Water Cycle • Precipitation, Runoff, Collection, Infiltration
advertisement

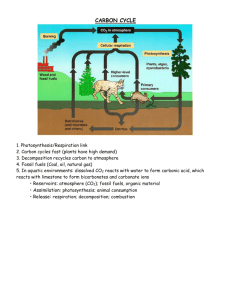
Water Cycle • Precipitation, Runoff, Collection, Infiltration, Percolation, Evaporation, Transpiration, Condensation o Sun powers evaporation, precipitation, and transpiration o Dust particles / condensation nuclei are needed for condensation to create raindrops. • Reservoirs – Surface water (oceans, rivers, lakes), Glaciers, Groundwater (Aquifers), Water Vapor Atmospheric Gas, Living Organisms Importance of Water • Water is essential to living organisms for a variety of reactions and cell processes • Water’s high heat storage distribute heat and determine regional and local climates • Water is a greenhouse gas – essential for maintaining Earth’s temperature – BUT too much greenhouse gas can be a bad thing • Sculpts landscapes (erosion and sedimentation) • It is the universal solvent, often interacts with the movement of other cycles because it transports them • The water cycle is nature’s natural water purifier (evaporation and underground bacteria) • Water is a necessity in industry, waste management Alteration of Water Cycle • Overuse – use faster than it can replenish o Think groundwater withdrawal and shrinking aquifers • Increase pollutants in runoff o Changing permeability of surfaces increases what runs off and increases in nonpoint source pollutants • Reduce infiltration o hard paved surfaces prevent recharging of groundwater and therefore increases runoff and contamination of surface water supplies • Accelerate topsoil erosion and runoff. o Areas that have little vegetation lack roots to hold soil in place, when surface water moves downhill it brings sediment with it. • Increase risk of flooding – when we drain and fill wetlands for farming or urban development o Changes permeability of the soil, even if areas aren’t paved over decreased water dries out soil making it harder for water to infiltrate. • Alter weather – deforestation reduces transpiration of rainforests, the primary source of rainfall in rainforests. Reduced shade also evaporates water before it can permeate the soil. • • • • • • • • • • • Carbon Cycle Natural occurrence through photosynthesis and cellular respiration Producers pull carbon from the atmosphere or water for the process of photosynthesis 6CO2 + 6H2O + SUNLIGHT à 6O2 + C6H12O6 Consumers take in carbon by ingesting producers or other consumers Producers and consumers release carbon as a product of respiration 6O2 + C6H12O6 à 6CO2 + 6H2O + ENERGY Found in the atmosphere as a gas (CO2), dissolvable in water, Carbon can be incorporated into marine and terrestrial sediments (CaCO3) After millions of years carbon can be compressed into fossil fuels Reservoirs – the ocean is the largest reservoir, calcium carbonate found in shells, marine sediments (decomposers release insoluble carbonates from dead marine organisms) forming limestone as well, fossil fuels (oil, natural gas, coal), tropical rain forests, tundra (permanently frozen plant matter), atmosphere, living organisms Importance of Carbon Cycle Required for all life. Make up carbohydrates, nucleic acids, lipids, proteins. Carbon dioxide is a greenhouse gas – essential for maintaining Earth’s temperature – BUT too much greenhouse gas can be a bad thing Due to combustion the amount of CO2 in atmosphere is much greater than what is removed through natural photosynthesis and absorption via water How humans alter Carbon cycle • The extraction and burning of huge quantities of fossil fuels that have taken millions of years to form have released large amounts of carbon dioxide to the atmosphere o Change from slow cycling carbon to rapid cycling carbon cycle • The clear-cutting of carbon-absorbing vegetation (especially tropical rain forests) have released large amounts of carbon to the atmosphere • • • • • • The increased concentration of carbon dioxide and other greenhouse gases (methane, water) are warming our planet and projected to change Earth’s climate this century Nitrogen Cycle N2 cannot readily be absorbed and used by plants and animals, so we rely on electrical discharges (lightening) and nitrogen fixing bacteria to make it usable for plants Animals consume plants or other consumers to obtain their nitrogen Reservoirs – the atmosphere (78%) Importance of Nitrogen Nitrogen is a key component in macromolecules such as proteins, vitamins, and nucleic acids (DNA and RNA) Limiting factor for plant and algae growth Nitrogen Fixation Nitrification Assimilation Ammonification Denitrification • • • Occurs in root nodules which convert N2 to NH3 (or NH4) whatever the plants do not assimilate enters soil Soil bacteria convert NH3 or NH4 into nitrates NO3- or Nitrites NO2- which can be assimilated by plants Plants or animals taking in the nitrogen and incorporate it into macromolecules (proteins, nucleic acids, etc Occurs when organisms die and begin to decompose the nitrogen from the organism is converted back into NH3 and ammonium NH4 in the soil to continue cycle Bacteria convert nitrogen back into the atmosphere as NO2 or N2 Human Impacts on Nitrogen Cycle We add nitric oxide NOx to the atmosphere as we burn fuel at high temperatures (cars, jets). o This gas is converted into nitrogen dioxide gas NO2 and nitric acid vapor HNO3 which falls to the earth as acid deposition aka acid rain. o Also associated with the combustion of these fuels is Photochemical smog Deforestation releases large quantities of nitrogen stored in soils and plants, back to a gas Agricultural runoff of fertilizers, animal manure, and sewage discharge add excess nitrates NO3- to bodies of water. o This can lead to eutrophication § algal blooms, which bring in more bacteria as algal decomposes § decreasing the oxygen in aquatic environment § often resulting in fish kills Phosphorus Cycle Very slow to cycle in comparison to water, carbon, and nitrogen because does not exist in the atmosphere or gaseous state o Water erodes away inorganic compounds, that include phosphates, from rocks o Water carries dissolved phosphates into the soil where they can be absorbed by producers o Consumers take in phosphates by ingesting producers § Become part of nucleic acid backbone § Adenosine TriPhosphate • Phosphates can be lost from the cycle for long periods when it is washed to the ocean and held in the marine sediment for millions of years • Reservoirs – Phosphate salts in terrestrial rock formations, and ocean bottom sediments. Importance of Phosphorous • Phosphate ions PO43- are incorporated into nucleic acids, ADP, and ATP, bones, and teeth Human Impacts on Phosphorous Cycle • Humans mine phosphate salts, which are added to fertilizers and applied to agricultural fields. • Excess phosphates from runoff can also cause eutrophicaton o algal blooms, which bring in more bacteria as algal decomposes o decreasing the oxygen in aquatic environment and increased B.O.D. o often resulting in fish kills Clearing of rain forests causes phosphates to wash away • • • • • Sulfur Cycle Natural sources of Sulfur in the atmosphere: o Volcanic eruptions release sulfur dioxide (a colorless, poisonous gas with a rotten egg smell). o Decomposers in flooded swamps, bogs, and tidal flats also release sulfur dioxide. Plants absorb sulfate ions, and incorporate them into proteins. Reservoirs – underground rocks and minerals in the form of sulfate salts S042Importance of Sulfur Essential nutrient – Used in amino acids, proteins, enzymes, keratin Human Impacts on Sulfur Cycle • Combustion of fossil fuels (coal that has high sulfur content or refined oil) can form sulfuric acid H2SO4 and can fall to the earth in the form of acid deposition/acid rain. o Can also impact water cycle by creating acid shock of waterways when pH decreases so much.