahl DA. 1998. Phylogeny of
Dissimilatory Sulfite Reductases Supports an Early Origin of Sulfate Respiration. Journal
of Bacteriology 180:2975-2982.
5.
Canfield DE, Olesen CA, Cox RP. 2006. Temperature and its control of isotope
fractionation by a sulfate-reducing bacterium. Geochimica et Cosmochimica Acta
70:548-561.
6.
Habicht KS, Gade M, Thamdrup B, Berg P, Canfield DE. 2002. Calibration of Sulfate
Levels in the Archean Ocean. Science 298:2372-2374.
7.
Leavitt WD, Halevy I, Bradley AS, Johnston DT. 2013. Influence of sulfate reduction
rates on the Phanerozoic sulfur isotope record. Proceedings of the National Academy of
Sciences 110:11244-11249.
8.
Sim MS, Ono S, Bosak T. 2012. Effects of iron and nitrogen limitation on sulfur isotope
fractionation during microbial sulfate reduction. Appl Environ Microbiol 78:8368-8376.
9.
Sim MS, Bosak T, Ono S. 2011. Large Sulfur Isotope Fractionation Does Not Require
Disproportionation. Science 333:74-77.
10.
Sim MS, Ono S, Donovan K, Templer SP, Bosak T. 2011. Effect of electron donors on
the fractionation of sulfur isotopes by a marine Desulfovibrio sp. Geochimica et
Cosmochimica Acta 75:4244-4259.
11.
Hoek J, Reysenbach AL, Habicht KS, Canfield DE. 2006. Effect of hydrogen
limitation and temperature on the fractionation of sulfur isotopes by a deep-sea
hydrothermal vent sulfate-reducing bacterium. Geochimica et Cosmochimica Acta
70:5831-5841.
12.
Johnston DT, Farquhar J, Canfield DE. 2007. Sulfur isotope insights into microbial
sulfate reduction: When microbes meet models. Geochimica Et Cosmochimica Acta
71:3929-3947.
13.
Chambers LA, Trudinger PA, Smith JW, Burns MS. 1975. Fractionation of sulfur
isotopes by continuous cultures of Desulfovibrio desulfuricans. Canadian Journal of
Microbiology 21:1602-1607.
14.
Detmers J, Brüchert V, Habicht KS, Kuever J. 2001. Diversity of sulfur isotope
fractionations by sulfate-reducing prokaryotes. Applied and Environmental Microbiology
67:888-894.
15.
Kleikemper J, Schroth MH, Bernasconi SM, Brunner B, Zeyer J. 2004. Sulfur
isotope fractionation during growth of sulfate-reducing bacteria on various carbon
sources. Geochimica et Cosmochimica Acta 68:4891-4904.
16.
Bruchert V, Knoblauch C, Jorgensen BB. 2001. Controls on stable sulfur isotope
fractionation during bacterial sulfate reduction in Arctic sediments. Geochimica Et
Cosmochimica Acta 65:763-776.
17.
Bolliger C, Schroth MH, Bernasconi SM, Kleikemper J, Zeyer J. 2001. Sulfur isotope
fractionation during microbial sulfate reduction by toluene-degrading bacteria.
Geochimica et Cosmochimica Acta 65:3289-3298.
18.
Lenski RE, Rose MR, Simpson SC, Tadler SC. 1991. Long-Term Experimental
Evolution in Escherichia coli. I. Adaptation and Divergence During 2,000 Generations.
The American Naturalist 138:1315-1341.
19.
Heidelberg JF, Seshadri R, Haveman SA, Hemme CL, Paulsen IT, Kolonay JF,
Eisen JA, Ward N, Methe B, Brinkac LM, Daugherty SC, Deboy RT, Dodson RJ,
Durkin AS, Madupu R, Nelson WC, Sullivan SA, Fouts D, Haft DH, Selengut J,
Peterson JD, Davidsen TM, Zafar N, Zhou L, Radune D, Dimitrov G, Hance M,
Tran K, Khouri H, Gill J, Utterback TR, Feldblyum TV, Wall JD, Voordouw G,
Fraser CM. 2004. The genome sequence of the anaerobic, sulfate-reducing bacterium
Desulfovibrio vulgaris Hildenborough. Nature biotechnology 22:554-559.
20.
He Z, Zhou A, Baidoo E, He Q, Joachimiak MP, Benke P, Phan R, Mukhopadhyay
A, Hemme CL, Huang K, Alm EJ, Fields MW, Wall J, Stahl D, Hazen TC, Keasling
JD, Arkin AP, Zhou J. 2009. Global transcriptional, physiological and metabolite
analyses of Desulfovibrio vulgaris Hildenborough responses to salt adaptation. Appl.
Environ. Microbiol.:AEM.02141-02109.
21.
Hemme CL, Wall JD. 2004. Genomic insights into gene regulation of Desulfovibrio
vulgaris Hildenborough. Omics 8:43-55.
22.
Wall J, Hemme C, Rapp-Giles B, Ringbauer J, Casalot L, Giblin T. 2003. Genes and
Genetic Manipulations of Desulfovibrio, p. 85-98.
23.
Zhou A, Baidoo E, He Z, Mukhopadhyay A, Baumohl JK, Benke P, Joachimiak
MP, Xie M, Song R, Arkin AP, Hazen TC, Keasling JD, Wall JD, Stahl DA, Zhou J.
2013. Characterization of NaCl tolerance in Desulfovibrio vulgaris Hildenborough
through experimental evolution. ISME J 7:1790-1802.
24.
Hillesland KL, Stahl DA. 2010. Rapid evolution of stability and productivity at the
origin of a microbial mutualism. Proceedings of the National Academy of Sciences:-.
25.
Kaplan IR, Rittenberg SC. 1964. Microbiological fractionation of sulphur isotopes. J.
Gen. Microbiol. 34:195-212.
26.
Zane GM, Yen H-cB, Wall JD. 2010. Effect of the Deletion of qmoABC and the
Promoter-Distal Gene Encoding a Hypothetical Protein on Sulfate Reduction in
Desulfovibrio vulgaris Hildenborough. Applied and Environmental Microbiology
76:5500-5509.
27.
Harrison AG, Thode HG. 1958. Mechanism of the bacterial reduction of sulphate from
isotope fractionation studies. Transactions of the Faraday Society 54:84-92.
28.
Carrasco P, Daros JA, Agudelo-Romero P, Elena SF. 2007. A real-time RT-PCR
assay for quantifying the fitness of tobacco etch virus in competition experiments.
Journal of virological methods 139:181-188.
29.
Meyer JR, Ellner SP, Hairston NG, Jr., Jones LE, Yoshida T. 2006. Prey evolution
on the time scale of predator-prey dynamics revealed by allele-specific quantitative PCR.
Proc Natl Acad Sci U S A 103:10690-10695.
30.
Tang Y, Pingitore F, Mukhopadhyay A, Phan R, Hazen TC, Keasling JD. 2007.
Pathway Confirmation and Flux Analysis of Central Metabolic Pathways in
Desulfovibrio vulgaris Hildenborough using Gas Chromatography-Mass Spectrometry
and Fourier Transform-Ion Cyclotron Resonance Mass Spectrometry. Journal of
Bacteriology 189:940-949.
31.
Lee M-C, Chou H-H, Marx CJ. 2009. Asymmetric, bimodal trade-offs during
adaptation of Methylobacterium to distinct growth substrates. Evolution 63:2816-2830.
32.
Kryazhimskiy S, Rice DP, Jerison ER, Desai MM. 2014. Global epistasis makes
adaptation predictable despite sequence-level stochasticity. Science 344:1519-1522.
33.
Barrick JE, Lenski RE. 2013. Genome dynamics during experimental evolution. Nature
Reviews Genetics 14:827-839.
34.
Cooper TF, Rozen DE, Lenski RE. 2003. Parallel Changes in Gene Expression after
20,000 Generations of Evolution in Escherichia coli. Proceedings of the National
Academy of Sciences of the United States of America 100:1072-1077.
35.
Fisher RA. 1930. The genetical theory of natural selection. Clarendon Press, Oxford.
36.
Muller HJ. 1932. Some Genetic Aspects of Sex. The American Naturalist 66:118-138.
37.
Muller HJ. 1964. The Relation of Recombination to Mutational Advance. Mutat Res
1:2-9.
38.
Crow JF, Kimura M. 1965. Evolution in Sexual and Asexual Populations. The
American Naturalist 99:439-450.
39.
Gerrish P, Lenski R. 1998. The fate of competing beneficial mutations in an asexual
population. Genetica 102-103:127-144.
40.
de Visser JA, Rozen DE. 2006. Clonal interference and the periodic selection of new
beneficial mutations in Escherichia coli. Genetics 172:2093-2100.
41.
Miralles R, Gerrish PJ, Moya A, Elena SF. 1999. Clonal interference and the evolution
of RNA viruses. Science 285:1745-1747.
42.
Joseph SB, Hall DW. 2004. Spontaneous mutations in diploid Saccharomyces
cerevisiae: more beneficial than expected. Genetics 168:1817-1825.
43.
Desai MM, Fisher DS, Murray AW. 2007. The speed of evolution and maintenance of
variation in asexual populations. Current biology : CB 17:385-394.
44.
Kao KC, Sherlock G. 2008. Molecular characterization of clonal interference during
adaptive evolution in asexual populations of Saccharomyces cerevisiae. Nature genetics
40:1499-1504.
45.
Chou HH, Chiu HC, Delaney NF, Segre D, Marx CJ. 2011. Diminishing Returns
Epistasis Among Beneficial Mutations Decelerates Adaptation. Science 332:1190-1192.
46.
Wiser MJ, Ribeck N, Lenski RE. 2013. Long-term dynamics of adaptation in asexual
populations. Science 342:1364-1367.
47.
Lang GI, Rice DP, Hickman MJ, Sodergren E, Weinstock GM, Botstein D, Desai
MM. 2013. Pervasive genetic hitchhiking and clonal interference in forty evolving yeast
populations. Nature 500:571-+.
48.
Travisano M, Mongold J, Bennett A, Lenski R. 1995. Experimental tests of the roles
of adaptation, chance, and history in evolution. Science 267:87 - 90.
49.
Jackson BE, McInerney MJ. 2002. Anaerobic microbial metabolism can proceed close
to thermodynamic limits. Nature 415:454-456.
50.
Bell G. 1996. The basics of selection. International Thompson Publishing.
51.
Canfield DE. 2001. Biogeochemistry of Sulfur Isotopes. Reviews in Mineralogy and
Geochemistry 43:607-636.
52.
Rees CE. 1973. A steady-state model for sulphur isotope fractionation in bacterial
reduction processes. Geochimica et Cosmochimica Acta 37:1141-1162.
53.
Canfield DE, Habicht KS, Thamdrup B. 2000. The Archean Sulfur Cycle and the
Early History of Atmospheric Oxygen. Science 288:658-661.
54.
Hoek J, Reysenbach A-L, Habicht KS, Canfield DE. 2006. Effect of hydrogen
limitation and temperature on the fractionation of sulfur isotopes by a deep-sea
hydrothermal vent sulfate-reducing bacterium. Geochimica et Cosmochimica Acta
70:5831-5841.
55.
Dekel E, Alon U. 2005. Optimality and evolutionary tuning of the expression level of a
protein. Nature 436:588-592.
56.
Lewis NE, Hixson KK, Conrad TM, Lerman JA, Charusanti P, Polpitiya AD,
Adkins JN, Schramm G, Purvine SO, Lopez-Ferrer D, Weitz KK, Eils R, Konig R,
Smith RD, Palsson BO. 2010. Omic data from evolved E-coli are consistent with
computed optimal growth from genome-scale models. Molecular Systems Biology 6.
Figure 1
40
Desulfobacula phenolicad
PRTOLe
20
34
ε (‰)
30
Archaeoglobus fulgidus strain Zd
10
'066íf
Desulfovibrio oxyclinaed
0
c
a
s
b
us
m
c
i
h
u
ecie
d
g
c
p
i
n
u
s
i
h
o
r
e
tor
trop
bor
Oth
uto
lfata
den
l
a
u
i
s
H
de
rium
ris
rmo
cte
lga
e
a
u
h
v
b
T
rio
ulfo
ovib
Des
f
l
u
Des
Figure 2
Desulfovibrio vulgaris
Hildenborough (wild-type)
Founders
Ancestral
lineages
Evolved
lineages
A
B
C
D
E
F
0.6mL
Desulfovibrio vulgaris
Hildenborough (mutant)
10mL
G
H
I
J
K
L
0
0.6mL
10mL
0.6mL
10mL
10mL
10
5
0.6mL
10mL
0.6mL
10mL
925
15
A
B
C
D
E
F
10mL
930
Increasing generations
Preservation
at -80oC
Generations 0, 560 & 927 are
selected for further analysis
Direct RT-PCR
competition assay
Phenotypic
characterization
“Fossil
record”
G
H
I
J
K
L
Preservation intervals of
less than 100 generations
Fitness
Isotope fractionation (34ε)
Growth rate (k)
Growth yield (Y)
Respiration rate (csSRR)
Figure 3
A
Generation
0
1.20
560
927
Lineage
1.15
A
Fitness
B
C
D
1.10
E
F
1.05
1.00
0
250
500
750
1000
generations
B
Selection interval
0.20
Paired fitness difference
0-560
560-927
0.15
Lineage
A
0.10
B
C
0.05
D
E
0.00
F
1.00
1.05
1.10
1.15
Paired average fitness
1.20
Figure 4
A
Lineages
Generation
DvH-wt
10
wt
mu
A
0
B
560
C
927
9
E
8
F
34
ε (‰)
D
7
DvH-mu
G
6
H
I
5
0
250
500
J
750
Generations
K
L
B
Selection interval
Paired difference in 34 ε (‰)
0-560
2
560-927
Lineage
A
0
B
C
í
D
E
í
F
1.00
1.05
1.10
1.15
Paired average fitness over interval
1.20
Figure 5
A
Generation
0
Growth rate (dayí)
6
560
927
5
Lineage
A
4
B
C
0
500
750
Yield6 cells µmoleSO42- í)
B
250
E
F
80
60
40
0
250
0
250
500
750
500
750
C
Respiration rate (csSRR)
(fmole cellí dayí)
D
80
40
Generation
Figure 6
A
Paired growth rate difference (dayí)
Selection interval
0-560
2
560-927
Lineage
A
B
C
0
D
E
í
F
0.00
0.05
0.20
50
Selection interval
2-
B
Paired yield diffHUHQFH6 cells µmoleSO4 í)
Paired fitness difference
0-560
560-927
25
Lineage
A
B
0
C
D
í
E
F
0.00
0.05
0.20
Paired fitness difference
Selection interval
C
Paired respiration rate (csSRR)
difference (fmole cellí dayí)
0-560
40
560-927
Lineage
A
0
B
C
D
í
E
F
0.00
0.05
Paired fitness difference
0.20
A
Selection interval
0-560
Paired difference in ε (‰)
Lineage
0
A
B
C
í
D
E
í
F
í
0
Paired growth rate difference (dayí)
B
Selection interval
Paired difference in ε (‰)
0-560
Lineage
0
A
B
C
í
D
E
í
F
í
0
Paired yield diffHUHQFH6 cells µmole SO í)
C
50
Selection interval
0-560
Paired difference in ε (‰)
Figure 7
Lineage
0
A
B
C
í
D
E
í
F
í
0
Paired respiration rate (csSRR) difference (fmole cellí dayí)
Ŭ;ĚĂLJͲϭͿ ʍ
z;ϭϬϲĐĞůůƐͬђŵŽůĞ^KϰͿ ʍ
ĐƐ^ZZ;ĨŵŽůĞͬĐĞůůͬĚĂLJͿ
>ŝŶĞĂŐĞŶĂŵĞ 'ĞŶĞƌĂƚŝŽŶ &ŝƚŶĞƐƐ;tͿΎ ʍ
ŶĐĞƐƚŽƌ
Ϭ ϰ͘ϬϬ Ϭ͘ϯϵ
ϱϲ͘ϳ
ϳϬ͘ϱ
ϱ͘ϭ
Ϭ ϰ͘Ϭϱ Ϭ͘ϯϯ
ϰϰ͘ϵ
ϵϬ͘ϯ
ϯ͘ϭ
Ϭ ϯ͘ϲϭ Ϭ͘ϯϵ
ϰϵ͘Ϭ
ϳϯ͘ϱ
ϱ͘ϭ
Ϭ
ϭ͘ϬϬϲ ϰ͘ϲϲ Ϭ͘Ϯϱ
ϱϰ͘ϱ
ϴϱ͘ϱ
Ϯ͘ϯ
Ϭ
ϭ͘ϬϬϬ ϰ͘Ϯϵ Ϭ͘Ϯϳ
ϰϴ͘Ϭ
ϴϵ͘ϰ
Ϯ͘ϰ
&
Ϭ
ϭ͘ϬϬϭ ϰ͘Ϯϵ Ϭ͘Ϯϯ
ϱϱ͘ϯ
ϳϳ͘ϲ
Ϯ͘ϲ
ŵĞĂŶ
ϭ͘ϬϬϮΎΎ Ϭ͘ϬϬϯ
ϰ͘ϭϱ Ϭ͘ϯϱ
ϱϭ͘ϰ
ϰ͘ϳ
ϴϭ͘ϭ
'ĞŶĞƌĂƚŝŽŶ
ϱϲϬ
ϳ͘ϲ
ϱϲϬ
ϭ͘ϭϳϲ Ϭ͘ϬϬϯ
ϰ͘Ϯϭ Ϭ͘ϲϴ
ϰϰ͘ϴ
ϵϰ͘Ϭ
ϵ͘Ϭ
ϱϲϬ
ϭ͘ϭϳϯ Ϭ͘ϬϬϰ
ϱ͘Ϯϯ Ϭ͘ϲϵ
ϲϲ͘ϳ
ϳϴ͘ϯ
ϭϬ͘Ϯ
ϱϲϬ
ϭ͘ϭϰϴ Ϭ͘ϬϭϬ
ϱ͘ϭϯ Ϭ͘ϳϰ
ϲϲ͘ϵ
ϳϲ͘ϲ
ϴ͘ϴ
ϱϲϬ
ϭ͘ϭϴϱ Ϭ͘Ϭϭϵ
ϱ͘ϭϮ Ϭ͘ϲϬ
ϳϬ͘ϰ
ϳϮ͘ϳ
ϭϳ͘ϯ
ϱϲϬ
ϭ͘ϭϳϳ Ϭ͘ϬϬϲ
ϰ͘ϱϯ Ϭ͘ϴϯ
ϳϴ͘ϭ
ϱϴ͘Ϭ
ϭϭ͘Ϯ
&
ϱϲϬ
ϭ͘ϭϳϱ Ϭ͘ϬϬϰ
ϰ͘ϳϰ Ϭ͘ϱϳ
ϳϵ͘ϲ
ϱϵ͘ϲ
ŵĞĂŶ
ϭ͘ϭϳϮ Ϭ͘ϬϭϮ
ϰ͘ϴϯ Ϭ͘ϰϬ
ϲϳ͘ϴ
ϭϮ͘ϱ
ϳϯ͘Ϯ
'ĞŶĞƌĂƚŝŽŶ
ϵϮϳ
ϳϳ͘Ϭ
ϵϮϳ
ϭ͘Ϯϭϯ Ϭ͘ϬϬϳ
ϯ͘ϳϳ Ϭ͘ϰϮ
ϰϴ͘ϵ
ϵ͘Ϯ
ϰϲ͘ϲ
ϵϮϳ
ϭ͘ϭϵϰ Ϭ͘Ϭϭϴ
ϱ͘ϳϭ Ϭ͘ϰϱ
ϭϮϮ͘ϲ
Ϯ͘ϭ
ϰϳ͘ϵ
ϵϮϳ
ϭ͘ϭϳϴ Ϭ͘ϬϬϯ
ϱ͘ϳϲ Ϭ͘ϱϯ
ϭϮϬ͘Ϯ
Ϯ͘ϰ
ϱϲ͘ϳ
ϵϮϳ
ϭ͘ϭϴϴ Ϭ͘Ϭϭϱ
ϱ͘ϳϭ Ϭ͘ϯϴ
ϭϬϬ͘ϲ
Ϯ͘Ϯ
ϲϬ͘ϰ
ϵϮϳ
ϭ͘ϮϭϮ Ϭ͘ϬϬϲ
ϲ͘Ϭϭ Ϭ͘ϱϲ
ϵϵ͘ϲ
ϯ͘Ϭ
ϱϱ͘ϳ
&
ϵϮϳ
ϭ͘ϭϵϲ Ϭ͘ϬϬϳ
ϱ͘ϴϯ Ϭ͘ϰϲ
ϭϬϰ͘ϳ
Ϯ͘ϱ
ŵĞĂŶ
ϭ͘ϭϵϳ Ϭ͘Ϭϭϰ
ϱ͘ϰϲ Ϭ͘ϴϰ
ϱϳ͘ϰ
ϭϭ͘Ϭ
ϵϵ͘ϰ
Ύ&ŝƚŶĞƐƐĂƐƐĂLJƐĂƌĞƉĞƌĨŽƌŵĞĚŝŶĚĞƉĞŶĚĞŶƚůLJŽĨƚŚĞŐƌŽǁƚŚĂƐƐĂLJƐŝŶƚŚĞƌĞƐƚŽĨƚŚĞƚĂďůĞ͘
ΎΎ&ŝƚŶĞƐƐŽĨƚŚĞĂŶĐĞƐƚŽƌůŝŶĞĂŐĞŝƐĞdžƉƌĞƐƐĞĚƌĞůĂƚŝǀĞƚŽshϬϲϬϬ͘dŚĞĨŝƚŶĞƐƐŽĨǀ,tdĂŶĚshϬϲϬϬĂƌĞĞĨĨĞĐƚŝǀĞůLJĞƋƵĂů͘
dĂďůĞϭ͗&ŝƚŶĞƐƐĂŶĚŐƌŽǁƚŚĐŚĂƌĂĐƚĞƌŝƐƚŝĐƐŽĨůŝŶĞĂŐĞƐ
ʍ
Ϯϲ͘ϳ
ϵ͘ϱ
ϭϬ͘ϱ
ϳ͘ϴ
ϭϮ͘ϲ
ϭϭ͘ϭ
ϴ͘Ϭ
ϭϯ͘ϯ
ϭϭ͘Ϭ
ϭϲ͘ϳ
ϭϮ͘ϱ
ϭϲ͘ϭ
ϭϰ͘ϴ
ϮϮ͘Ϭ
ϴ͘ϰ
ϱ͘ϲ
ϳ͘Ϯ
ϱ͘ϴ
ϭϬ͘ϵ
ϵ͘ϲ
ϵ͘ϰ
Table 2: Mass balance and isotopic characteristics of individual lines during the evolution
experiment.
Generation#
0
560
927
Lineage id
A
B
C
D
E
F
G (DVH-mut)
H (DVH-mut)
I (DVH-mut)
J (DVH-mut)
K (DVH-mut)
L (DVH-mut)
L (DVH-mut, replicate)
A
B
C
D
E
F
F (replicate)
G (DVH-mut)
H (DVH-mut)
I (DVH-mut)
J (DVH-mut)
K (DVH-mut)
L (DVH-mut)
A
B
C
D
E
F
G (DVH-mut)
H (DVH-mut)
I (DVH-mut)
J (DVH-mut)
K (DVH-mut)
L (DVH-mut)
34
f
0.97
0.97
0.98
0.95
0.83
0.96
0.96
0.99
0.81
0.75
0.97
0.88
0.98
0.99
0.99
0.92
0.92
0.94
0.93
0.99
0.95
0.98
0.97
0.92
0.97
0.97
0.94
0.96
0.93
0.93
0.94
0.96
0.80
0.90
0.85
0.89
0.88
0.80
34
İ
10.06
7.60
7.78
6.83
7.00
6.55
8.66
6.34
5.46
5.74
6.39
5.14
7.05
10.04
9.54
8.19
8.94
10.09
7.34
6.99
6.73
5.90
8.34
7.90
7.17
6.58
6.12
7.17
8.58
8.25
6.00
6.22
6.02
6.49
6.54
5.05
6.54
6.09
33
Ȝ
0.5060
0.5073
0.5082
0.5067
0.5081
0.5130
0.5077
0.5078
0.5046
0.5100
0.5088
0.5044
0.5047
0.5084
0.5080
0.5123
0.5070
0.5087
0.5133
0.5091
0.5087
0.5141
0.5121
0.5094
0.5131
0.5110
0.5087
0.5085
0.5100
0.5109
0.5109
0.5092
0.5078
0.5082
0.5106
0.5077
0.5111
0.5089
ı34İ
0.13
0.13
0.13
0.13
0.13
0.13
0.13
0.14
0.13
0.14
0.13
0.13
0.14
0.14
0.14
0.13
0.13
0.13
0.13
0.13
0.14
0.13
0.13
0.13
0.13
0.13
0.14
0.13
0.13
0.14
0.13
0.14
0.13
0.13
0.13
0.14
0.13
0.13
ı 33Ȝ
0.0016
0.0016
0.0017
0.0018
0.0019
0.0012
0.0014
0.0021
0.0022
0.0019
0.0019
0.0024
0.0017
0.0012
0.0012
0.0013
0.0015
0.0014
0.0012
0.0017
0.0018
0.0021
0.0015
0.0016
0.0017
0.0019
0.0020
0.0017
0.0014
0.0015
0.0020
0.0020
0.0021
0.0019
0.0019
0.0025
0.0019
0.0020
Paper 2 – Evolutionary adaptation of a
sulfate reducing bacterium and its sulfur
isotope phenotype
Appendix
Supplemental material
Table S1
The growth characteristics of the DvH lineages at generation 0 (ancestor), 560 and 927 as
well as the interpreted values growth rate, yield and csSRR. The data was acquired at T0, T1 but
also at T2 which corresponds to time when the cultures enter stationary phase. Initially, because
the biomass accumulation from T0 to T2 was greater, it was thought they may provide a more
reliable estimate of yield. Different yield and csSRR estimates are based on the utilization of
growth data from T0, T1 and T2 but ultimately the data from T2 was not retained for further
analysis.
Figure S1
The relationship between
34
İ and Ȝ throughout the microbial evolution experiment. Circles
denote measurements from the ancestor, triangles are from generation 560 and the squares are
from generation 927. The data obtained from cultures started from DvH wild type are colored
according to lineage while the data obtained from DVU0600 are pooled together as light brown.
A
B
C
D
E
F
A
B
C
D
E
F
927
927
927
927
927
927
0.278
0.309
0.267
0.625
0.282
0.332
0.285
0.308
0.3
0.398
0.24
0.356
7.81832
9.21669
7.37753
8.59446
6.53394
8.4795
7.28633
8.5099
8.5251
8.4643
6.14635
9.42948
Lineage OD
[H2S]
A
0.482 13.2522
B
0.482 13.2522
C
0.482 13.2522
D
0.482 13.2522
E
0.482 13.2522
F
0.482 13.2522
560
560
560
560
560
560
Generation
0
0
0
0
0
0
Growth data
Table S1
YES
YES
YES
YES
YES
YES
YES
YES
YES
YES
YES
YES
13.5
13.5
13.5
13.5
13.5
13.5
0
0
0
0
0
0
0
0
0
0
0
0
0.029
0.026
0.022
0.031
0.021
0.025
0.027
0.025
0.023
0.029
0.021
0.031
0.005
0.005
0.005
0.005
0.005
0.005
0.005
0.005
0.005
0.005
0.005
0.005
3.3E+07
2.9E+07
2.5E+07
3.5E+07
2.4E+07
2.9E+07
3.1E+07
2.9E+07
2.6E+07
3.3E+07
2.4E+07
3.5E+07
INOC
T0
MICROSCOPY Age (hrs) Time (hrs) OD
ı
cells/mL
0
0.031
0.005 3.5E+07
0
0.037
0.005 4.2E+07
0
0.032
0.005 3.6E+07
Yes
0
0.047
0.005 5.4E+07
Yes
0
0.045
0.005 5.1E+07
Yes
0
0.051
0.005 5.8E+07
Page 1
ı
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
5.7E+06
[H2S]
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.2
0.2
0.2
0.3
0.1
0.3
0.0
0.1
0.1
0.1
0.0
0.1
ı
0.2
0.3
0.1
0.4
0.2
0.3
Table S1
10.5
10.5
10.5
10.5
10.5
10.5
7.5
7.5
7.5
7.5
7.5
7.5
0.15
0.31
0.27
0.37
0.29
0.32
0.10
0.13
0.12
0.14
0.09
0.14
T1
Time (hrs) OD
ı
10.5
0.18
10.5
0.22
10.5
0.16
10.5
0.36
10.5
0.29
10.5
0.33
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
0.01
1.7E+08
3.6E+08
3.1E+08
4.2E+08
3.3E+08
3.6E+08
1.1E+08
1.5E+08
1.3E+08
1.6E+08
1.0E+08
1.6E+08
cells/mL
2.0E+08
2.5E+08
1.8E+08
4.1E+08
3.4E+08
3.8E+08
ı
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
Page 2
[H2S]
1.9
7.1
6.1
6.9
5.1
6.1
2.1
2.0
1.8
2.2
1.1
1.8
3.1
4.9
2.9
6.9
6.1
6.2
ı
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
24
24
24
24
24
24
23.5
23.5
23.5
23.5
23.5
23.5
0.675
0.677
0.653
0.739
0.688
0.693
0.599
0.623
0.626
0.713
0.626
0.653
T2
Time (hrs) OD
ı
49
0.53
49
0.54
49
0.523
49
0.507
49
0.516
49
0.539
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
0.02
2.3E+07
2.3E+07
2.3E+07
2.3E+07
2.3E+07
2.3E+07
2.3E+07
2.3E+07
2.3E+07
2.3E+07
2.3E+07
2.3E+07
6.8E+08
7.1E+08
7.1E+08
8.1E+08
7.1E+08
7.4E+08
7.7E+08
7.7E+08
7.4E+08
8.4E+08
7.8E+08
7.9E+08
14.2022
12.7962
12.4694
13.6246
15.1218
15.2966
13.1762
14.9622
15.1218
14.4682
14.0654
14.2858
cells/mL ı
[H2S]
ı
6E+08 2.3E+07
14.339
6.2E+08 2.3E+07 14.3466
6E+08 2.3E+07 14.1338
5.8E+08 2.3E+07 14.2858
5.9E+08 2.3E+07 13.7918
6.1E+08 2.3E+07 14.2326
Table S1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
3.8
5.7
5.8
5.7
6.0
5.8
4.2
5.2
5.1
5.1
4.5
4.7
k (day-1)
T1-T0
ı
4.0
4.1
3.6
4.7
4.3
4.3
0.4
0.5
0.5
0.4
0.6
0.5
0.7
0.7
0.7
0.6
0.8
0.6
0.4
0.3
0.4
0.3
0.3
0.2
77.0
46.6
47.9
56.7
60.4
55.7
44.8
66.7
66.9
70.4
78.1
79.6
9.2
2.0
2.4
2.2
3.0
2.5
7.6
9.0
10.2
8.8
17.3
11.2
50.3
46.3
46.1
55.1
49.5
50.5
49.6
52.0
58.6
58.1
59.7
50.4
50.1
94.0
78.3
76.6
72.7
58.0
59.6
73.2
48.9
122.6
120.2
100.6
99.6
104.7
8.0
11.1
12.6
7.8
10.5
9.5
22.0
14.8
16.1
12.5
16.7
11.0
72.4
97.4
99.2
95.5
119.3
116.2
83.6
112.8
111.2
92.9
91.6
93.9
11.8395
8.81708
10.3645
7.36315
12.5747
10.5806
19.6061
21.2559
23.3576
15.9462
26.4114
17.317
Y(10^6cells/µmole SO4)
csSRR (fmole/cell/day)
(T1-T0) ı
(T2-T0) Y:T1-T0 ı
Y:T2-T0 ı
56.7
5.1
70.5
9.4
44.9
3.1
90.3
9.6
49.1
5.1
73.5
10.9
54.5
2.3
85.5
5.8
48.0
2.4
89.4
7.2
55.3
2.6
77.6
5.6
/ŶƚĞƌƉƌĞƚĂƚŝŽŶƐŽĨŐƌŽǁƚŚƌĂƚĞ͕LJŝĞůĚĂŶĚĐƐ^ZZ
Page 3
Figure S1
0.516
Interval
Ancestor
Generation 560
0.512
Generation 927
Lineage
33λ
A
B
0.508
C
D
E
F
0.504
All DVU0600
5
6
7
8
34ε
9
10
Detailed methods
A-1 : 16S rRNA contamination check
DNA was extracted from the ancestral strain and the 12 evolved lineage strains of DvH. In
order to test for contaminations within the liquid cultures, we amplified and sequenced the 16S
ribosomal gene from each lineage including the ancestral strain using general primers 27F (5’AGA GTT TGA TCC TGG CTC AG-3’) and 728R (5’-CTA CCA GGG TAT CTA ATC C-3’)
and Sanger sequencing. The program cycle used to amplify the 16S gene is as follows: initial
denaturation at 94°C for 15min followed by an initial 15 cycles of denaturation at 94°C for 1min,
primer annealing at 52°C for 45sec and elongation at 72°C for 1min. This is then immediately
followed by 20 cycles of denaturation at 94°C for 1min, primer annealing at 49.5°C for 45sec
and elongation at 72°C for 1min. The final elongation step was at 72°C for 7min. All PCR were
prepared in a laminar flow hood using aerosol resistant pipette tips and performed using Qiagen
hotstart©. All PCR reactions were carried out on the Eppendorf Mastercycler Pro S. All the
sequencing was done by the Génome Québec Innovation Centre.
The gene sequences from each lineage we’re aligned using clustalW and trimmed to remove
any low quality reads. In the end all sequences had a 692bp common overlap. Since all 13 tests
were a 100% match to the 16S rRNA gene of DvH from Heidelberg et al. (2008), no subsequent
analysis was undertaken.
A-2: Measurement of the sulfur isotopes and estimation of the isotope phenotype (34İ) and
33
Ȝ
The media containing both the sulfate and sulfide fractions of S was filtered through a 0.22um
filter to remove all ZnS as well as cells and other precipitates from the filtrate containing the
sulfate. The filter was subsequently reacted with concentrated hydrochloric acid to produce acidvolatile H2S (AVS) from the precipitated ZnS.
This was carried by a N2 gas through a
distillation column, a water trap and bubbled through an acidic zinc acetate trap which reprecipitated the H2S as a purified ZnS that did not contain cells or precipitates from the growth
media. The sulfate in the filtrate was reacted with a ‘Thode’ reducing solution, a mixture of HI,
H2PO4 and HCl that reduced the sulfate to H2S when heated to §100°C and followed the same
trajectory as with the AVS extraction. The purified ZnS samples were then reacted with a silver
nitrate solution to produce silver sulfide. The silver sulfide was removed from the filtrate by
filtering through a 0.22um filter, which was washed with 1 M ammonium hydroxide and rinsed
three times with deionized water and then dried at 50°C for 2 days. Approximately 3mg of the
dried samples were weighed into aluminum packets in preparation for mass spectrometry.
Subsequently, the samples were converted to sulfur hexafluoride and the gas purified using
the procedure outlined in (1) (Paper 1) before analysis of the sample with a MAT 253 running in
dual inlet mode in the Stable Isotope Laboratory of the Earth and Planetary Sciences Department
at McGill University, Montreal, Canada. Isotopic compositions are reported using delta notation
ଷ
ܴ ௦
ߜ ଷܵ ൌ ቆ ଷ
െ ͳቇ ൈ ͳͲͲͲ
ܴ ି்
where 3iR = 3iS/32S, i is 3 or 4 and V-CDT refers to the Vienna-Cañon Diablo Troilite
international reference scale. All sulfur isotope data reported relative to Vienna Cañon Diablo
Troilite (V-CDT), against which the international reference material IAEA-S-1 is taken to have
the following isotopic composition: δ33S = -0.061‰ and δ34S ≡ -0.3‰. The precision (1ı) on
individual measurements is better than 0.05 ‰ for į34S values, and 0.01‰ for ǻ33S values.
Accuracy of the measurements is controlled by the analytical reproducibility (1ı), which for the
full measurement procedure is better than 0.1 ‰ for į34S values and 0.01‰ for ǻ33S values.
Repeat analyses of the international reference materials IAEA-S-1, IAEA-S-2, and IAEA-S-3
always matched their accepted values within these uncertainties..
Data processing
In order to correct for the Rayleigh effect produced by the closed system of a batch culture,
three isotopic measurements were required to produce estimates of
34
İ. We used: (1) the initial
isotopic composition of the starting sulfate (T0,SO4) (2) the isotopic composition of the sulfate at
the time of sampling (T1,SO4) and (3) the isotopic composition of the sulfide produced by
dissimilatory sulfate reductions up to the time of sampling (T1,H2S).
First, the fraction of remaining sulfate (f), can be calculated with the assumption that the
isotopic composition of the sulfide will be equal to that of the starting reactant when the reaction
goes to completion. This results in:
݂ൌ
ߜ ଷସܵ ்ǡௌைସ
ߜ ଷ ்ܵଵǡுଶௌ
ቆ ͳͲͲͲ
ͳቇ െ ቆ ͳͲͲͲ
ͳቇ
ߜ ଷ்ܵଵǡௌைସ
ߜ ଷ்ܵଵǡுଶௌ
ͳቇ െ ቆ ͳͲͲͲ
ͳቇ
ቆ ͳͲͲͲ
The fractionation factor (Į) was calculated with a Rayleigh distillation correction while
assuming isotopic mass balance between sulfate and sulfide (Hoek 2006, Johnston 2007, Sim
2011)
ଷ
ሺͳ െ ݂ ሻ ߜ ଷ ்ܵଵǡுଶௌ ͳͲͲͲ
ͳ
ߙൌെ
݈݊ ቆͳ
ଷ
ቇ
݈݂݊
݂
ߜ ்ܵଵǡௌைସ ͳͲͲͲ
We refer commonly to a form of the fractionation factor,34İ as the isotope phenotype where
ߝ ൌ ሺ ଷସߙ െ ͳሻ ͲͲͲͳ כ
ଷସ
The relationship between the fractionation of 33S relative to 32S and the heavier 34S relative to
32
S is expressed as 33Ȝ.
ߣൌ
ଷଷ
The uncertainty on the f,
34
İ, and
33
ሺ ଷଷߙ ሻ
ሺ ଷସߙ ሻ
Ȝ was estimated through a Monte Carlo simulation (2).
The initial uncertainties on į values were estimated from the variability observed in the pooled
ߜ ଷସܵ ்ǡௌைସ of all the experiments. This was 0.11 ‰ (see APPENDIX A-3).
A-3 : Estimate of uncertainty on 34İ and 33Ȝ
We assume that our results follow a Gaussian distribution and obtain an uncertainty estimate
by Monte Carlo simulation. All procedures were performed in R. We created Gaussian
distributions of 5000 replicates for each of our measurement of ߜ ଷସܵ ்ǡௌைସ, ߜ ଷସܵ ்ଵௌைସ and
ߜ ଷସܵ ்ଵுଶௌ and assume that these distributions represent a good approximation of the true
probability distribution of each measurement. These distributions center on a mean of
ߜ ଷସܵ௦௨ௗ and standard deviation of ıఋ యరௌ
ೌೞೠೝ
The standard deviation value was set at
0.11‰ because this was the uncertainty estimate obtained from pooling the entire dataset of
ߜ ଷସܵ ்ǡௌைସ values for all experiments. We utilized this as the estimator of uncertainty rather than
simply the mass spectrometer uncertainty of individual measurements because it is a more
relevant measure of the true uncertainty encompassing the whole-system handling. It takes into
account the machine uncertainty, the extraction uncertainty, as well as manipulation uncertainty
and any isotopic variation which may occur stochastically between bottles. The measure of
ߜ ଷସܵ ்ǡௌைସ has not yet been subject to the bacteriological activity we wish to measure yet
follows the entire rest of the preparation protocol.
The uncertainties between į34S and į33S are correlated in large part. We therefore produced
probability distributions for ߜ ଷଷܵ ்ǡௌைସ,
ߜ ଷଷܵ ்ଵௌைସ and ߜ ଷଷܵ ்ଵுଶௌ respectively by
transforming the Gaussian distributions we had created for ߜ ଷସܵ ்ǡௌைସ ,
ߜ ଷସܵ ்ଵௌைସ and
ߜ ଷସܵ ்ଵுଶௌ . There are however deviations from the predicted relationship betweenߜ ଷଷܵ and
ߜ ଷସܵ as well as a small uncorrelated uncertainty which is equivalent to ıοయయ ୗ , the machine
uncertainty or 0.01‰. Both are taken into account in οଷଷ ܵௗௗ
The transformation of the probability distribution from į34S to į33S is:
ߜ ܵௗௗ
ଷଷ
Ǥହଵହ
ߜ ଷସܵௗௗ
ൌ ቆ
ͳቇ
ͳͲͲͲ
Where οଷଷ ܵௗௗ is defined as
ο ܵௗௗ
ଷଷ
െ ͳ൩ ൈ ͳͲͲͲ οଷଷ ܵௗௗ
ߜ ଷସܵ௦௨ௗ
ൌ ߋሺͲǡ ıοయయ ୗ ሻ ߜ ܵ௦௨ௗ ቆ
ͳቇ
ͳͲͲͲ
ଷଷ
Ǥହଵହ
െ ͳ൩ ൈ ͳͲͲͲ
ߋሺͲǡ ıοయయ ୗ ሻ is the uncorrelated uncertainty and ߜ ଷଷܵ௦௨ௗ is the experimentally
determined į33S.
With the distributions of ߜ ଷଷܵ ்ǡௌைସ , ߜ ଷଷܵ ்ଵௌைସ and ߜ ଷଷܵ ்ଵுଶௌ , ߜ ଷସܵ ்ǡௌைସ, ߜ ଷସܵ ்ଵௌைସ
and ߜ ଷସܵ ்ଵுଶௌ in hand we calculated the distributions of 34İ and 33Ȝ. The standard deviation on
the obtained distributions of 34İ and 33Ȝ were taken as a reliable estimate of the uncertainty.
A-4 : qPCR method
In order to successfully monitor the frequency of the two competitors during a competition
experiment, it was necessary for them to have a differentiable trait. This trait was already
available in the form of the unique DNA barcode present in the mutant DVU0600 strain, which
we dubbed the “reference” strain. The stock of DVU0600 strain was therefore utilized as the
reference strain in the competition experiment and every time a competition was run, it a new
aliquot of the reference was utilized.
The two competing strains were removed from the -80oC freezer and “revived” back to health
by serial transfers for three days (three transfers) prior to the start of the competition experiment.
On the day of the start of the competition experiment, the initial aliquots of contestant and
reference were grown until they reached stationary phase (10e9 cells/mL) but were not allowed
to remain longer than a few hours in this state of growth. This replicates perfectly the transfer
dynamics of the long term evolution experiment. 3mL aliquots were then taken from the pure
cultures of the strains to be competed using a needle and syringe and were mixed together in a 15
mL falcon tube. The tube was vortexed for 10s to ensure homogeneity of the mix. This mixed
culture we defined as “T=0” and is the common “parent” culture to all three competition
experiment replicates. In order to ensure reproducibility of the result, each competition assay is
composed of three individual competitions. Volumes of 0.6mL of T0 were taken from the falcon
tube and put into 20mL serum bottles containing 10mL of MOLS media (for a total volume of
10.6mL) and a headspace of 100% Nitrogen. Therefore, the only variable which is different than
a normal serial transfer during the long term evolution experiment is that the culture is now a
mixed population rather than a pure culture. Each triplicate was numerated according to the
current transfer (eg. The first transfer for replicate one is labelled T1-A). Over the course of the
next 24 hours the strains enter balanced growth and consumed the entire pool of lactate and
sulfate in the media. Assuming that the cell density in the new stationary phase is the same as the
previous stationary phase, in the case of the mixing ratio used (0.6mL/10.6mL, inoculum
vol/total vol) one growth cycle is equivalent to 4.14 doublings (# doubling = ln(dilution)/ln2).
The competitions are then serially transferred every 24 hours using the same dilution (0.6mL into
10mL) of fresh media and labelled progressively higher (eg. T2A, T3A etc.). The remaining
spent media leftover after the serial transfer is centrifuged at 7000xg for 15 minutes to pellet the
cells. The supernatant is removed and the pellet frozen at -20oC until the DNA extraction step.
The competitions lasted for a total of 10 serial transfers (equivalent to 41.4 doublings) and at
each serial transfer, cells were preserved. Mostly, only three transfers were selected for further
analysis. The first competition transfer (T1) was used as a reference to quantify relative changes
in the frequency of the mutant in later transfers (usually T5 and T10). For timepoint 0, we
competed the ancestor wild type (parent to the evolved lines) in three distinct competition assays.
In order to have a closer look at the variability of the results within one of these competitions, we
tested at multiple different serial transfers (T0,T2,T3,T4,T8 and T9). As for the assays of the
evolved DVH WT at 560 and at 927, each of six lines were assayed individually at T1,T5 and
T10.
Competition theory
We track the rate of disappearance of the reference strain from the population. This has been
modelled in chemostats (3) and in batch cultures (4).
The growth rate of the two strains vary throughout each batch but over an entire growth cycle,
each strain will have an average growth rate, which, if different, will have an impact on the
abundance of a strain in a population at the time of serial transfer. We monitor this net change in
frequency over multiple serial transfers. During serial transfer in batch culture, the effective
exponential growth rate (the ‘malthusian parameter’, Lenski et al., 1991) of a strain (i) can be
modelled through
ܰ ሺݔሻ ൌ ܰ ሺͲሻሺݎ ݔሻ
where x is the number of serial transfers, Ni(x) is the number of cells of strain i at transfer x,
Ni(0) is the number of cells present at the first transfer and ri is the malthusian parameter of strain
i in units of transfers. When a second strain (j) is present such as during a competition
experiment, the ratio of the abundances can be modelled as
ܰ ሺݔሻ ܰ ሺͲሻሺݎ ݔሻ
ൌ
ܰ ሺݔሻ ܰ ሺͲሻሺݎ ݔሻ
However, we cannot estimate the numbers of two strains in the population because in our
case, only the mutant barcode strain has the unique marker which can distinguish it from the rest
of the population. We have only the frequency of the mutant and the total population. We can
use the ratio of the abundances, which will be the same as the ratio of the frequencies because
ܰ
ܰ ܰ ܰ
ൌ
ܰ
ܰ
ܰ ܰ
and since there are only two strains in the competition experiment we can express this ratio of
abundances
ൌ
Finally,
൬
ே
ே ାேೕ
and ݍൌ ͳ െ
௫
൰ ൌ ൬ ൰ ൫ݎ െ ݎ ൯ݔ
ݍ௫
ݍ
where ൫ݎ െ ݎ ൯ is the selection-rate constant (sij) in units of transfers-1, which can be
determined in our case by taking multiple samples during the competition experiment, the first
one being ቀబ ቁ, and fitting a straight line to logarithmically transformed frequency ratios
బ
Finally, in order to express the selection coefficient in units of generations-1, we calculate the
number of doublings (Dx)
ܦ௫ ൌ ݔ
ሺ݀ሻ
ሺʹሻ
where d is the dilution factor of each serial transfer (10.6 mL/0.6mL = 17.67). This allows us
to calculate the selection coefficient (sg) in units of generation-1.
௫
൬ ൰ ൌ ൬ ൰ ݏ ൈ ܦ௫
ݍ௫
ݍ
From this, we can calculate the fitness (W) of strain i relative to strain j, as
ܹ ൌ ͳ ݏ
This fitness definition is slightly different than that used in other experimental evolution
studies, which is the direct ratio of malthusian parameters for strain i relative to strain j.
However, if the actual average malthusian parameter in the mixed competition culture is constant
and equal to the theoretical limit set by d, both fitness definitions give equivalent numerical
values.
Extraction of DNA
DNA of serial transfers was extracted using a QIAGEN QIAquick DNA preparation columns
as well as a negative control to assess contamination. The suggested protocol for gram negative
bacteria was followed. We did not use DNase or RNase treatements. The final yield of DNA was
quantified by measuring a 2ul subsample of the final extract on a Nanodrop spectrophotometer.
qPCR target information
Two targets were used for this competition experiment. The first is located on the dsrA gene
of all sulfate reducing bacteria including the Wild type DvH and mutant DVU 0600 and
produces a product of 222 bp. The second target region was designed to bind on the barcode
inserted into DVU 0600, a deletion mutant generated through a double recombination process
which swapped a gene coding for L-lactate dehydrogenase (TIGR) protein (DVU0600, accession
number YP_009822) with an antibiotic resistance cassette as well as two unique barcodes
flanked by common sequences to all mutants created in the Wall lab. We utilized one of the
unique barcodes as a primer as well as a sequencing primer known to work on this mutant to
produce a PCR product that is of an optimal size for a qPCR assay. The primers used are listed in
the table below. They were ordered from Invitrogen Life Sciences as desalted purity.
Table S2: List of primers used for competition experiment
Primer pair
Sequence (5’ 3’)
Target gene
Product size (bp)
Reference
DSR 1F
ACSCACTGGAAG
dsrA
222
(5)
Antibiotic
157
Dr.
CACG
RH3-dsr-R
GGTGGAGCCGTG
CATGTT
2Fsequencing
2R-barcode
CTACCCGTGATAT
TGCTGAAGA
GACCGTAATGAT
cassette,
Judy Wall
Artificial barcode
ACTACACG
Real time PCR.
Real-time PCR analysis was performed using the primers shown in Table 1. Quantifying the
total recovery of genomic DNA from the DNA extraction was done using a Nano drop
spectrophotometer. We then diluted this genomic DNA to a final concentration of
2.5attomoles/ul assuming 3570585 bp/genome (6) and used this concentration as the template for
the reaction. The PCR reaction had a total volume of 12.5 ul/well. This mix was 6.75 ul of SYBR
Green Supermix (Bio-Rad),(a 2X solution of 100 mM KCl, 40 mM Tris-HCI, pH 8.4, 0.4 mM of
each dNTP (dATP, dCTP, dGTP,and dTTP), iTaq DNA polymerase, 50 units/ml, 6 mM MgCl2,
SYBR Green I, 20 nM fluorescein, and stabilizers) 0.5 ul of each forward and reverse primer, 2ul
of template DNA and 2.75ul of DEPC treated water. The final concentration of each primer was
0.4µM. Final concentration of template DNA was 5.92 ng/ul. Thermal cycling was performed on
a Bio-rad IQ cycler using an optical grade 96well plate and film. The thermal conditions were as
follows: 3min at 95oC followed by 40 cycles of 10 seconds at 95oC, 30 seconds at 56oC. To
verify that a single PCR product was produced and to assay purity of the PCR product a melt
curve sequence was performed from 55oC to 95oC after the initial cycling.
Pure culture
template DNA of D. Vulgaris Hildenborough was used as positive control for the dsrA primer
pairs. The same template was utilized to ensure that the barcode-sequencing primer pairs did not
produce a PCR product. Pure culture of JW9019 was used as positive control for the barcodesequencing primer pairs. Because of poor reproducibility between runs on the qPCR instrument,
it was necessary to include all reactions which needed to be compared for relative growth rates
on the same plate. Or, alternatively, two or more plates were used but the reference was run on
all plates and each plate corrected to the performance of the reference during its respective run.
Essentially, inter-plate results were not directly compared.
The PCR efficiency was optimized using pure cultures of JW9019 with standard dilution
curves. Well-specific efficiency measurements were obtained through post-run processing of the
background substracted fluorescence data of each run imported into the program LinRegPCR
(7). This program allows the calculation of well-specific reaction efficiencies as well as adjusted
Ct values depending on set thresholds and limits which can be user-controlled.
Data processing
Since PCR efficiency car vary from plate to plate and from well to well, the PCR efficiency
was calculated using the LinRegPCR program with default settings. This programs utilizes an
algorithm to fit the measured fluorescence increases to exponential growth models available in
the literature (8). We assigned each well an amplicon group (dsrA or barcode) from which an
average amplicon efficiency was calculated within each plate. In order to ascertain that the
efficiency of the primer pairs followed a normal distribution and to measure the variation in
efficiency in-between plates, we visually inspected the distribution of efficiencies of each
amplicon group with histograms and plotted theoretical and sample quantiles. We obtained
reproducible calculated average efficiency (Ɯሻ and we utilized them to interpret the ct values
signal using Pfaffl et al. (2001). This yielded the corrected expressions of the dsrA amplicons
(A) as well as the barcode amplicons (B).
Ɯௗ௦
Ɯ
ି௧
ି௧
ൌܣ
ൌܤ
The results from the first transfer (T1a, T1b, T1c) were used as the reference signal of either
dsrA or barcode in the respective competitions. This is the reference from which the other
transfer points (typically T5a-c and T10a-c) were corrected to obtain the change in signal during
the competition experiment. The barcode signal is then divided by the dsrA signal to obtain the
relative frequency of the barcode in a given replicate, at a given transfer during the competition
experiment (Barcode/dsrA at T1, T5, T10). This corrected ct value derived expression is
equivalent the ratio of abundances discussed previously.
ܣ௫ Τ்ܣଵ
௫
൬ ൰ ൌ ݈݊
ܤ௫ Τ்ܤଵ
ݍ௫
Uncertainty estimates
Each competition experiment was performed three times. The uncertainty on the relative
growth rates, which include the variability caused by manipulations during the competition
(handling of cultures, bottles and media volumes), the manipulations during the PCR reaction
setup (pipetting error, volumes of reagents) as well as the instrumental error on qPCR efficiency
and Ct values expressed in the standard deviation on the mean ı, obtained for each experiment.
References
ϭ͘
Ϯ͘
ϯ͘
ϰ͘
ϱ͘
ϲ͘
ϳ͘
ϴ͘
WĞůůĞƌŝŶ͕tŝŶŐ͕ZŽƵŐŚD͕DƵĐĐŝ͕ĂŶĨŝĞůĚ͕Ƶŝd,͘ϮϬϭϰ͘ZĞŽdžŝĚĂƚŝǀĞƐƵůĨƵƌĐLJĐůŝŶŐŝŶ
ƚŚĞƐƵůĨŝĚŝĐĐĂƌďŽŶͲƌŝĐŚƐĞĚŝŵĞŶƚƐŽĨDĂŶŐƌŽǀĞ>ĂŬĞ͕ĞƌŵƵĚĂ͘'ĞŽĐŚŝŵŝĐĂĞƚŽƐŵŽĐŚŝŵŝĐĂ
ĐƚĂ;ƐƵďŵŝƚƚĞĚ͕'ͲͲϭϰͲϬϬϮϲϳͿ͘
WĂƉĂĚŽƉŽƵůŽƐ͕zĞƵŶŐ,͘ϮϬϬϭ͘hŶĐĞƌƚĂŝŶƚLJĞƐƚŝŵĂƚŝŽŶĂŶĚDŽŶƚĞĂƌůŽƐŝŵƵůĂƚŝŽŶŵĞƚŚŽĚ͘
&ůŽǁDĞĂƐƵƌĞŵĞŶƚĂŶĚ/ŶƐƚƌƵŵĞŶƚĂƚŝŽŶϭϮ͗ϮϵϭͲϮϵϴ͘
LJŬŚƵŝnjĞŶ͕,Ăƌƚů>͘ϭϵϴϯ͘^ĞůĞĐƚŝŽŶŝŶĐŚĞŵŽƐƚĂƚƐ͘DŝĐƌŽďŝŽůŽŐŝĐĂůƌĞǀŝĞǁƐϰϳ͗ϭϱϬͲϭϲϴ͘
>ĞŶƐŬŝZ͘ϭϵϵϭ͘YƵĂŶƚŝĨLJŝŶŐĨŝƚŶĞƐƐĂŶĚŐĞŶĞƐƚĂďŝůŝƚLJŝŶŵŝĐƌŽŽƌŐĂŶŝƐŵƐ͘ŝŽƚĞĐŚŶŽůŽŐLJϭϱ͗ϭϳϯͲ
ϭϵϮ͘
ĞŶͲŽǀ͕ƌĞŶŶĞƌ͕<ƵƐŚŵĂƌŽ͘ϮϬϬϳ͘YƵĂŶƚŝĨŝĐĂƚŝŽŶŽĨ^ƵůĨĂƚĞͲƌĞĚƵĐŝŶŐĂĐƚĞƌŝĂŝŶ
/ŶĚƵƐƚƌŝĂůtĂƐƚĞǁĂƚĞƌ͕ďLJZĞĂůͲƚŝŵĞWŽůLJŵĞƌĂƐĞŚĂŝŶZĞĂĐƚŝŽŶ;WZͿhƐŝŶŐ
Θůƚ͖ŝΘŐƚ͖ĚƐƌΘůƚ͖ͬŝΘŐƚ͖ĂŶĚΘůƚ͖ŝΘŐƚ͖ĂƉƐΘůƚ͖ͬŝΘŐƚ͖'ĞŶĞƐ͘DŝĐƌŽďŝĂůĐŽůŽŐLJϱϰ͗ϰϯϵͲϰϱϭ͘
,ĞŝĚĞůďĞƌŐ:&͕^ĞƐŚĂĚƌŝZ͕,ĂǀĞŵĂŶ^͕,ĞŵŵĞ>͕WĂƵůƐĞŶ/d͕<ŽůŽŶĂLJ:&͕ŝƐĞŶ:͕tĂƌĚE͕
DĞƚŚĞ͕ƌŝŶŬĂĐ>D͕ĂƵŐŚĞƌƚLJ^͕ĞďŽLJZd͕ŽĚƐŽŶZ:͕ƵƌŬŝŶ^͕DĂĚƵƉƵZ͕EĞůƐŽŶt͕
^ƵůůŝǀĂŶ^͕&ŽƵƚƐ͕,ĂĨƚ,͕^ĞůĞŶŐƵƚ:͕WĞƚĞƌƐŽŶ:͕ĂǀŝĚƐĞŶdD͕ĂĨĂƌE͕ŚŽƵ>͕ZĂĚƵŶĞ
͕ŝŵŝƚƌŽǀ'͕,ĂŶĐĞD͕dƌĂŶ<͕<ŚŽƵƌŝ,͕'ŝůů:͕hƚƚĞƌďĂĐŬdZ͕&ĞůĚďůLJƵŵds͕tĂůů:͕
sŽŽƌĚŽƵǁ'͕&ƌĂƐĞƌD͘ϮϬϬϰ͘dŚĞŐĞŶŽŵĞƐĞƋƵĞŶĐĞŽĨƚŚĞĂŶĂĞƌŽďŝĐ͕ƐƵůĨĂƚĞͲƌĞĚƵĐŝŶŐ
ďĂĐƚĞƌŝƵŵĞƐƵůĨŽǀŝďƌŝŽǀƵůŐĂƌŝƐ,ŝůĚĞŶďŽƌŽƵŐŚ͘EĂƚƵƌĞďŝŽƚĞĐŚŶŽůŽŐLJϮϮ͗ϱϱϰͲϱϱϵ͘
dƵŽŵŝ:D͕sŽŽƌďƌĂĂŬ&͕:ŽŶĞƐ>͕ZƵŝũƚĞƌ:D͘ϮϬϭϬ͘ŝĂƐŝŶƚŚĞƋǀĂůƵĞŽďƐĞƌǀĞĚǁŝƚŚ
ŚLJĚƌŽůLJƐŝƐƉƌŽďĞďĂƐĞĚƋƵĂŶƚŝƚĂƚŝǀĞWZĐĂŶďĞĐŽƌƌĞĐƚĞĚǁŝƚŚƚŚĞĞƐƚŝŵĂƚĞĚWZĞĨĨŝĐŝĞŶĐLJ
ǀĂůƵĞ͘DĞƚŚŽĚƐϱϬ͗ϯϭϯͲϯϮϮ͘
<ĂƌůĞŶz͕DĐEĂŝƌ͕WĞƌƐĞŐƵĞƌƐ^͕DĂnjnjĂ͕DĞƌŵŽĚE͘ϮϬϬϳ͘^ƚĂƚŝƐƚŝĐĂůƐŝŐŶŝĨŝĐĂŶĐĞŽĨ
ƋƵĂŶƚŝƚĂƚŝǀĞWZ͘DďŝŽŝŶĨŽƌŵĂƚŝĐƐϴ͗ϭϯϭ͘
Connection 2: Experimental evolution with DvH to evolution with DBac.
In the previous section (Paper 2) we assessed the evolutionary response of DvH to 927
generations of evolutionary adaptation in batch culture. We documented the trajectory
34
İ that
occurred with the evolutionary adaptation that occurred throughout the experiment. We found
that evolutionary adaptation appears to recapitulate physiological effects of
34
İ. However, the
previous study left us with many unanswered questions because it was performed at relatively
high growth rates where the sensitivity of
34
İ might be less than at lower growth rates. In the
next section (Paper 3), we investigated whether this response is similar at lower growth rates in a
different species of sulfate reducing bacteria: Desulfomicrobium baculatum which has a slower
growth rate as well as a different starting isotope phenotype under the exact same environmental
conditions which were utilized with DvH.
Paper 3 : Evolutionary response of S
isotope fractionation is predicted by
phenotypic plasticity
Evolutionary response of S isotope fractionation is predicted by
phenotypic plasticity
Andre Pellerin, Luke Anderson-Trocme and Boswell Wing
[1] Department of Earth and Planetary Sciences and GEOTOP, McGill University, 3450
University Street, Montréal, Canada, H3A 2A7
Correspondance to: André Pellerin (andrepellerin@gmail.com)
ABSTRACT
Establishing the relationship between evolutionary adaptation and isotope phenotype is
critical to unlocking the isotopic record of dissimilatory sulfate reduction that is preserved in
modern and ancient sediments. To address this issue, pure cultures of Desulfomicrobium
baculatum were evolved in batch culture for 300 generations. A greater than twofold increase in
growth rate over the course of the experiment was measured as well as a change in isotope
phenotype (34İ) from approximately 15 to 12 ‰. The response of 34İ to evolutionary adaptation
resembles the isotopic response of physiological adaptations to changing environmental
conditions. This similitude suggests that the evolutionary adaptation which occurred during the
experiment was not functional but rather regulatory. These results show that variations in S
isotope fractionation during dissimilatory sulfate reduction do not require environmental change.
The same effect can be obtained through evolutionary adaptation of the sulfate reducing
microorganism.
1. Introduction
The relationship between evolutionary adaptation of sulfate reducing microorganism and the
sulfur isotope phenotype has only recently become apparent and remains largely unanswered.
Even a first-order understanding of the relationship can potentially provide clues to the evolution
of one of Earth’s earliest metabolism for a span of nearly four billion years (Shen et al., 2001).
Early work on dissimilatory sulfate reduction (DSR) in pure cultures of sulfate reducing
microorganisms has lead to a comprehensive understanding of the fundamental control that the
environment (Canfield et al., 2006; Habicht et al., 2002; Hoek et al., 2006; Kaplan and
Rittenberg, 1964) and cellular physiology (Johnston et al., 2007; Leavitt et al., 2013; Sim et al.,
2011a; Sim et al., 2012; Sim et al., 2011b) exert on the resulting sulfur isotope fractionation.
However, the genetic makeup of sulfate reducers may be an important but underexplored control
on S isotope fractionation (Bolliger et al., 2001; Bruchert et al., 2001; Canfield, 2001a, b;
Detmers et al., 2001; Kaplan and Rittenberg, 1964; Kleikemper et al., 2004). Work investigating
the isotope phenotype response to evolutionary adaptation during a 927 generation evolution
experiment led to the suggestion that the sulfate reducing bacterium Desulfovibrio vulgaris
Hildenborough (DvH) might respond in a manner reminiscent of physiological adaptation
(Pellerin et al. accepted to AEM (Paper 2)). Under the high initial growth conditions of the
experiment, the low sensitivity of the isotope phenotype may have rendered the isotopic effects
of evolutionary adaptation more challenging to observe. In the present study, we report the
results of a 300 generation evolution experiment with Desulfomicrobium baculatum, a sulfate
reducing microbe whose ancestor has a much slower initial growth rate. The reasoning behind
starting evolution experiment at slower initial growth rates is that at low metabolic rates, the
sensitivity of the isotope phenotype is known to be relatively large in comparison to at higher
metabolic rates (Kaplan and Rittenberg, 1964; Leavitt et al., 2013; Sim et al., 2011a; Sim et al.,
2011b). Changes in isotope phenotype may therefore be more sensitive to evolutionary
adaptation when a population starts at low rates.
In simple evolution experiments with unlimited growth resources, adaptive success mostly
means increasing growth rate. In these conditions, we therefore expect that evolutionary
adaptation should predictably affect fractionation, through rate. Three variables may affect
growth rate; yield, csSRR and maintenance metabolism. This relationship is summarized in
(Hoehler and Jorgensen, 2013)
݇ = ܻ (ܿ ܴܴܵݏെ ݉)
where k is the growth rate, Y is the cellular yield, m is the maintenance metabolism and
csSRR is the cell specific sulfate reduction rate (respiration rate). All three parameters may be
optimized to increase growth rate and lead to evolutionary success but only one, cssRR has a pre
established relationship with 34İHJ(Harrison and Thode, 1958; Kaplan and Rittenberg, 1964;
Leavitt et al., 2013)). Should evolutionary adaptation and isotope phenotype be related via
optimization of csSRR, a smaller 34İwould be expected in identical culture conditions.
2. Methods
Much of the methodology used in these experiments is described in detail in (Pellerin et al.
accepted to AEM (Paper 2)). Here we describe the general methodology and highlight
differences from our earlier work. For reference, we reproduce the detailed methods of (Pellerin
et al. accepted to AEM (Paper 2)) in the appendix.
2.1 Choice of model organism
The sulfate reducing bacterium, Desulfomicrobium baculatum (Dbac) was selected as model
organism for this study because of its low growth rate in the growth medium MOLS4. Another
advantage of utilizing Dbac was its fully sequenced genome (Copeland et al., 2009) which can
simplify the analysis of genetic data, if necessary. The stock of this culture was purchased from
the Leibniz institute DSMZ but the evolution experiment was the product of a single colony.
2.2 Design of microbial evolution experiment
Rather than investigating selective responses to novel environmental, physiological, or
genetic stressors, the evolution experiments were designed to select for increased growth rate in
a simple and reproducible manner.
Three replicate lines of Dbac were propagated in a chemically defined growth media in batch
culture for 300 generations. Each batch cycle corresponded to roughly 4.1 binary fissions which
we equate to generations (Appendix A). In typical evolution experiment this batch culture cycle
is often a daily occurrence. However, the initial growth rates of Dbac were slow. The time for the
culture to reach stationary phase varied from 5 days at the beginning of the evolution experiment
to under 24 hours as the experiment progressed. The transfers were therefore performed when
the culture reached an optical density greater than 0.6 which indicated the culture had entered
stationary phase.
The genetic composition of the evolved microbial community was assessed in order to
ascertain that the cultivated lineages were composed of Dbac. We extracted DNA from the
growing evolved cultures and amplified a portion of the 16S rRNA gene. A more detailed
procedure is available in Appendix A. The 625bp overlap in the sequences obtained from all four
lineages (the ancestor and the three evolved lineages) had 100% sequence similarity. This
sequence was identical to the 16S rRNA sequence reported by (Copeland et al., 2009). We
interpret this to show that exogenous strains with higher fitness did not take over any of the
populations of the evolution experiment.
2.3 Measurement of exponential growth characteristics
Specific growth rates (k day-1) of exponentially growing cells were measured as well as yield
(Y, in 106 cells per µmol SO 4 -2 consumed) and cell specific sulfate reduction rate (csSRR, in
femtomoles SO 4 -2 consumed cell-1 day-1) as outlined in (Pellerin et al. accepted to AEM (Paper
2)), (Appendix A). One remarkable operational difference was that this data was gathered from
longer lengths of growth for the ancestor than for the evolved lineages. In addition, real time
monitoring of the growth throughout the experiment was monitored with a homemade logger of
optical density (Appendix A).
2.4 Characterization of the sulfur isotope phenotype
We measured the S isotope phenotypes of the ancestral Dbac wild-type and of all three
lineages in triplicate over the same interval as the exponential growth characteristics (300
generations) following the methodology outlined in (Pellerin et al. accepted to AEM (Paper 2)),
(Appendix A). Again for the sulfur isotope phenotype, one difference in methodology for this
study was that in some instances the slow growth rate of Dbac caused the experiment to run for
periods of a few days before enough sulfide was accumulated for measurement.
To characterize the isotope phenotype we use the definitions of
(accepted to AEM) (Paper 2).In the text and in the figures, the
thousand (‰). İ and 33ORf Pellerin et al.
34
İ is expressed as parts per
34
3. Results & discussion
3.1 Growth rate differences between evolved and ancestors are the product of
evolutionary adaptation
The mean growth rate (day-1) (Figure 1) that was measured from replicate growth experiments
of the ancestral line was of 0.99 ± 0.16, which corresponds to 1.4 generations per day. The mean
growth rate of individual lineages at generation 300 was of 2.85 ± 0.23, 2.69 ± 0.05 and 2.91 ±
0.05 for lines A, B and C respectively which amounts to a mean growth rate of 2.82 ± 0.16 or 4.0
generations per day (Table 1). There were no significant differences in mean growth rates
between the three evolved lines at the 95% confidence interval (p=0.21) but the difference in
growth rate between the ancestor and evolved lines is visually (Figure 1) and statistically
unquestionable (p<<0.0001). Real-time logging of the growth of ancestor and evolved lineages
show that changes in maximum growth rate during exponential growth phase are immense and
likely the predominant parameter which appears to determine the overall fitness of individuals in
the experimental conditions provided (Appendix B-1). Beneficial adaptations such as hoarding,
which have provided fitness advantages in other evolution experiments in the past (Lenski and
Travisano, 1994) would be dwarfed in comparison. There were no observed reductions in the lag
phase. The potential fitness advantage of increasing growth rate during the evolution experiment
may simply have been the dominant strategy because it conferred overwhelming advantages.
We equate the relative growth rates obtained in this study directly to fitness in the limited context
of our experiment while keeping in mind that other growth parameters may have a small impact
on fitness. A similar conclusion, that relative growth rate and fitness are very similar features,
was reached by comparing fitness and growth rate with DvH in identical environmental and
selective conditions although the gains in growth rate/fitness were smaller (Pellerin et al.
accepted to AEM (Paper 2)). Increases on the order that was observed in the experimental
evolution with Dbac were observed with DvH in co-culture with Methanococcus Maripaludis
over a 300 generation evolution experiment (Hillesland and Stahl, 2010).
Yields remained relatively constant (Figure 1) with no significant differences between
ancestor and evolved lines (p=0.22). The ancestor had a mean yield (expressed in 106 cells
µmole SO 4 2- -1) of 47.78 ± 5.57 whereas the mean yield of the evolved lineages were of 55.25±
4.91, 49.46± 4.74, 50.75± 4.20 respectively for a combined average of 52.118 ± 4.708 (Table 1).
Overall the yield results suggest that the strong growth rate increases observed during this
experiment are not a result of significant changes in yield.
csSRR (femto mole cell-1 day-1) (Figure 1) changed significantly (p<<0.0001) between the
evolved and ancestor but similarly to growth rate, no significant differences were observed
between the evolved lines (p=0.24). Mean csSRR of the ancestor was of 20.61 ± 1.47 whereas
for the evolved lineages A, B and C it was of 51.71 ± 1.55, 54.87 ± 3.92 and 57.68 ± 5.08
respectively for a combined average between all three lineages of 54.73 ± 4.22 (Table 1).
The difference in mean csSRR between evolved and ancestor is close to the same relative
change in growth rate. A close inspection of growth rate and csSRR, reveals a slightly larger
mean increase in growth rate than in csSRR. The mean growth rate between the ancestor and
evolved lines corresponds to a 2.85 ± 0.12 fold increase. The mean increase in csSRR appears to
be slightly lower at 2.65 ± 0.15 fold. The difference between the mean change in growth rate and
csSRR is not significant at the 95% confidence interval (p=0.136). Had the fold changes in
growth rate and csSRR between ancestors and evolved been significant, we might have
suspected that fitness was not only caused by an increase in csSRR but also by another parameter
affecting growth. However, this was not the case and the results support the hypothesis that in
the environmental conditions provided, increasing growth rate is driven by increasing csSRR.
In the following paragraphs we ask whether this evolutionary-driven change in csSRR
translates to a change in isotopic phenotype and we investigate the mechanism connecting the
genotype to the isotope phenotype if any.
3.2 The evolutionary response of 34İLVmeasurable
The growth rate and csSRR differences described above between ancestor and evolved
lineages are correlated with a decrease in isotope phenotype. Ancestor Dbac measurements
produced a mean 34İRI15.42±0.67 ‰ (mean 33ȜRI0.5096 ±0.0008) (Figure 3). In contrast, the
evolved lineages had mean
İ of 12.54 ±0.50, 11.96±0.17, 12.55±0.23‰ (mean
34
Ȝ RI
33
0.5112±0.0012, 0.5097±0.0017 0.5010±0.0017) respectively (Figure 3). Each individual isotope
assay had an uncertainty on 34İ between 0.13 and 0.14 based on reasonable assumptions of the
combined uncertainty associated with mass spectrometry, sulfate/sulfide chemical extractions
and media preparations and preservation. The uncertainty between replicate lines was greater
than the estimates of uncertainty on each replicate line (Table 2). This means that the
reproducibility of microbial growth was a major source of variability in
İbetween replicates
34
except in the case of the lineage “B” where the replicate uncertainty was close to the individual
uncertainty. Significant differences between the ancestor and each evolved lines are evident
visually (Figure 2), statistically at the 95% confidence interval (p=0.001, 0.018 and 0.028
respectively) and suggest that the evolved
34
İ LV VLJQLILFDQWO\ GLIIHUHQW WR WKH DQFHVWRU¶V in all
cases. By comparison, the 33ȜRIWKHHYROYHGOLQHVZHUHLQQRFDVHs significantly different to that
of the ancestor at the 95% confidence interval (p= 0.089,0.991 and 0.235 respectively). This
result was expected given that the experiment only covered a small range of possible
relationship which typically characterizes
İ and
34
34
İ. The
Ȝ LV SRVLWLYH YHU\ VKDOORZ DQG DOVR KDV D
33
large degree of variability. The values obtained in this study fit in the typical 34İ-33Ȝrelationship
“field” normally associated with pure cultures of sulfate reducing microorganisms (Johnston et
al., 2005; Pellerin et al., 2014; Sim et al., 2011a; Sim et al., 2011b).
In contrast to the results of
İ between ancestor and evolved, there are no significant
34
İ
34
differences between the three evolved lines at the 95% confidence interval (p=0.11). This
suggests that the
İ expression of evolutionary adaptation might be a relatively stable
34
characteristic in set environmental conditions. While this study lacks evidence establishing
whether the genotypes of the replicate lines evolved in parallel, the low diversity of
İ in the
34
evolved populations are in line with the phenotypic parallelism observed in E. Coli populations
when microbial populations are large and lineages are grown in identical conditions (Lenski and
Travisano, 1994). They also support the suggestion that adaptation is predictable despite the fact
that evolution can be a highly stochastic process at the genotype level (Kryazhimskiy et al.,
2014).
While the evolution experiment of this study does show a measurable and statistically
significant decrease in 34İ, one may ask if the evolutionary signal measured in this study is large
enough to matter in the “real world” where the potential fractionation is up to 66‰ (Sim et al.,
2011a) and natural populations typically span roughly 15 to 45 ‰ (Habicht and Canfield, 1997).
The evolutionary driven change in
34
İ that is documented in this study occurs effectively
instantaneously when considering the age of the DSR metabolism. While the authors would
strongly advise against taking the rate of
İ change observed in this study and applying it
34
liberally to longer evolutionary periods, it should be evident that on geological timescales, the
potential for evolutionary adaptation is much greater. Indeed, a quick comparison of the results
of this study and the study of (Pellerin et al. accepted to AEM, (Paper 2)) shows a difference in
İRI§ÅEHWZHHQ Dbac and DvH. Although it is impossible to speculate on the ancestry of
34
DvH and Dbac, they are ultimately related and at some point their common ancestor displayed
only one isotope phenotype in conditions where both now have a distinct one. This simple
comparison highlights the fact that larger effects on
İ are attainable and that evolutionary
34
adaptation is not of negligible contribution on a grander scale than this study. What remains to be
determined is the evolutionary mechanism behind changes in isotope phenotype .
3.3 The evolutionary response of
34
İ UHVHPEOHV WKH SK\VLRORJLFDO UHVSRQVH EHFDXVH WKH
phenotypic expression of evolutionary adaptation is an extension of physiological
adaptation
The previous paragraphs offer evidence for an important role played by evolutionary
adaptation on the resulting 34İ. Though evolution is incontestably the driver of the change in 34İ
observed in this study, the mechanistic processes which cause this change are not exclusive (to
evolutionary adaptation). A deeper analysis of the mechanisms responsible for the change is
necessary if we are to understand the role played by evolutionary adaptation in the continuum of
biological processes which affect
İ. For one thing, the phenotypic changes associated with
34
evolutionary adaptations on generational timescales are largely an extension of physiological
adaptations which take place on an organismal timescale and should be correlated (Milo et al.,
2007). Physiological adaptation affects the phenotype by modifying protein expression levels
while evolutionary adaptation affects the phenotype by modifying genotype. Indeed, in the case
of physiological adaptation, when a microorganism is subject to increasing levels of growth
substrate, cellular enzyme levels will typically scale linearly with abundance of substrate after a
minimum threshold is reached and up to a certain physiological limit (Segel, 1975). The high
concentration of growth substrate of the evolution experiment is a situation where the
physiological limit that enables the maximum possible growth rate may be reached. It is after this
physiological limit is reached that a significant fitness advantage exists for individuals capable of
utilizing substrate at a higher rate and where evolutionary processes become important. Two
types of genetic changes control phenotypic functional innovation; those affecting protein
structure and those affecting protein expression (Blank et al., 2014). Assuming, perhaps
reasonably, a certain level of preconditioned optimality in protein structure, genetic changes
affecting protein expression will have larger fitness advantage and have a better chance of
getting fixed. Empirical evidence suggests that protein expression variations are indeed first
order evolutionary responses; In large microbial populations, a greater fraction of regulatory
mutations are fixed (Blank et al., 2014) suggesting expression plays a dominant role in fitness.
Microbial experiments where the evolutionary strategy is to maximize growth rate, find that most
fitness gains are caused not by functional innovation but rather by the loss of unused catabolic
functions (Sniegowski et al., 1997). In the simplest of cases, one may be led to believe that
simply increasing the limiting enzyme concentration by a process such as gene duplication may
confer a significant fitness advantage (Kondrashov et al., 2002). Fitness changes via protein
expression levels preserve the fundamental structure of the metabolism because the core
enzymes are not structurally changing. This is not to say that structural mutations are irrelevant.
On long evolutionary timescales or when a population adapts to drastically different growth
conditions, structural mutations undoubtedly play a fundamental role on fitness. However, since
this study considers only the early stages of evolutionary adaptation in conditions that select for
increasing growth rate, the experimental situation is likely analogous to the physiological
adaptation to increasing substrate levels. The evolutionary results on 34İof this study therefore
could be explained by the same common metabolic mechanism as the one involving
physiological adaptation of
34
İ – simply increasing enzyme concentrations. Therefore, similar
relationships between csSRR and 34İ would be expected.
While the deduction postulated above appears logical and straightforward, just obtaining
empirical evidence of a similarity between the evolutionary and physiological responses on
İ
34
may pose a challenge. The physiological relationship which exists between csSRR and İ is in
34
practical terms limited by the resolution of available datasets. With physiological response
studies, increases in csSRR and the resulting decrease in
İ is evident when large changes (in
34
csSRR) are considered (Appendix B-2). However, this relationship is much less evident within
the range of csSRR (18-62 fmol/cell/day) obtained in our evolution experiment; no significant
trajectory in
İis observable (Appendix B-3). High precision measurement of csSRR and
34
İ
34
might be an easy feat in batch culture for evolution experiments but may be more difficult for
physiological studies which need to control the supply of growth substrate to obtain a given
İ. Therefore, while the general tendency of physiological adaptation
34
csSRR and its resulting
and evolutionary adaptation both decrease
34
İ by increasing csSRR, their analogous behaviour
remains to be empirically confirmed within the same range.
3.4 Sensitivity of 34İto evolutionary adaptation and preservation of evolutionary history
One important growth component which has remained in excess for evolutionary adaptation
throughout this study but which has liberally varied for physiological adaptation is the
availability of growth substrate. This section considers how changing growth conditions may
affect the response of
İ of evolved populations relative to the response of an ancestor. The
34
sensitivity of evolved populations to different growth conditions may suggest whether 34İis truly
sensitive to evolutionary adaptation in an environmental context.
Growth rate and csSRR are often limited by the availability of organic growth substrate
(Hoehler and Jorgensen, 2013) and not, as in the case of this study, the genetic inability to grow
faster. If the behaviour of
İ from evolved population to substrate-limited environmental
34
conditions does not differ from that of the ancestor, then the isotope phenotype displayed in the
environment has a low sensitivity to evolutionary adaptation. This would be explained by the
levels of metabolic activity remaining in check by environmental constraints. In turn this means
that genetic modifications which result from short term evolutionary adaptation to different
conditions does not affect the metabolism in a manner which can be expressed through
34
İ.
However, the opposite may also be true. The evolutionary adaptation may have tweaked the
metabolism for specific environmental conditions which result in a measurable effect on
İ
34
under substrate limited conditions. This would suggest that 34İis more sensitive to evolutionary
adaptation.
The first case would suggest that the isotope phenotype is a record of longer term divergence
processes than the second instance where the divergence of the isotope phenotype would be a
record of short term evolution. An empirical investigation into this matter would add powerful
interpretive potential to evolution experiments because it would offer a means of applying
experimental results to environmental observations.
4. Conclusion
Three hundred generations of unconstrained growth produces observable growth changes in
an initially isogenic Dbac culture. These changes, in turn result in a significant decrease in 34İ.
The results of this study constitute the first empirical evidence demonstrating such a relationship
between evolutionary adaptation and the S isotope fractionation. This evolutionary response on
İ appears to behave similarly to the physiological response of
34
34
İ to an increasing supply of
growth substrate. This is because the mechanisms involved in increasing growth rate in the
evolution experiment and the ones which increase growth rate in physiological studies are likely
correlated. The most important implication of these results is that variations in S isotope
fractionation do not necessarily require a change in environmental conditions. One of the
remaining challenges is to understand the how sensitivity of
İ to evolutionary adaptation in
34
evolution experiments may provide a mean to assess the importance of evolutionary processes on
the variability seen in environmental observations.
Figure Captions
Figure 1: Growth characteristics monitored as a function of progression throughout the
experiment. Data is jittered along the x axis for visualization purposes. Errobars indicate the 1 ı
uncertainty on measurements which is propagated from measurements of optical density and
[H 2 S]. Symbols are assigned to the individual lineages while colors are meant to allow for better
replicate visualization. Left: Growth rate. Middle: cell specific sulfate reduction rate (csSRR).
Right: Yield.
Figure 2: Measurements of
İ (y-axis) suggest that the isotope phenotype of the ancestor
34
(squares) and the evolved lineages (circles) are different and negatively correlated with csSRR
(x-axis). Colored bars are meant to allow for better replicate visualization and correspond to the
1ı uncertainty on the measurement propagated from the primary cell and H 2 S measurements
whereas on x-axis while the y axis colored lines report the estimate of uncertainty on
34
İbased
on a Monte Carlo simulation (Appendix A).
Figure 3: The relationship between
linear. Green points show the larger
İ DQG Ȝ WKURXJKRXW WKH evolution experiment is mostly
34
34
İ of the ancestor compared with the red, turquoise and
SXUSOHRIWKHHYROYHGOLQHDJHVDWJHQHUDWLRQ;DQG<D[LVFRORUHGOLQHVFRUUHVSRQGWRWKHı
uncertainty on measurement based on a Monte Carlo simulation (Appendix A).
Table 1: Growth characteristics of ancestor and evolved lineages of Dbac. Normal fonts are
individual measurements, bold fonts are the mean of lineages, italicized are the estimates of
uncertainty on individual measurements while bold italicized are standard deviation on the mean
of lineages.
Table 2: Compilation of the measurements of 34İ and 33ȜIURPDQFHVWRUDQGHYROYHGOLQHDJHVRI
Dbac. Normal fonts are individual measurements, bold fonts are the mean of lineages, italicized
are the estimates of uncertainty on individual measurements while bold italicized are standard
deviation on the mean of lineages.
References
Blank, D., Wolf, L., Ackermann, M., Silander, O.K., 2014. The predictability of molecular
evolution during functional innovation. Proceedings of the National Academy of Sciences.
Bolliger, C., Schroth, M.H., Bernasconi, S.M., Kleikemper, J., Zeyer, J., 2001. Sulfur isotope
fractionation during microbial sulfate reduction by toluene-degrading bacteria. Geochimica et
Cosmochimica Acta 65, 3289-3298.
Bruchert, V., Knoblauch, C., Jorgensen, B.B., 2001. Controls on stable sulfur isotope
fractionation during bacterial sulfate reduction in Arctic sediments. Geochimica Et
Cosmochimica Acta 65, 763-776.
Canfield, D.E., 2001a. Biogeochemistry of Sulfur Isotopes. Reviews in Mineralogy and
Geochemistry 43, 607-636.
Canfield, D.E., 2001b. Isotope fractionation by natural populations of sulfate-reducing bacteria.
Geochimica et Cosmochimica Acta 65, 1117-1124.
Canfield, D.E., Olesen, C.A., Cox, R.P., 2006. Temperature and its control of isotope
fractionation by a sulfate-reducing bacterium. Geochimica et Cosmochimica Acta 70, 548-561.
Copeland, A., Spring, S., Göker, M., Schneider, S., Lapidus, A., Glavina Del Rio, T., Tice, H.,
Cheng, J.-F., Lucas, S., Chen, F., Nolan, M., Bruce, D., Goodwin, L., Pitluck, S., Ivanova, N.,
Mavromatis, K., Ovchinnikova, G., Pati, A., Chen, A., Palaniappan, K., Land, M., Hauser, L.,
Chang, Y.-J., Jeffries, C.D., Meincke, L., Sims, D., Brettin, T., Detter, J.C., Han, C., Chain, P.,
Bristow, J., Eisen, J.A., Markowitz, V., Hugenholtz, P., Klenk, H.-P., Kyrpides, N.C., 2009.
Complete genome sequence of Desulfomicrobium baculatum type strain (X T ).
Detmers, J., Brüchert, V., Habicht, K.S., Kuever, J., 2001. Diversity of sulfur isotope
fractionations by sulfate-reducing prokaryotes. Applied and Environmental Microbiology 67,
888-894.
Habicht, K.S., Canfield, D.E., 1997. Sulfur isotope fractionation during bacterial sulfate
reduction in organic-rich sediments. Geochimica et Cosmochimica Acta 61, 5351-5361.
Habicht, K.S., Gade, M., Thamdrup, B., Berg, P., Canfield, D.E., 2002. Calibration of Sulfate
Levels in the Archean Ocean. Science 298, 2372-2374.
Harrison, A.G., Thode, H.G., 1958. Mechanism of the bacterial reduction of sulphate from
isotope fractionation studies. Transactions of the Faraday Society 54, 84-92.
Hillesland, K.L., Stahl, D.A., 2010. Rapid evolution of stability and productivity at the origin of
a microbial mutualism. Proceedings of the National Academy of Sciences, -.
Hoehler, T.M., Jorgensen, B.B., 2013. Microbial life under extreme energy limitation. Nature
reviews. Microbiology 11, 83-94.
Hoek, J., Reysenbach, A.-L., Habicht, K.S., Canfield, D.E., 2006. Effect of hydrogen limitation
and temperature on the fractionation of sulfur isotopes by a deep-sea hydrothermal vent sulfatereducing bacterium. Geochimica et Cosmochimica Acta 70, 5831-5841.
Johnston, D.T., Farquhar, J., Canfield, D.E., 2007. Sulfur isotope insights into microbial sulfate
reduction: When microbes meet models. Geochimica Et Cosmochimica Acta 71, 3929-3947.
Johnston, D.T., Farquhar, J., Wing, B.A., Kaufman, A.J., Canfield, D.E., Habicht, K.S., 2005.
Multiple sulfur isotope fractionations in biological systems: A case study with sulfate reducers
and sulfur disproportionators. Am J Sci 305, 645-660.
Kaplan, I.R., Rittenberg, S.C., 1964. Microbiological fractionation of sulphur isotopes. J. Gen.
Microbiol. 34, 195-212.
Kleikemper, J., Schroth, M.H., Bernasconi, S.M., Brunner, B., Zeyer, J., 2004. Sulfur isotope
fractionation during growth of sulfate-reducing bacteria on various carbon sources. Geochimica
et Cosmochimica Acta 68, 4891-4904.
Kondrashov, F.A., Rogozin, I.B., Wolf, Y.I., Koonin, E.V., 2002. Selection in the evolution of
gene duplications. Genome Biol 3, RESEARCH0008.
Kryazhimskiy, S., Rice, D.P., Jerison, E.R., Desai, M.M., 2014. Global epistasis makes
adaptation predictable despite sequence-level stochasticity. Science 344, 1519-1522.
Leavitt, W.D., Halevy, I., Bradley, A.S., Johnston, D.T., 2013. Influence of sulfate reduction
rates on the Phanerozoic sulfur isotope record. Proceedings of the National Academy of Sciences
110, 11244-11249.
Lenski, R.E., Travisano, M., 1994. Dynamics of adaptation and diversification: a 10,000generation experiment with bacterial populations. Proc Natl Acad Sci U S A 91, 6808-6814.
Milo, R., Hou, J.H., Springer, M., Brenner, M.P., Kirschner, M.W., 2007. The relationship
between evolutionary and physiological variation in hemoglobin. Proceedings of the National
Academy of Sciences 104, 16998-17003.
Pellerin, A., Wing, B.A., Rough, M., Mucci, A., Canfield, D.E., Bui, T.H., 2014. Massdependent sulfur isotope fractionation during reoxidative sulfur cycling: A case study from
Mangrove Lake, Bermuda. Geochimica et Cosmochimica Acta (submitted, GCA-D-14-00267).
Segel, I.H., 1975. Enzyme kinetics. Wiley, New York.
Shen, Y., Buick, R., Canfield, D.E., 2001. Isotopic evidence for microbial sulphate reduction in
the early Archaean era. Nature 410, 77-81.
Sim, M.S., Bosak, T., Ono, S., 2011a. Large Sulfur Isotope Fractionation Does Not Require
Disproportionation. Science 333, 74-77.
Sim, M.S., Ono, S., Bosak, T., 2012. Effects of iron and nitrogen limitation on sulfur isotope
fractionation during microbial sulfate reduction. Appl Environ Microbiol 78, 8368-8376.
Sim, M.S., Ono, S., Donovan, K., Templer, S.P., Bosak, T., 2011b. Effect of electron donors on
the fractionation of sulfur isotopes by a marine Desulfovibrio sp. Geochimica et Cosmochimica
Acta 75, 4244-4259.
Sniegowski, P.D., Gerrish, P.J., Lenski, R.E., 1997. Evolution of high mutation rates in
experimental populations of E. coli. Nature 387, 703-705.
Growth rate (dayí)
1.0
1.5
2.0
2.5
3.0
3.5
Figure 1
0
Generations
300
20
30
40
50
60
70
0
Generations
300
Yield (106 cells/umole SO4)
csSRR (fmol/cell/day)
40
45
50
55
60
0
Generations
300
C
B
A
Evolved
Ancestor
Figure 2
16
Ancestor
Evolved
14
A
34
ε (‰)
15
B
13
C
12
20
30
40
50
csSRR (fmole/cell/day)
60
70
Figure 3
0.513
33
λ (‰)
0.512
Ancestor
0.511
Evolved
A
0.510
B
C
0.509
0.508
12
13
34
14
ε (‰)
15
16
TABLE 1: Growth characteristics of ancestor and evolved lineages of Dbac. Normal fonts are
individual measurements, bold fonts are the mean of lineages, italicized are the estimates of
uncertainty on individual measurements while bold italicized are standard deviation on the mean
of lineages.
generation
0
300
Rep1
Rep2
Rep3
mean, sd
A-Rep1
A-Rep2
A-Rep3
mean, sd
B-Rep1
B-Rep2
B-Rep3
mean, sd
C-Rep1
C-Rep2
C-Rep3
mean, sd
Y
53.80
46.74
42.80
47.78
59.91
55.73
50.12
55.25
52.82
46.12
ı
4.17
4.67
3.34
5.58
3.10
2.59
3.11
4.91
3.53
2.96
49.47
49.12
55.53
47.61
50.75
4.74
2.42
2.70
1.94
4.20
k
1.17
0.89
0.90
0.99
3.01
2.97
2.59
2.86
2.75
2.66
2.67
2.69
2.96
2.88
2.90
2.91
ı
0.18
0.15
0.14
0.16
0.40
0.38
0.37
0.23
0.40
0.39
0.39
0.05
0.37
0.36
0.35
0.05
csSRR
21.77
18.96
21.12
20.62
50.20
53.30
51.63
51.71
52.10
57.64
ı
3.79
3.80
3.73
1.47
7.12
7.27
8.00
1.55
8.36
9.14
54.87
60.35
51.82
60.87
57.68
3.92
8.19
6.95
7.67
5.08
TABLE 2: Compilation of the measurements of 34İ and Ȝ from ancestor and evolved lineages of
Dbac. Normal fonts are individual measurements, bold fonts are the mean of lineages, italicized
are the estimates of uncertainty on individual measurements while bold italicized are standard
deviation on the mean of lineages.
generation Lineage- rep#
Rep1
Rep2
0
Rep3
mean, sd
A-Rep1
A-Rep2
A-Rep3
mean, sd
B-Rep1
B-Rep2
300
B-Rep3
mean, sd
C-Rep1
C-Rep2
C-Rep3
mean, sd
İ
15.27
16.16
14.85
15.43
12.44
13.08
12.08
12.53
11.98
11.78
12.11
11.96
12.43
12.40
12.82
12.55
34
ı34İ
0.13
0.14
0.13
0.67
0.13
0.14
0.13
0.50
0.13
0.14
0.14
0.17
0.14
0.13
0.14
0.23
Ȝ
0.5112
0.5123
0.5107
0.5114
0.5115
0.5132
0.5109
0.5119
0.5115
0.5085
0.5116
0.5105
0.5076
0.5111
0.5095
0.5094
33
ı33Ȝ
0.0010
0.0010
0.0010
0.0008
0.0010
0.0010
0.0010
0.0012
0.0010
0.0010
0.0010
0.0017
0.0010
0.0010
0.0010
0.0017
Paper 3 Evolutionary response of S isotope
fractionation is predicted by phenotypic
plasticity
Appendix
Appendix A – Additional methods
A-1 Growth media
The experiments were performed in a Tris-buffered chemically defined media (MOLS4) that
consists of a final concentration of 30mM sulfate, 60mM lactate, 8mM MgCl2, 20mM NH4Cl,
2mM K2HPO4-NaH2PO4, 30mM Tris-HCl as well as solutions of trace elements, Thauer
vitamins and trace rezasurin as an oxygen indicator. The pH was adjusted to 7.2. For the
evolution experiments, 10mL of MOLS4 was placed into 20mL serum bottles, while 80mL of
MOLS4 was placed into120mL serum bottles for the isotope assays. Bottles were crimp sealed
with butyl rubber stoppers from which the headspace was purged of oxygen by flushing with
pure N2 gas. After gassing, individual crimp-sealed media bottles were sterilized in an autoclave.
A - 2 Design of microbial evolution experiment
Rather than investigating selective responses to novel environmental, physiological, or genetic
stressors, the evolution experiments were designed to select for increased growth rate in a simple
and reproducible manner.
Three replicate lines of Dbac were propagated in a chemically defined growth media. Each of the
replicate lines was propagated in batch culture by inoculating 0.6mL of the previous culture into
a fresh media bottle containing 10mL of defined MOLS4 media. Cultures were grown in 20mL
serum bottles capped with blue butyl rubber stoppers.
The headspace was 100% N2 gas
(99.995% purity). Cultures were incubated at 33°C and shaken at 110 rpm. Approximately every
10th transfer, the three replicate lines were preserved in a glycerol stock solution at -80°C to
preserve a “fossil” record of the evolution experiment. These frozen stocks were revived at later
times for isotopic and growth rate and fitness measurements. All inoculations, sampling, and
transfers were performed under strictly anaerobic conditions.
The evolution experiment was performed in batch culture for 300 generations. Each batch cycle
corresponded to roughly 4.1 binary fissions which we equate to generations. In typical evolution
experiment this batch culture cycle is often a daily occurrence. However, the initial growth rates
of Dbac were slow. The time for the culture to reach stationary phase varied from 5 days to
under 24 hours. The transfers were therefore performed when the culture reached an optical
density greater than 0.6 which indicated the culture had entered stationary phase. This happened
slowly at first and more quickly as the experiment progressed.
A-3 - 16S rRNA contamination check
DNA was extracted from the ancestral strain and evolved lineage strains of Dbac. In order to test
for contaminations within the liquid cultures, we amplified and sequenced the 16S ribosomal
gene from each lineage including the ancestral strain using general primers 27F (5’-AGA GTT
TGA TCC TGG CTC AG-3’) and 728R (5’-CTA CCA GGG TAT CTA ATC C-3’) and Sanger
sequencing. The program cycle used to amplify the 16S gene is as follows: initial denaturation at
94qC for 15min followed by an initial 15 cycles of denaturation at 94qC for 1min, primer
annealing at 52qC for 45sec and elongation at 72qC for 1min. This is then immediately followed
by 20 cycles of denaturation at 94qC for 1min, primer annealing at 49.5qC for 45sec and
elongation at 72qC for 1min. The final elongation step was at 72qC for 7min. All PCR were
prepared in a laminar flow hood using aerosol resistant pipette tips and performed using Qiagen
hotstart©. All PCR reactions were carried out on the Eppendorf Mastercycler Pro S. All the
sequencing was done by the Génome Québec Innovation Centre. The gene sequences from each
lineage we’re aligned using clustalW and trimmed to remove any low quality reads. All
bioinformatics analyses we’re performed using MEGA5 software.
A-4 - Measurement of exponential growth characteristics
Strains were revived from storage at -80oC and cultured back to activity on MOLS4. Growing
cultures were transferred three times in order to acclimatize all populations to similar
environmental conditions prior to measuring growth characteristics. Specific growth rates (k in
day-1) of exponentially growing cells were calculated as
݇ൌ
ሺܥଵ ሻ െ ሺܥ ሻ
ܶଵ െ ܶ
where T0 is the time of the initiation of the experiment (in days), T1 is the first sampling time (in
days) and C0 and C1 are the cell concentrations (in cells mL-1) at these times. We estimated cell
concentrations by measuring the optical density (OD) of an actively growing culture at 600nm on
a Genesys 10S UV-VIS spectrophotometer. These OD600 measurements were converted to cell
concentrations via a single constant conversion factor (1.01 x 109). The conversion factor was
obtained by counting individual cells in dilute, DAPI-stained aliquots of actively growing
ancestral and evolved lines under an epifluoresence microscope. We used a single conversion
factor for all cultures since conversion factors between OD600 and cell numbers were similar in
all assayed lineages. Uncertainty estimates on growth rate was obtained by propagating the
uncertainty on measurements of OD.
Determinations of yield (Y, in 106 cells per µmol SO4-2 consumed) and cell specific sulfate
reduction rate (csSRR, in femtomoles SO4-2 consumed cell-1 day-1.) were based on
concentrations of hydrogen sulfide produced by actively growing cultures in exponential phase.
Concentrations were measured with a commercial sulfide kit based on the colorimetric method
of Cline (1969). Absorbance was measured on a Genesys 10S UV-VIS spectrophotometer at
670nm. The spectrophotometer was calibrated with mixed standards of dissolved sodium sulfide
and zinc chloride that were reproducible to ± 0.1mM.
Once H2S concentrations and cell numbers were measured, we estimated yield during
exponential growth as
ܻൌ
ܥ௫ െ ܥ
ͳ
ൈ
ሾܪଶ ܵሿ௫ െ ሾܪଶ ܵሿ ͳͲ
where [H2S]0 is the concentration of H2S (in mM) at the initiation of the experiment, [H2S]x
concentration of H2S (in mM) at later sampling times, and the factor of 106 converts from moles
to micromoles. This expression assumes a 1-to-1 stoichiometry between SO4-2 consumed and
H2S produced. Uncertainty estimates on yield was obtained by propagating the uncertainty on
measurements of OD and [H2S].The cell specific sulfate reduction rate during exponential
growth was calculated from estimates of growth rate and the yield as
ܿ ܴܴܵݏൌ
݇
ൈ ͳͲଷ
ܻ
where the factor of 103 converts from nanomoles to femtomoles. Uncertainty estimates on csSRR
was obtained by propagating the uncertainty on measurements of k and Y.
Real time monitoring of growth
We built a home-made device (POD) to monitor the growth rate of cultures in Real-time. 20mL
culture tubes were filled with 10mL of media and utilized to grow strains of bacteria. The device
consisted essentially of a photoresistor and an IR LED embedded into a plastic mounting plate
which was specifically designed to house the culture tube in which the growth curve was
performed. Each plate was mounted on a magnetic stirrer. Six of these devices were connected to
a breadboard which was operated by an Arduino and Ethernet shield. The entire device was
mounted on an incubator shelf and was inserted into the incubator. The Arduino was operated via
a USB cable which was connected to a computer, outside of the incubator. The Arduino operated
the apparatus through a simple program which monitored the voltage on the photoresistor as well
as the time since the start of the experiment and saved it to an SD card that was inserted in the
Arduino. The intensity of the voltages measured scaled linearly with increasing OD in the range
measured during the experiments. The data was measured in millivolts and the background
varied between each specific pairs of LED and photoresistor and were calibrated individually. To
convert the voltages obtained from the POD into the more common “OD”, we measured
OD@600nm with a spectrophotometer equipped with a bottle adapter for our POD bottles at the
start and the end of each experiment. The average of 10 voltage measurements around the time
of the OD measurement were used as the mV calibration fitted with the “true OD” by fitting a
linear model through the two points.
A-5 - Characterization of the sulfur isotope phenotype
We measured the S isotope phenotypes of the ancestral Dbac wild-type and of all three lineages
over the same interval as exponential growth characterics (300 generations).
Strains were
revived from storage at -80oC and cultured back to activity on MOLS4. Growing cultures were
transferred three times at 24hr intervals prior to performing the isotope assay. At the start of the
assay, 5 mL of a mid-exponential phase culture (OD600 ~ 0.2) was inoculated into gassed and
sterile assay bottles containing 80 mL of MOLS4 and a magnetic stir bar. The assay bottles were
vigorously sitrred while being simultaneously purged with pure N2 gas for two to three hours to
remove any sulfide that was carried-over with the inoculum. Repeated tests showed that the
sulfide blank in the assay media after purging was <5 ppm, which would have have a negligible
effect on the isotopic assay. Immediately after purging we took a sample (labelled T0) to
characterize the initial S isotope composition of the sulfate in the media. Assay cultures were
incubated at 33oC and shaken at 110 rpm. We halted cell growth and sulfate respiration once
enough sulfide was produced for a reliable isotope measurement, typically when <10% of the
initial sulfate had been consumed. The assay was stopped by adding 10mL of an acidic, 4% zinc
acetate solution. This also preserved all the sulfide that had been produced since T0 as ZnS.
This sample (labelled T1) provided the S isotope composition of the product sulfide as well as the
residual sulfate.
The 34S-32S and 33S-32S ratios of sulfate at T0 and T1, and sulfide at T1 provided six data points
that could be used to constrain the three critical parameters influencing the S isotope phenotype
at the assay conditions, as well as their uncertainty.
These are the fraction of sulfate left
unconsumed during the assay (f), the intrinsic discrimination of
respiration ( İ), and the characteristic discrimination of
34
33
S from
34
32
S from
32
S during sulfate
S during sulfate respiration
(34Ȝ) (see below).
A-6 - Measurement of the sulfur isotopes and estimation of the isotope phenotype (34İ) and
33
Ȝ
The media containing both the sulfate and sulfide fractions of S was filtered through a 0.22um
filter to remove all ZnS as well as cells and other precipitates from the filtrate containing the
sulfate. The filter was subsequently reacted with concentrated hydrochloric acid to produce acidvolatile H2S (AVS) from the precipitated ZnS.
This was carried by a N2 gas through a
distillation column, a water trap and bubbled through an acidic zinc acetate trap which reprecipitated the H2S as a purified ZnS that did not contain cells or precipitates from the growth
media. The sulfate in the filtrate was reacted with a ‘Thode’ reducing solution, a mixture of HI,
H2PO4 and HCl that reduced the sulfate to H2S when heated to §100°C and followed the same
trajectory as with the AVS extraction. The purified ZnS samples were then reacted with a silver
nitrate solution to produce silver sulfide. The silver sulfide was removed from the filtrate by
filtering through a 0.22um filter, which was washed with 1 M ammonium hydroxide and rinsed
three times with deionized water and then dried at 50°C for 2 days. Approximately 3mg of the
dried samples were weighed into aluminum packets in preparation for mass spectrometry.
Subsequently, the samples were converted to sulfur hexafluoride and the gas purified using the
procedure outlined in (Pellerin et al., 2014) (Paper 1) before analysis of the sample with a MAT
253 running in dual inlet mode in the Stable Isotope Laboratory of the Earth and Planetary
Sciences Department at McGill University, Montreal, Canada.
Isotopic compositions are
reported using delta notation
where
3i
R =
3i
S/32S,
ଷ
ܴ ௦
ߜ ଷܵ ൌ ቆ ଷ
െ ͳቇ ൈ ͳͲͲͲ
ܴ ି்
i is 3 or 4 and V-CDT refers to the Vienna-Cañon Diablo Troilite
international reference scale. All sulfur isotope data reported relative to Vienna Cañon Diablo
Troilite (V-CDT), against which the international reference material IAEA-S-1 is taken to have
the following isotopic composition:
33
S = -0.061‰ and
34
S { -0.3‰. The precision (1ı) on
individual measurements is better than 0.05 ‰ for į34S values, and 0.01‰ for ǻ33S values.
Accuracy of the measurements is controlled by the analytical reproducibility (1ı), which for the
full measurement procedure is better than 0.1 ‰ for į34S values and 0.01‰ for ǻ33S values.
Repeat analyses of the international reference materials IAEA-S-1, IAEA-S-2, and IAEA-S-3
always matched their accepted values within these uncertainties..
Data processing
In order to correct for the Rayleigh effect produced by the closed system of a batch culture, three
isotopic measurements were required to produce estimates of 34İ. We used: (1) the initial isotopic
composition of the starting sulfate (T0,SO4) (2) the isotopic composition of the sulfate at the time
of sampling (T1,SO4) and (3) the isotopic composition of the sulfide produced by dissimilatory
sulfate reductions up to the time of sampling (T1,H2S).
First, the fraction of remaining sulfate (f), can be calculated with the assumption that the isotopic
composition of the sulfide will be equal to that of the starting reactant when the reaction goes to
completion. This results in:
݂ൌ
ߜ ଷସܵ ்ǡௌைସ
ߜ ଷ்ܵଵǡுଶௌ
ቆ
ͳቇ െ ቆ
ͳቇ
ͳͲͲͲ
ͳͲͲͲ
ቆ
ߜ ଷ்ܵଵǡௌைସ
ߜ ଷ்ܵଵǡுଶௌ
ͳቇ െ ቆ
ͳቇ
ͳͲͲͲ
ͳͲͲͲ
The fractionation factor (Į) was calculated with a Rayleigh distillation correction while assuming
isotopic mass balance between sulfate and sulfide (Hoek 2006, Johnston 2007, Sim 2011)
ଷ
ሺͳ െ ݂ ሻ ߜ ଷ்ܵଵǡுଶௌ ͳͲͲͲ
ͳ
݈݊ ቆͳ
ଷ
ቇ
ߙൌെ
݈݂݊
݂
ߜ ்ܵଵǡௌைସ ͳͲͲͲ
We refer commonly to a form of the fractionation factor,34İ as the isotope phenotype where
ଷସ
ߝ ൌ ሺ ଷସߙ െ ͳሻ ͲͲͲͳ כ
The relationship between the fractionation of 33S relative to 32S and the heavier 34S relative to 32S
is expressed as 33Ȝ.
ଷଷ
The uncertainty on the f,
34
İ,
and
33
ߣൌ
ሺ ଷଷߙ ሻ
ሺ ଷସߙ ሻ
Ȝ was estimated through a Monte Carlo simulation
(Papadopoulos and Yeung, 2001). The initial uncertainties on į values were estimated from the
variability observed in the pooled ߜ ଷସܵ ்ǡௌைସ of all the experiments. This was 0.11 ‰ (see
APPENDIX A-7).
A-7 - Estimate of uncertainty on 34İ and 33Ȝ
We assume that our results follow a Gaussian distribution and obtain an uncertainty estimate by
Monte Carlo simulation. All procedures were performed in R. We created Gaussian distributions
of 5000 replicates for each of our measurement of ߜ ଷସܵ ்ǡௌைସ , ߜ ଷସܵ ்ଵௌைସ and ߜ ଷସܵ ்ଵுଶௌ and
assume that these distributions represent a good approximation of the true probability
distribution of each measurement. These distributions center on a mean of ߜ ଷସܵ௦௨ௗ and
standard deviation of ıఋ యరௌ
ೌೞೠೝ
The standard deviation value was set at 0.11‰ because this
was the uncertainty estimate obtained from pooling the entire dataset of ߜ ଷସܵ ்ǡௌைସ values for all
experiments. We utilized this as the estimator of uncertainty rather than simply the mass
spectrometer uncertainty of individual measurements because it is a more relevant measure of
the true uncertainty encompassing the whole-system handling. It takes into account the machine
uncertainty, the extraction uncertainty, as well as manipulation uncertainty and any isotopic
variation which may occur stochastically between bottles. The measure of ߜ ଷସܵ ்ǡௌைସ has not
yet been subject to the bacteriological activity we wish to measure yet follows the entire rest of
the preparation protocol.
The uncertainties between į34S and į33S are correlated in large part. We therefore produced
probability distributions for ߜ ଷଷܵ ்ǡௌைସ ,
ߜ ଷଷܵ ்ଵௌைସ and ߜ ଷଷܵ ்ଵுଶௌ respectively by
transforming the Gaussian distributions we had created for ߜ ଷସܵ ்ǡௌைସ ,
ߜ ଷସܵ ்ଵௌைସ and
ߜ ଷସܵ ்ଵுଶௌ . There are however deviations from the predicted relationship betweenߜ ଷଷܵ and
ߜ ଷସܵ as well as a small uncorrelated uncertainty which is equivalent to ıοయయ ୗ , the machine
uncertainty or 0.01‰. Both are taken into account in οଷଷ ܵௗௗ
The transformation of the probability distribution from į34S to į33S is:
ଷଷ
ߜ ܵௗௗ
ߜ ଷସܵௗௗ
ൌ ቆ
ͳቇ
ͳͲͲͲ
Where οଷଷ ܵௗௗ is defined as
Ǥହଵହ
െ ͳ൩ ൈ ͳͲͲͲ οଷଷ ܵௗௗ
ଷଷ
ο ܵௗௗ
ߜ ଷସܵ௦௨ௗ
ൌ ߋሺͲǡ ıοయయ ୗ ሻ ߜ ܵ௦௨ௗ ቆ
ͳቇ
ͳͲͲͲ
ଷଷ
Ǥହଵହ
െ ͳ൩ ൈ ͳͲͲͲ
ߋሺͲǡ ıοయయୗ ሻ is the uncorrelated uncertainty and ߜ ଷଷܵ௦௨ௗ is the experimentally determined
į33S.
With the distributions of ߜ ଷଷܵ ்ǡௌைସ , ߜ ଷଷܵ ்ଵௌைସ and ߜ ଷଷܵ ்ଵுଶௌ , ߜ ଷସܵ ்ǡௌைସ , ߜ ଷସܵ ்ଵௌைସ and
ߜ ଷସܵ ்ଵுଶௌ in hand we calculated the distributions of 34İ and Ȝ. The standard deviation on the
obtained distributions of 34İ and Ȝ were taken as a reliable estimate of the uncertainty.
References
Papadopoulos,C.E.,Yeung,H.,2001.UncertaintyestimationandMonteCarlosimulationmethod.Flow
MeasurementandInstrumentation12,291Ͳ298.
Pellerin,A.,Wing,B.A.,Rough,M.,Mucci,A.,Canfield,D.E.,Bui,T.H.,2014.Reoxidativesulfurcyclingin
the sulfidic carbonͲrich sediments of Mangrove Lake, Bermuda. Geochimica et Cosmochimica Acta
(submitted,GCAͲDͲ14Ͳ00267).
Appendix B captions
Appendix B-1
Collage of growth curves from all experimental lineages when monitored in real-time with a
home-built monitor of optical density. The first column is the ancestor, the second column is the
evolved lineage A. Column 3 is the evolved lineage B. Column 4 is the evolved lineage C. Rows
are replicates of the lineages. Within each graph, the increase in optical density is utilized as a
proxy for the increase in abundance of with time. The X axis is the time in hours and Y axis is
the optical density measured at 600nm. From these growth curves, one can extrapolate the
growth rate. It is evident from the growth curves that the ancestor’s growth is much slower than
the evolved lineages.
Appendix B-2
The relationship between
34
İ
and csSRR when controlled by physiological adaptation is
hyperbolically decreasing at low csSRR. Red dots are a partial dataset from the study of Hoeck
et al. (2006) utilizing Thermodesulfatator indicus growing on a low concentration of H2 as
growth substrate at high temperatures. Purple dots are from the studies of Sim et al. 2011a,
2011b and 2012 with strain DMSS-1 where csSRR was controlled by the nature of the organic
carbon substrate. Green dots are from the study of Leavitt et al. (2013) with DvH where the
csSRR is controlled by limiting the abundance of organic carbon substrate in a chemostat
experiments. Colored horizontal and vertical lines correspond to the uncertainty on
measurements when available. Black lines are a Loess extrapolation of the relationship between
csSRR and 34İ. All manipulations were perfomed in R with ggplot2.
Appendix B-3
The relationship between
34
İ and csSRR when the datasets are limited to a range of csSRR
between 18 and 62 fmol/cell/day show no reliable predictive relationship at the 95% confidence
level. Green dots are from the study of Leavitt et al. (2013) with DvH where the csSRR is
controlled by limiting the abundance of organic carbon substrate in chemostat experiments
whereas purple dots are from the studies of Sim et al. (2011a,b, 2012). Colored horizontal and
vertical lines correspond to the uncertainty on measurements, if available. The grey overlay
represents the 95% confidence interval on the linear regression that was utilized to establish the
relationship. All manipulations were performed in R with ggplot2.
Appendix B-4
The growth characteristics of the Dbac lineages at generation 0 (ancestor) and 300 as well as
critical information about the state of the inoculum. This data was utilized to interpret values of
growth rate, yield and csSRR (Figure 1, Table 1).
Appendix B-5
The isotopic measurements of sulfate and sulfides expressed in delta notation and utilized to
produce 34İ and 33Ȝ estimates (Figure 3, Table 2).
OD@600nm
OD@600nm
OD@600nm
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
0
0
0
5
5
5
15
15
15
time(hrs)
10
Ancestor rep3
time(hrs)
10
Ancestor rep2
time(hrs)
10
Ancestor rep1
Appendix B-1
20
20
20
25
25
25
OD@600nm
OD@600nm
OD@600nm
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
0
0
0
15
time(hrs)
10
15
time(hrs)
10
5
15
time(hrs)
10
EvolvHG$íUHS
5
EvolvHG$íUHS
5
EvolvHG$íUHS
20
20
20
25
25
25
OD@600nm
OD@600nm
OD@600nm
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
0
0
0
15
time(hrs)
10
15
time(hrs)
10
5
15
time(hrs)
10
EvolvHG%íUHS
5
EvolvHG%íUHS
5
EvolvHG%íUHS
20
20
20
25
25
25
OD@600nm
OD@600nm
OD@600nm
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
0.0
0.1
0.2
0.3
0.4
0
0
0
10
15
time(hrs)
10
15
time(hrs)
5
15
time(hrs)
10
EvolvHG&íUHS
5
EvolvHG&íUHS
5
EvolvHG&íUHS
20
20
20
25
25
25
Appendix B-2
Hoeck et al. (2008)
Leavitt et al. (2013)
60
34ε (‰)
Sim et al. (2011a,
2011b, 2012)
40
20
0
0
50
100
150
csSRR (fmole/cell/day)
200
Appendix B-3
30
34ε (‰)
Author
Leavitt et al. (2013)
20
Sim et al. (2011a,
2011b, 2012)
This study
10
0
20
40
csSRR (fmole/cell/day)
60
Ancestor
Ancestor
Ancestor
Evolved
Evolved
Evolved
Evolved
Evolved
Evolved
Evolved
Evolved
Evolved
Lineage id Experiment name
Rep 1
Rep 2
Rep 3
A
Rep 1
A
Rep 2
A
Rep 3
B
Rep 1
B
Rep 2
B
Rep 3
C
Rep 1
C
Rep 2
C
Rep 3
WWE/yͲϰ
Age
48hrs
48hrs
48hrs
18hrs
18hrs
18hrs
18hrs
18hrs
18hrs
18hrs
18hrs
18hrs
Inoculum
OD @600nm [H2S] mM Inoculum Vol. (mL)
0.2089
3.82
7
0.2089
3.82
5
0.2089
3.82
5
0.2152
7.74
5
0.2152
7.74
5
0.2152
7.74
5
0.2133
7.55
5
0.2133
7.55
5
0.2133
7.55
5
0.2281
8.27
5
0.2281
8.27
5
0.2281
8.27
5
WĂŐĞϭ
[H2S]
bdl
bdl
bdl
bdl
bdl
bdl
bdl
bdl
bdl
bdl
bdl
bdl
OD
0.012
0.012
0.012
0.013
0.013
0.013
0.013
0.013
0.013
0.013
0.013
0.013
ı
0.005
0.005
0.005
0.005
0.005
0.005
0.005
0.005
0.005
0.005
0.005
0.005
T0
cells/mL
1.4E+07
1.4E+07
1.4E+07
1.4E+07
1.4E+07
1.4E+07
1.4E+07
1.4E+07
1.4E+07
1.5E+07
1.5E+07
1.5E+07
ı
Elapsed time (hrs) T1 OD ı
5.7E+06
54
0.171
5.7E+06
64.5
0.133
5.7E+06
69
0.165
5.7E+06
24
0.256
5.7E+06
25
0.279
5.7E+06
26
0.209
5.7E+06
24
0.197
5.7E+06
25
0.200
5.7E+06
25
0.202
5.7E+06
24
0.260
5.7E+06
25
0.269
5.7E+06
26
0.310
WWE/yͲϰ
0.010
0.010
0.010
0.010
0.010
0.010
0.010
0.010
0.010
0.010
0.010
0.010
cells/mL
2.0E+08
1.5E+08
1.9E+08
2.9E+08
3.2E+08
2.4E+08
2.2E+08
2.3E+08
2.3E+08
3.0E+08
3.1E+08
3.5E+08
ı
WĂŐĞϮ
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
1.1E+07
T1
5.72
5.24
7.10
[H2S] mM
ı
3.37
2.94
4.07
4.63
5.46
4.46
3.97
4.63
0.11
0.11
0.11
0.11
0.11
0.11
0.11
0.11
0.11
0.11
0.11
0.808589925
0.824712069
0.76264931
f calculated from [H2S]
0.887247271
0.901538696
0.863779535
0.845004438
0.817502759
0.850714648
0.867136612
0.84502488
A
A
A
B
B
B
C
C
C
Evolved
Evolved
Evolved
Evolved
Evolved
Evolved
Evolved
Evolved
Evolved
Rep 1
Rep 2
Rep 3
Rep 1
Rep 2
Rep 3
Rep 1
Rep 2
Rep 3
Lineage id Experiment name
Rep 1
Rep 2
Rep 3
Ancestor
Ancestor
Ancestor
WWE/yͲϱ
-0.40093
-0.30352
-0.27127
-0.27262
-0.33577
-0.26556
-0.30151
-0.29546
-0.31595
-0.7721
-0.64116
-0.54812
-0.55421
-0.69597
-0.54947
-0.6371
-0.59616
-0.62323
0.42035
0.59065
0.30883
0.36056
0.37769
0.35115
0.66119
0.67194
0.92756
0.78766
1.12092
0.56029
0.69123
0.72168
0.65807
1.27825
1.31818
1.79625
-6.37327
-6.58455
-6.18046
-6.07868
-6.00579
-6.16367
-6.21136
-6.17072
-6.27183
-12.454
-12.8577
-12.089
-11.9272
-11.7807
-12.0676
-12.1438
-12.0703
-12.2761
T0 Sulfate
T1 Sulfate
T1 Sulfide
34
33
34
33
į S
į S
į S
į S
į S
į34S
-0.32703 -0.65876 0.56512 1.08201 -7.70008 -15.0745
-0.39489 -0.79951 0.40825 0.79239 -8.26406 -16.1717
-0.15169 -0.32887 0.43983 0.83266 -7.4589 -14.6093
33
WĂŐĞϭ
Conclusion
In the first paper, the investigation of the multiple S isotope signature of porewater sulfate in
Mangrove Lake shows relatively small
ଷସ
ߝ௧ coupled with large
ଷଷ
ߣ௧ , a behavior that is
inconsistent with a S cycle driven solely by microbial sulfate reduction. A simple diagenetic
model accounts for whole-core abundance and isotopic variability of porewater sulfate with a
single set of
ଷସ
ߝ௧ and
ଷଷ
ߣ௧ values. Splitting these net fractionations into the contributions
from individual components of the S cycle enabled us to estimate the contribution of reoxidation
in Mangrove Lake at between 50 and 80% of the microbial sulfate reduction flux. This model
implies that sulfide oxidation to S0, followed by the disproportionation of S0 to sulfide and
sulfate, is critical in generating the isotopic signature. The new understanding developed from
the S cycle in Mangrove Lake highlights the diagnostic capabilities of the multiple sulfur isotope
approach in identifying the importance of the reoxidative cycle in sedimentary porewaters.
In the second paper, the investigation shows that short-term evolutionary adaptation at initially
high growth rates can consistently affect the S isotope phenotype of sulfate-reducing
microorganisms. Our experimental design led to the mean fitness increases of ~20 % after
nearly §1000 generations of selection. There were consistent changes observed within different
selection intervals between
34
İ and fitness. Although the fitness changes were associated with
changes in exponential growth rate, the changes in 34İ were not. Changes in 34İ appeared to be a
response to changes in the parameters that govern the overall growth rate: yield and cell-specific
sulfate respiration rate. We hypothesize that these act together through the direct influence of
cell-specific sulfate respiration rate on
34
İ, such that higher yields at a constant growth rate
would lead to slower respiration and higher 34İ.
In the third paper, we follow up on the discoveries of paper 2 where the 300 generations of
unconstrained growth produces marked changes in the evolved lineages of Dbac which includes
a measurable effect the isotope phenotype with increasing growth rate and fitness. This
evolutionary response on 34İ appears to behave similarly to the physiological response of 34İ to
an increasing supply of growth substrate. Because of this similarity, the isotope phenotype might
be a predictable characteristic through periods of evolutionary adaptation when the
environmental and evolutionary conditions are well understood. It remains to be seen whether
conditions outside of this experimental framework would result in a similar response. If so, then
the metabolic evolution of DSR may very well be extractable from the waste products of sulfate
reducing microorganisms of past times.