MT2_2013_practice2
advertisement
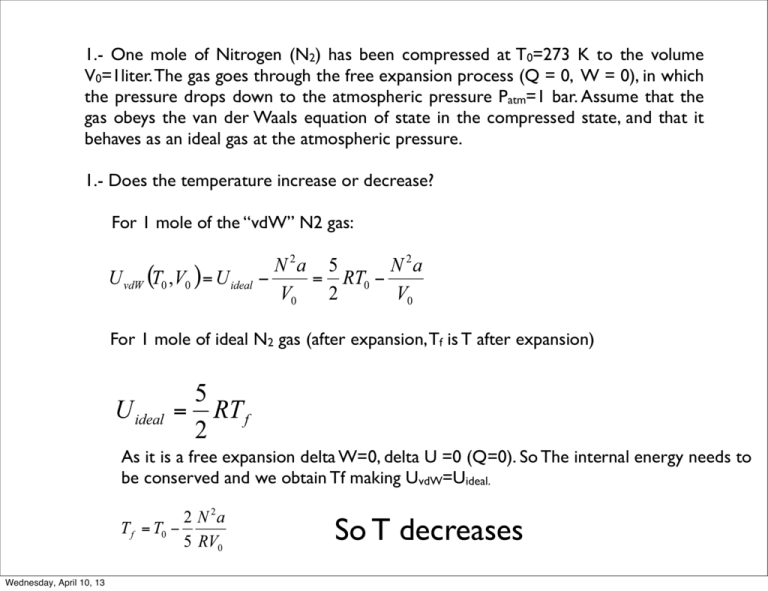
1.- One mole of Nitrogen (N2) has been compressed at T0=273 K to the volume V0=1liter. The gas goes through the free expansion process (Q = 0, W = 0), in which the pressure drops down to the atmospheric pressure Patm=1 bar. Assume that the gas obeys the van der Waals equation of state in the compressed state, and that it behaves as an ideal gas at the atmospheric pressure. 1.- Does the temperature increase or decrease? For 1 mole of the “vdW” N2 gas: For 1 mole of ideal N2 gas (after expansion, Tf is T after expansion) As it is a free expansion delta W=0, delta U =0 (Q=0). So The internal energy needs to be conserved and we obtain Tf making UvdW=Uideal. So T decreases Wednesday, April 10, 13 2.- Why? T decreases because in the vdW U we have kinetic (temperature) and potential energy terms. In the ideal U we only have the kinetic term. This means that the potential energy region was attractive on expanding the gas molecules work against attraction forces and this work comes at the expense of kinetic energy. 3.- Will the temperature always change in the same direction as you answered in 1 for any free expansion process like this where the compressed state is a vdW gas and the expanded state is an ideal gas? Yes, according to the equation any free expansion should always decrease the T. Wednesday, April 10, 13 2.- The pressure of the saturated water vapor at T = 0.010C is 0.006 bar (these T and P correspond to the triple point of water). The latent heat of the solid-liquid transformation at 0.010C is 335 kJ/kg, the latent heat of the liquid-gas transformation is 2500 kJ/kg. Find the pressure of the saturated water vapor at T = -10C. HINT: The vapor gas behaves like an ideal gas. HINT2: The volume of the solid is negligible as compared to the gas To solve this problem we just need to use Clausius-Clapeyron. Latent heat of sublimation at the triple point satisfies: Wednesday, April 10, 13 Above I neglected the volume of solid as compared to the gas and expressed the gas volume using the ideal gas law. n is the number of moles in 1 kg. Wednesday, April 10, 13 3.- 1 kg of water at 200C is converted into ice at -100C (all this happens at P = 1 bar). The latent heat of ice melting Lmelt = 334 kJ/kg, the heat capacity of water at constant pressure is 4.2 kJ/(kg·K) and that of ice 2.1 kJ/(kg·K), the heat of fusion of ice at 00C is 336 kJ/kg. (a) What is the total change in entropy of the water-ice system? (b) If the density of water at 00C is taken as 10% greater than that of ice, what is the slope of the melting curve of ice at this temperature? Give both sign and size. (c)Is this sign normal? Wednesday, April 10, 13 (a) This is almost identical to the problem we did on tuesday, but now we are asked for the change in entropy. We need to calculate 3 entropy changes ((1) water from 20 oC to 0 oC; (2) freezing of water;(3) cooling of ice from 0 oC to -10 oC). (1) (2) (3) Wednesday, April 10, 13 (b) We use Clausius-Clapeyron (b) The sign is not normal, for an ideal substance the solid should be more dense than the liquid. Wednesday, April 10, 13 4.- The phase diagram of a solution of B in A, at a pressure of 1 bar, is shown in the Figure. The upper bounding curve (the dew-point curve) of the twophase region can be represented by The lower bounding curve (the bubble-point curve) can be represented by A beaker containing equal mole numbers of A and B is brought to its boiling temperature at the bubble-point curve. What is the composition of the vapor as it first begins to boil off? Does boiling tend to increase or decrease the mole fraction of B in the remaining liquid? T0 T* T1 A Wednesday, April 10, 13 B We are asked to compute what is XB in the dew point curve at T=T*. We first need to compute T* and then replace this value in the dew point curve to solve for XB. - boiling tends to decrease the mole fraction of B in the remaining liquid (because there is much more B in the gas). Wednesday, April 10, 13 P liquid solid gas Ttr Wednesday, April 10, 13 T 5.- The schematic phase diagram shown is for hydrogen H2. Here are the things that we know about the system (all values near the triple point): 1.- The triple point T is Ttr=14 K 2.- The molar weight of H2=2g/mole 3.- Latent heat of vaporization Lv=1.01 kJ/mol 4.- Molar density of liquid H2 is rho=71 kg/m3 5.- 4.- Molar density of solid H2 is rho=81 kg/ m3 6.- Melting temperature Tm=13.99+P/3.3 (Tm in K, P in MPa=106 N/m2) 7.- Vapor pressure equation: P=P0exp(-Lvap/RT), with P0=90 MPa. (a) What is the volume difference Vliq-Vsol per mole when solid H2 is melted by increasing T at constant P? (b) Calculate the latent heat of melting (c) Calculate the latent heat of sublimation (d)Compute the slope and sign of the vapor pressure curve (dP/dT) for H2 near the triple point, in units of MPa/K. Assume that H2 can be treated as an ideal gas. Wednesday, April 10, 13 (a) What is the volume difference Vliq-Vsol per mole when solid H2 is melted by increasing T at constant P? Vliq Vsol mliq (2g/mole)(1kg/1000g) = = = 2.817 · 10 ⇢liq 71kg/m3 msol (2g/mole)(1kg/1000g) = = = 2.469 · 10 ⇢sol 81kg/m3 V = VS VL = 0.348 · 10 5 5 5 3 m /mole m3 /mole 3 m /mole During the melting the volume increases, which is in agreement with the negative slope f the P-T (L/S) coexistence line Wednesday, April 10, 13 (b) Calculate the latent heat of melting We use Clausius-Clapeyron: Lmelt Ttr (Vl Vs ) = dTm /dP 1K 6 2 dTm /dP = = 0.303K/(10 N/m ) 3.3 · 106 N/m2 Lmelt Wednesday, April 10, 13 (14K)(0.348 · 10 5 m3 /mole) = = 160.8J/mole 0.303K/(106 N/m2 ) (c) Calculate the latent heat of sublimation: Sublimation: Solid-->gas We do not have enough data to compute it from the information provided, however, the latent heat of sublimation, can be computed form the sum of latent heats of vaporization and evaporation, because we are near the triple point: Lvap=Ttr(SGas-Sliq); Lmelt=Ttr(Sliq-SSol) ; Lsub=Ttr(SGas-SSol) Lsub=Ttr(SGas-SSol)= Lmelt + Lvap=160.8 J/mole + 1010 J/mole = 1170.8 J/mole. Wednesday, April 10, 13 (d)Compute the slope and sign of the vapor pressure curve (dP/dT) for H2 near the triple point, in units of MPa/K. Assume that H2 can be treated as an ideal gas. Use Clausius-Clapeyron: Ideal gas: Lvap Lvap dTm /dP = ⇠ Ttr (VG Vs ) Ttr (VG ) RTtr P Ttr VG = = P P0 e Lvap /RT Replacing numbers we obtain VG=7.7 10-3m3/mole and dP/dT=0.0094 MPa/K Wednesday, April 10, 13 6.- The vdW gas undergoes an isothermal expansion from volume V1 to volume V2. Calculate the change in the Helmholtz free energy. In the isothermal process, the change of the Helmholtz free energy is We can compare with Wednesday, April 10, 13






