CHM 111 - Gravimetric Chloride Experiment (r7) 1/5 Purpose You
advertisement

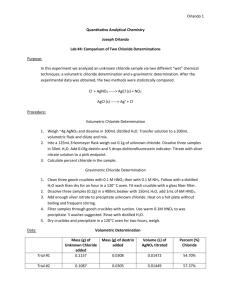
CHM 111 - Gravimetric Chloride Experiment (r7) 1/5 Purpose You will perform one of the basic types of quantitative analysis - the gravimetric analysis. You will be asked to determine the percentage of chloride in an unknown sample. Equipment ● ● ● ● ● ● ● ● ● Filter crucibles (3) Rubber adapter (1) Suction flask (1) Beakers, 400 mL or 250 mL (as needed) Glass stirring rods (3) Watch glasses (as needed) Hotplate Drying oven Analytical balance Chemicals required ● ● ● ● ● AgNO3 solid concentrated HNO3 solution HNO3 (nitric acid) wash solution Deionized water Concentrated NH3 solution Chemical Hazards. ● ● ● Concentrated nitric acid, HNO3, can cause severe chemical burns if you get it on your skin or in your eyes. Use extreme care when handling concentrated HNO3. Rinse with plenty of water immediately if you spill any on yourself. A nitric acid burn will appear first as a yellow stain on your skin which will later turn brown. Concentrated ammonia, NH3, can cause severe chemical burns if you get it on your skin or in your eyes. NH3 is a volatile compound with a pungent odor, and can incapacitate you if you inhale a large quantity of the vapor. Use extreme care when handling concentrated NH3. Rinse with plenty of water immediately if you spill any on yourself. Silver nitrate, AgNO3, should be used carefully. While not as hazardous as the concentrated acid and base solutions, AgNO3 solutions will stain skin on contact. These stains will look almost black initially and will fade to brown/yellow over the course of several days to several weeks. You can avoid these stains by washing your hands immediately after a silver nitrate spill (before you notice any dark color forming). If the stain is visible, it will probably not wash off with soap and water. CHM 111 - Gravimetric Chloride Experiment (r7) 2/5 Theoretical Overview of the experiment Gravimetric analysis relies on the laws of conservation of mass and the law of definite proportions. In a gravimetric analysis, you determine the amount of a substance present in an unknown by reacting it with another substance to make a chemical compound that can be isolated and weighed. Since a given compound contains the same proportion of elements by mass no matter how it is produced, this lets us calculate the amount of the desired substance based on the mass of the new compound. Your unknown will be a soluble chloride salt. You will use a solution of silver nitrate to precipitate out the chloride as silver chloride. The ionic equation for the reaction looks like this. Ag+(aq) +Cl-(aq) --> AgCl(s) Your unknown will release its chloride ions when dissolved, and these ions will react with the silver ions from silver nitrate solution to form silver chloride, which you will filter out and weigh. You can calculate the mass of chloride in the original sample based on the mass of AgCl recovered. Gravimetric analysis has several good points. It normally generates very precise data. It requires no calibration, since the mass of unknown may be directly calculated from the mass of the precipitate. It is also an easy measurement to understand - requiring little more than basic understanding of chemical reactions and calculations. However, the method has disadvantages. Some substances aren't suitable for gravimetric analysis since they don't readily form precipitates. The method is almost always timeconsuming, and many techniques will give you data that is almost as precise in only a fraction of the time. In fact, this laboratory procedure is an abbreviated version of the procedure that an analyst would use to determine the chloride content of a sample - trimmed down to fit in our academic lab schedule. Procedure Sample preparation and analysis First, weigh three samples of your unknown onto weighing paper. Each sample should be approximately 0.23 grams. (Do not try to weigh out 0.2300 grams of sample each time. Just get approximately 0.23 grams ... and record the actual weight, to four significant figures, of each sample!) Put each sample into a clean, dry, and numbered 400 mL beaker. Dissolve the samples in CHM 111 - Gravimetric Chloride Experiment (r7) 3/5 deionized water until each beaker contains 150 mL of solution. You may use the graduations on the beaker to determine how much water to add. Add about half a milliliter of concentrated nitric acid to each beaker. Be extremely careful with the concentrated nitric acid, since it can cause severe burns to skin and eyes! Stir the solutions to make sure that the samples are thoroughly mixed, but use a separate stirring rod for each sample. Do not remove the stirring rods from the samples until you are ready to filter them. Prepare 150 mL of 0.1 M AgNO3 solution. This will require that you dissolve 2.54 grams of AgNO3 solid in 150 mL of deionized water. Since the exact concentration of this solution is not vital to your calculations, you may measure out the AgNO3 solid using the balance, add it to a beaker, and then add 150 mL of water measured with a graduated cylinder to the AgNO3. Dispose of any excess 0.1 M AgNO3 solution in the marked bottle before leaving the lab. Tap water contains chloride ion, and if you add tap water to your samples or solutions at any time, it will affect your results! Slowly add up to 47 mL of 0.1 M AgNO3 solution to each sample solution while stirring. You should see a white precipitate of silver chloride form. The precipitate may turn pink or light purple in a few minutes. The color change indicates that some AgCl has broken down after exposure to light. This will reduce the weight of the precipitate slightly, but is not a problem for your results unless the precipitate is exposed to bright light for a long time. Heat the sample solutions (on a hotplate) to boiling. You should observe the silver chloride precipitate clumping together and the solution clearing up. The solution will not be perfectly clear like pure water, but it should be noticeably clearer than it was before you began heating it. Remove the beakers from the hotplate and let them cool for two or three minutes. Add a few drops of the 0.1 M AgNO3 solution. If you see new cloudiness form, keep adding more AgNO3 solution until you don't see any more cloudiness forming. If you saw additional cloudiness when adding AgNO3 solution, reheat them as described above until the solutions are clear with precipitate at the bottom. Put the beakers of precipitate in a lab drawer and allow them to sit - in the dark - for fortyfive minutes. Prepare a wash solution by slowly adding 1 mL of concentrated nitric acid to 300 mL deionized water. Set this solution aside - you'll use it to filter the precipitates. Be extremely careful with the concentrated nitric acid, since it can cause severe burns to skin and eyes! Mark (so you will be able to identify the crucibles later) and weigh three filter crucibles. When marking the crucibles, use a pencil and mark on the white area of the filter crucible. Do not use a label, as the glue in the label may not be made to withstand the temperatures of the oven. Set up a suction flask and your first filter crucible for suction filtration. Clamp the suction CHM 111 - Gravimetric Chloride Experiment (r7) 4/5 flask to a ring stand to prevent the flask from falling over during the experiment. Then, attach the next of the suction flask to the aspirator on your sink. Put a rubber adapter into the suction flask, then position the filter crucible into the adapter. Illustration 1 - Crucible, adapter, and flask Turn on the sink to start the suction. After you have verified that the crucible is securely held in the rubber adapter, slowly filter each sample (one per crucible). Rinse each sample with small portions of the nitric acid wash solution prepared earlier. Put the crucibles into a beaker to prevent them from falling over, then place them into the 125 oC drying oven. Allow the crucibles to dry until the next laboratory period. Allow the crucibles to cool, then weigh each crucible and calculate the amount of AgCl in each crucible. Calculate the mass of Cl and the percentage of chloride by mass in each sample. Find the average percentage of chloride in your unknown, and the standard deviation of the percentage chloride. Cleanup Silver waste should be disposed of in the designated containers. To clean filter crucibles (this must be done after drying and weighing), remove the solid AgCl precipitate with a spatula, then put the crucible in a beaker. Pour 5 mL of concentrated ammonia solution into each crucible, cover with a watch glass and place in the hood for ten minutes. Draw the ammonia through each crucible with a suction filtration setup, then rinse each crucible with deionized water. Be extremely careful with the concentrated ammonia, since it releases strong vapors that can burn nose, lungs, and eyes! Report In your report, present the full data for all three samples, including mass of sample, mass of chloride, percent of chloride. Make sure to mention which unknown you have, since part of your grade will be based on how accurately you have determined the percentage of chloride CHM 111 - Gravimetric Chloride Experiment (r7) 5/5 in your unknown. Discuss potential sources of error in the experiment and mention how these sources of error would affect the percentage of chloride calculated. The report should be presented in the same format as the lab report you have already done in CHM 111 - with one additional section. A section called "Sample Calculations" should be included at the end of the report. In this section, provide a complete set of calculations for the percentage chloride in the first sample..