Bio-Rad Protein Assay (Lowry)
advertisement

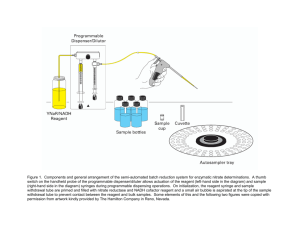
Bio-Rad Protein Assay (Lowry) The assay is based on the reaction of protein with an alkaline copper tartrate solution and Folin reagent. In alkaline solution proteins react with copper and subsequently the copper-treated proteins reduce Folin reagent by loss of oxygen atoms. The resulting reduced protein species have a characteristic blue color with maximum absorbance at 750 nm. Samples are measured with the HP8452A spectrophotometer using an automated analysis method. 1. Calibration - Prepare Reagent A* by adding 20 µl Reagent S per 1 ml Reagent A. - Place 0 µl, 5 µl, 10 µl, 15 µl, 20 µl, 25 µl (0-25 µg) of a 1 mg/ml stock solution of BSA (or BGG) in a microfuge tube, add H2O to 25 µl. - Add 125 µl Reagent A* and vortex. - Add 1000 µl Reagent B and vortex immediately. Transfer to 1 ml plastic cuvette and wait 15 min before starting the measurement. - Turn on HP8452A and computer (Pwd = "thch") 10 min before measuring so that the lamp light is stable. - Open the HP8452A software and select the Advanced Quant module. - Load parameter file: Files > Recall Parameter > LOWRY.QSP - Manually select an integration time of 5 s: Parameter > Instrumental > Integration time 5.0 s - Delete old standards: Edit > Delete Standards > All - Move transport to position 1: Parameter > Multicell Transport > Position > 1 - Measure blank (H2O): Measure > Blank - Measure standards: Measure > Standard [Specify Name: BSA or BGG, Concentration: 0-25, Units: µg] - When all standards have been measured make calibration: Task > Calibrate - Before saving, check quality of calibration curve: Graphics > Calibration Curve > Calibration Curve Calculation 1 - When calibration is ok save it: Files > Store Calibration [Specify Filename: LOWRYBSA or LOWRYBGG] - When asked Override File LOWRYBSA.QCL or LOWRYBGG.QCL say OK - Print new calibration and place copy in Protocols Folder: Hardcopy > Print Calibration 2. Samples - Prepare Reagent A* by adding 20 µl Reagent S per 1 ml Reagent A. - Place sample containing up to 25 µg of protein in a microfuge tube and add H2O to 25 µl. - In each series, include controls without protein (only H2O) and with 10 µg BSA or BGG. - Add 125 µl Reagent A* and vortex. - Add 1000 µl Reagent B and vortex immediately. Transfer to 1 ml plastic cuvette and wait 15 min before starting the measurement. - Turn on HP8452A and computer (Pwd = "thch") 10 min before measuring so that the lamp light is stable. - Open the HP8452A software and select the Advanced Quant module. - Load parameter file: Files > Recall Parameter > LOWRY.QSP - Manually select an integration time of 5 s: Parameter > Instrumental > Integration time 5.0 s - Place a cuvette with H2O in position 1 and your samples in positions 2-7 of the multicell transport. - Start measurement: Files > Run Method > LOWRY.QAU (Results are stored in LOWRY.QRS) - To view results on the screen: Task > Analyze > Display - To print results: Hardcopy > Report > LOWRY.QRS 3. Important considerations - If the controls are more than 2 µg off their expected value, repeat the assays including additional controls. If these are also off the expected values, make a new calibration. - The most accurate measurements can be made in the range of 5-20 µg, adjust sample volume accordingly. - For substances interfering with the Lowry method consult the Biorad Protein Assay Kit manual. 4. Reagents - Reagent A: - Reagent A*: - Reagent B: - Reagent S: - BSA: - BGG: alkaline copper tartrate solution, store at RT contains 20 µl Reagent S per 1 ml of Reagent A, prepare immediately before use diluted Folin reagent, store at RT aequous solution of SDS, store at RT bovine serum albumin, dissolved in H2O at 1 mg/ml, store at -20°C bovine gamma globulin, dissolved in H2O at 1 mg/ml, store at -20°C 1 CONTRIBUTED BY: Hubert Schwelberger (hubert.schwelberger@i-med.ac.at) LAST MODIFIED: 2012-06-21 2