CHM412 EXPERIMENT 1_fazni-1
advertisement

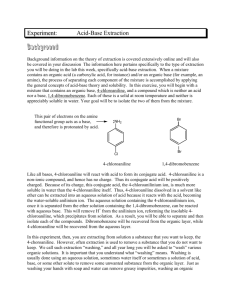
Page 1 of 3 CHM 412 EXPERIMENT 1 EXTRACTION AND DRYING OF AN AQUEOUS SOLUTION Objective To learn the techniques of separating toluene from water (and other inorganic compounds) by extraction and to learn the techniques of drying a dehydrated solution. Pre lab assignment: 1) Write a paragraph what do you understand about polar solvents and give at least three examples of polar solvents. 2) What is the meaning for the term immiscible solution? 3) Draw a diagram of separating funnel and a simple distillation apparatus set up. Introduction Removal of molecular solute from a liquid commonly practice from aqueous/water medium via immiscible medium is known as solvent or liquid-liquid extraction. This technique commonly in organic laboratory, where mixture of two immiscible liquids in a separating funnel, will be shaken thoroughly after both the tap and stopper are closed. Any pressure built up from the solvent vapor will be released from time to time by opening the tap.( refer to the diagram provided in figure 1) In this experiment the solvent is diethyl ether, ether family is well known having such as low boiling point, which made the solvent easily distilled off and recycled. As one of the volatile organic solvent the ether family is highly flammable. Through observation both water/aqueous layer are immiscible, but water in some extend dissolved/migrate in ether medium, (as air having water vapour /moist) can be seen by naked eyes. Due to this fact the after the extraction the first stage of drying process involving the usage of anhydrous substance such as magnesium sulfate or calcium chloride or any suitable drying agent, then followed by distillation for further purification. In solvent extraction procedure; to obtained higher percentage of recovery of product or extraction it can be obtained through successive/multiple extractions, where equal of amount of the solvent required will be used in multiple extractions. Page 2 of 3 Opening the stopper to let out the gas Figure:1 Chemicals 30ml of a mixture 50:50 ratio of water and toluene solution 40 ml of ether Adequate amount of anhydrous magnesium sulfate Apparatus 1 separating funnel, 150ml 1 retort stand 1 100 ml round bottom flask 1 condenser 1 still head 1 thermometer 1 filter paper 1 heating mantle 1 150 ml measuring cylinder 2 rubber hoses 1 adapter 1 pocket thermometer 1 filter funnel 1 150ml/250ml conical flask Separating the bottom layer Figure:2 Page 3 of 3 Procedure 1. Add 30 ml mixture 50:50 ratio of water and toluene solution into a separating funnel. 2. Add 20 ml of ether and shake vigorously. While shaking, open the stopcock occasionally to reduce the pressure built within, stop this process when no more or very small amount of pressure left. Leave the funnel to stand until the separation of the two layers stable or clearly seen. 3. Test a few drops of the bottom layer by mixing with distilled water in a beaker, after identify which is organic and aqueous layers. Drain the ether layer containing toluene (i.e. organic layer) into a clean and dry conical flask. Use the remaining solution (aqueous layer) layer for a second extraction. Note: the aqueous layer will be returned back to the separating funnel with a fresh batch of 20ml ether. 4. Repeat steps 2 and 3 using another/fresh batch 20 ml ether. 5. Dry the combined ether layers with adequate amount of anhydrous magnesium sulfate (or anhydrous calcium chloride), this can be indicated by no more of the anhydrous solid stuck at the bottom of the flask and a clear solution will be obtained. Filter out the drying agent with gravitational filter funnel. 6. Separate toluene from ether by distillation. 7. Record the volume of toluene and ether obtained. Calculate the percentage of recovery for both solvents. 8. Draw the apparatus needed for extraction and distillation. Questions a) Anhydrous magnesium sulfate or anhydrous calcium chloride are examples of drying agents which remove water reversibly. Why is it necessary to filter off these drying agents before distillation? b) Although most organic solvents are less dense than water, chloroform and dichloromethane are not. Explain briefly how you would separate chloroform or dichloromethane extract from an aqueous solution using a separating funnel.

![AL Chem Written Practical (Organic Chemistry) [F.7]](http://s2.studylib.net/store/data/005797652_1-4911d95dd6c8a0840f727bd387aa6027-300x300.png)