Experimen tt: Acid
advertisement

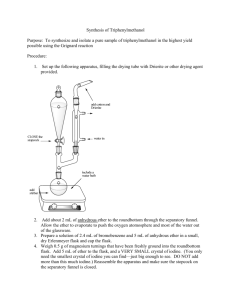
Experiment: Acid-Base Extraction Background information on the theory of extraction is covered extensively online and will also be covered in your discussion The information here pertains specifically to the type of extraction you will be doing in the lab this week, specifically acid-base extraction. When a mixture contains an organic acid (a carboxylic acid, for instance) and/or an organic base (for example, an amine), the process of separating each component of the mixture is accomplished by applying the general concepts of acid-base theory and solubility. In this exercise, you will begin with a mixture that contains an organic base, 4-chloroaniline, and a compound which is neither an acid nor a base, 1,4-dibromobenzene. Each of these is a solid at room temperature and neither is appreciably soluble in water. Your goal will be to isolate the two of them from the mixture. This pair of electrons on the amine functional group acts as a base, and therefore is protonated by acid. NH2 Cl 4-chloroaniline Br Br 1,4-dibromobenzene Like all bases, 4-chloroaniline will react with acid to form its conjugate acid. 4-chloroaniline is a non-ionic compound, and hence has no charge. Thus its conjugate acid will be positively charged. Because of its charge, this conjugate acid, the 4-chloroanilinium ion, is much more soluble in water than the 4-chloroaniline itself. Thus, 4-chloroaniline dissolved in a solvent like ether can be extracted into an aqueous solution of acid because it reacts with the acid, becoming the water-soluble anilinium ion. The aqueous solution containing the 4-chloroanilinium ion, once it is separated from the ether solution containing the 1,4-dibromobenzene, can be reacted with aqueous base. This will remove H+ from the anilinium ion, reforming the insoluble 4chloroaniline, which precipitates from solution. As a result, you will be able to separate and then isolate each of the compounds. Dibromobenzene will be recovered from the organic layer, while 4-chloroaniline will be recovered from the aqueous layer. In this experiment, then, you are extracting from solution a substance that you want to keep, the 4-choroaniline. However, often extraction is used to remove a substance that you do not want to keep. We call such extraction “washing,” and all year long you will be asked to “wash” various organic solutions. It is important that you understand what “washing” means. Washing is usually done using an aqueous solution, sometimes water itself or sometimes a solution of acid, base, or some other solute to remove some unwanted substance from the organic layer. Just as washing your hands with soap and water can remove greasy impurities, washing an organic solvent, contaminated with a small amount of acid impurity, with an aqueous solution of base (NaOH, Na2CO3) will remove that acid impurity by forming a water soluble salt. You will be doing both extraction and washing in today’s experiment. Your instructor will go over the procedure with you during pre-lab discussion. The procedure is straightforward and simple if you understand what you are doing. It is terribly confusing if you don't. Pay careful attention in pre-lab and ask questions if you don't understand something. The most important thing about this experiment is keeping track of where everything is in the experiment. You will start with a solution of 1,4-dibromobenzene and 4-chloroaniline in diethyl ether. As the experiment proceeds, you will extract the 4-chloroaniline, in the form of its acid salt, into the water. Since water and ether are immiscible, they will form layers which can easily be separated, thereby separating the two starting organic materials from each other. You will drain the aqueous solution (which contains the 4-chloroanilinium ion) from the ether solution (which now contains only dibromobenzene). The ether solvent will be evaporated from the dibromobenzene, and the 4-chloroanilinium ion will be converted back to the free 4chloroaniline using dilute base. The solid which forms will then be separated from the water by filtration. It can get confusing, and unless you know what you are doing, you may wind up throwing something you want down the drain. Be careful, and discuss everything with your partner before doing it. Pre-lab Preparation 1. Review your notes from discussion and watch the podcast demonstrating the procedure for extraction. Watching the podcast will make the lab faster for you and will help you answer quiz questions your instructor may ask from this podcast. So watch the entire podcast carefully. To find the podcast, go to the Montgomery College homepage and scroll to the very bottom. Under “News and Events”, select “Podcasts” (tiny font at the very bottom of the page). Under “Browse by Topic”, select “Science and Technology”. View the Podcast labeled “Separatory Funnels and Extractions”. 2. In your notebook, have the following information completed before coming to lab. a) Using a reliable reference, write a table containing the relevant physical properties (BP and density for liquids, MP for solids and solubility for both) for the following compounds (always list your reference source): 1,4-dibromobenzene 4-chloroaniline (look up under aniline, 4-chloro) diethyl ether b) Using the solubility information from your table of constants, make a flow chart, showing the procedure you would use to separate 4chloroaniline from 1,4-dibromobenzene. Indicate what compound is in each solvent by writing the structures at the appropriate locations in the flow chart. Do this for all steps of the procedure, showing what is removed at each step. c) Write an equation (using chemical structures, not formulas) for the reaction of 4-chloroaniline with HCl (aq) and another equation for the product of the first equation with NaOH(aq). Write under the product for each reaction whether it is soluble or insoluble in water. Experimental Procedure ! Safety Considerations ! 1,4-dibromobenzene is harmful. Avoid skin contact and minimize inhalation of vapors. ! Most aromatic amines are toxic and some are carcinogenic. Consequently, be careful with 4chloroaniline. Use rubber gloves during this experiment to keep this material off your skin. When recovering the dry solid, avoid breathing any dust from it. Normal precautions will keep you safe. Wash your hands thoroughly at the end of the lab. ! Ether is extremely flammable. Do all work with ether in the fume hoods. Never pour ether solutions into the sink. ! Do not pour mixtures into the sink unless told to do so. Use appropriate waste containers. Be sure that you and your partner both get to use the separatory funnel to make certain that both of you learn the necessary organic laboratory skills. Perform this entire lab procedure in the hood because ether is very flammable, and all solutions and glassware will have at least some ether in them. Do not remove anything from the hood unless you can no longer smell any ether on it or unless the container is tightly stoppered. 1. Obtain 20.0 mL of the 1,4-dibromobenzene/4-chloroaniline solution, which has already been prepared for you. Record the concentration listed on the bottle. Place the mixture into a 50-mL separatory funnel and add 10 mL of 1 M HCl. See the diagram for more information. ground glass stopper requires grease to prevent the joint from “freezing” in place (making it difficult or impossible to remove) and to prevent leaking rubber tubing on ring clamp prevents separatory funnel from falling through make certain that the valve is properly closed before adding liquid Place the stopper in the funnel, invert the funnel and immediately vent it by opening the stopcock. Ether is very volatile. Then shake the funnel well, venting frequently. Support the separatory funnel on a ring clamp attached to a ring stand. Remove the stopper and allow the two layers to settle. 2. Once the layers have separated, use the stopcock to drain off the bottom aqueous layer into a 100-mL beaker. Do not discard the aqueous layer. With the organic layer in the separatory funnel, add another 10 mL of 1 M HCl and repeat. Drain the aqueous layer into the same beaker as above, combining the two aqueous acid washes into one. 3. To the beaker containing the aqueous layer which now also contains the protonated amine, add 20 mL of 5% NaOH. After swirling to mix, use litmus paper to test the liquid to make sure it is basic. If it is not, continue adding NaOH solution in 5-mL portions until the solution turns the litmus blue. Allow this beaker to stand; you will come back to it later. 4. Now return to the organic layer in the separatory funnel, which should be a solution of 1,4-dibromobenzene dissolved in ether. There may also be a bit of residual hydrochloric acid. To neutralize this acid, wash the ether layer with 10 mL of an aqueous solution of sodium carbonate. You will observe bubbles of CO2 forming in the separatory funnel from the reaction of sodium carbonate with residual HCl in the ether layer. Vent the funnel frequently. Remove the aqueous layer and set it aside for waste disposal. Repeat these “washings” until you observe no more bubbling when the sodium carbonate solution is mixed with the organic layer. 5. Remove the organic layer by pouring it out of the top of the separatory funnel into a clean, dry Erlenmeyer flask. Add small amounts of anhydrous sodium sulfate drying agent until no further clumping occurs. Stopper the flask tightly and allow it to sit in the hood. After about five minutes, decant the ether solution carefully from the drying agent into a clean, tared beaker which you have weighed with a label containing your name, your partner’s name, and your instructor’s name, plus the contents of the beaker. Rinse the drying agent with a small amount of ether, and decant this ether into the other ether solution. Place the beaker in the back of the hood to allow the ether to evaporate. After at least 12 hours, when the liquid has completely evaporated, weigh the beaker to see how much 1,4-dibromobenzene you have recovered. 6. Now return to the aqueous layer that was set aside earlier. (It should still be standing in the hood). In the hood, collect the solid, 4-chloroaniline, using suction filtration. This requires a Büchner funnel, rubber adapter and side-arm flask. (If necessary, review how to perform suction filtration by reading online.) After collecting the solid, allow this to dry in your locker overnight or longer (this solid does not sublime), and then weigh the dry solid. 7. Dispose of all ether-containing mixtures in the appropriate waste containers. Aqueous solutions can be poured down the sink in the hood. Be certain to remove all equipment from your hood space. Remember, your instructor may deduct points for failure to properly clean your space and the common areas. Post-Lab and Report Requirements 1. A detailed explanation of the theory of extraction. Make sure to include both the general theory and the theory of separating acid and base solutes using extraction. 2. Data from the 1,4-dibromobenzene/4-chloroaniline extraction. a. Mass of 1,4-dibromobenzene and 4-chloroaniline isolated from the original solution in this experiment. b. Calculation of the amount of 1,4-dibromobenzene and 4-chloroaniline in your original solution before extraction. [Use the information from the reagent bottle that you recorded in your observations to determine the initial amounts.] c. Determination of the percentage of each of the two solutes recovered from the original solution. 3. Analysis of losses - In this portion of the report, discuss places in the experiment where procedural losses (often unavoidable) or human errors caused you to get less than a 100% recovery of 1,4-dibromobenzene or 4-chloroaniline. This is an important part of the report, so spend some time on this. This is the part of the report where it is going to be very tempting to get help from a friend on what these sources of error are. DON'T DO IT! This and all parts of the report should be your own work only. 4. 5. Answer the following questions: a. Describe how you would separate naphthalene from para-bromobenzoic acid, if both were dissolved in ether . (Look up structures online.) b. What was the purpose for extracting the original aqueous solution with two 10-mL portions of aqueous HCl? Why didn’t the procedure simply tell you to extract with one 20-mL portion? Solve the following problem: A 100-mL sample of aqueous solution contains 2.0 grams of compound “A” as solute. Extraction of this solution with 25 mL of ether produces an ether solution which after drying, is allowed to evaporate, leaving behind 1.4 grams of “A.” Calculate the distribution coefficient, Kd, of “A” in this ether/water extraction system. Kd = [A]ether [A]water Note that this partition coefficient indicates that even if you achieve “perfect” physical separation of the layers with the separatory funnel, some product will still be lost to the other layer. The amount lost will depend on the difference in solubility of the product in each of the immiscible layers. The larger the difference in solubility, the less you will loose to material staying dissolved in the wrong layer. The partition coefficient, then, represents a loss of yield that will occur with any experiment that utilizes extractions or washes. Don’t forget to write conclusions summarizing and analyzing your data.

![AL Chem Written Practical (Organic Chemistry) [F.7]](http://s2.studylib.net/store/data/005797652_1-4911d95dd6c8a0840f727bd387aa6027-300x300.png)