Jacques Charles - AMCEverydayChemistry
advertisement

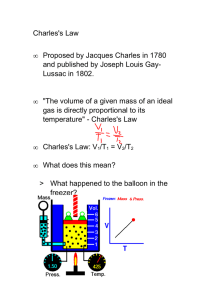
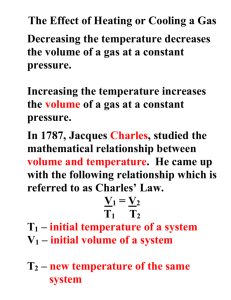
Jacques Charles Jacques Alexandre César Charles (November 12, 1746 – April 7, 1823) was a French inventor, scientist, mathematician, and balloonist. First flight by Prof. Jacques Charles with Ainé Roberts, December 1, 1783. Illustration from the late 19th Century. Charles was born in Beaugency-sur-Loire, and made the first flight of a hydrogen balloon on August 27, 1783.This balloon was destroyed by terrified peasants when it landed outside of Paris. On December 1, 1783, a mere ten days after the manned flight of the Montgolfier hot-air balloon, Charles with Ainé Roberts, ascended to a height of about 1,800 feet (550 m) in his balloon "La Charlière". Montgolfier's principal scientific collaborator was M. Charles, ... who had been the first to propose the gas produced by vitriol instead of the burning, dampened straw and wood that he had used in earlier flights. Charles himself was also eager to ascend but had run into a firm veto from the King, who from the earliest reports had been observing the progress of the flights with keen attentiveness. Anxious about the perils of a maiden flight, the King had then proposed that two criminals be sent up in a basket, at which Charles and his colleagues became indignant.[1] He developed several useful inventions, including a valve to let hydrogen out of the balloon and other devices, such as the hydrometer and reflecting goniometer, and improved the Gravesand heliostat and Fahrenheit's aerometer. In addition he confirmed Benjamin Franklin's electrical experiments. Around 1787 Charles did an experiment where he filled 5 balloons to the same volume with different gases. He then raised the temperature of the balloons to 80oC and noticed that they all increased in volume by the same amount. This unpublished experiment was later referenced by Gay-Lussac in 1802 when he published a paper on the precise relationship between the volume and temperature of a gas, which Gay-Lussac named "Charles' Law" in honor of Jacques Charles' original experiment, and also because he didn't want to confuse people by having two laws named after himself. Charles' Law states that under constant pressure, an ideal gas' volume is proportional to its absolute temperature. The volume of a gas at constant pressure increases linearly with the absolute temperature of the gas. The formula he created was V1/T1=V2/T2.