Vanillin Reduction & Esterification: Organic Chemistry Lab
advertisement

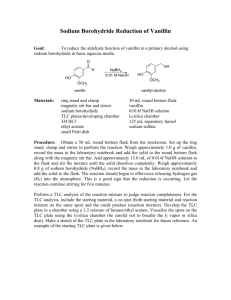
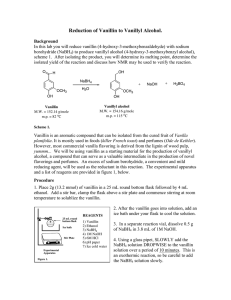
REDUCTION OF VANILLIN The reduction of vanillin, 4-hydroxy-3-methoxybenzaldehyde (1) to the primary alcohol, 4hydroxy-3-methoxybenzyl alcohol (2), can be accomplished using sodium borohydride. H O C CH2-OH 1. NaBH4/OHCH3 2. H+, H2O CH3 O OH (1) O OH (2) Procedure: 1. In a 125 mL Erlenmeyer flask, equipped with a stir bar, dissolve 2.00 grams of vanillin in 18.0 mL of 1M NaOH solution (use the 6 M NaOH to prepare the 1M NaOH) on a stirrer. CAUTION: THE NaOH SOLUTIONS ARE VERY CAUSTIC! Gently stir the solution to obtain a yellow, homogeneous solution. 2. Cool the solution to 10-15 C by immersing the flask in an ice-water bath. Continue to stir the solution while cooling the flask. 3. Stir the reaction solution and add 0.40 grams of sodium borohydride (HYGROSCOPIC) in three or four portions over a period of 8 minutes while maintaining the temperature between 10-15 C. 4. Let the reaction mixture stand for 30 minutes, then immerse the flask in an ice-water bath and add 6.0 M HCl dropwise until the solution is CLEAR and ACIDIC to litmus paper. NOTE: THE SOLUTION MUST BE ACIDIC BEFORE PROCEEDING TO THE NEXT STEP! CAUTION: THE HCl SOLUTION IS CORROSIVE! Why is HCl added to the reaction mixture? 5. Continue to cool the flask and gently scratch the wall of the flask with a stirring rod to induce crystallization. 6. Collect the crude product by vacuum filtration then wash the product three times with 12 mL of cold water. 7. Transfer the crude product to a pre weighed watch glass and allow to air dry for 5 minutes. 8. Recrystallize the crude product using a 60:40 V/V) mixture of ethyl acetate and petroleum ether. The most important feature of recrystallizing is to select an appropriate solvent. This is one in which the solute exhibits high solubility when hot and low solubility when cold. The solvent chosen for vanillinol is a 60:40 mixture of ethyl acetate and petroleum ether. 9. Add the weighed dried crude sample to the solvent mixture in an Erlenmeyer flask (15 mL solvent/1g sample) 10. Dissolve the crude sample by heating the flask on a hot plate in the hood. 11. Cool the mixture to room temperature to allow the solution to crystallize. Scratch the inside of the flask with a stirring rod to help initiate crystallization. 12. Cool the flask in an ice bath for 5 minutes and then collect the crystals by vacuum filtration. Wash the crystals once with 20 mL of ice cold ethyl acetate: petroleum ether (60:40) and then dry the crystals for 10 minutes under vacuum. Leave the crystals on a watch glass in your locker until the next lab. Analysis of Product 1. Determine the mass of the recrystallized 4-hydroxy-3-methoxybenzyl alcohol (2) isolated. 2. Determine the melting point of the product (2). Literature melting point: 113-114 C. 3. Calculate the % yield of 4-hydroxy-3-methoxybenzyl alcohol (2). 4. Run an infrared spectrum (IR) of the product (2). IR Procedure for Solid Samples Using a small metal spatula add a small amount (1/8 spatula) of your product to the ATR (Attenuated Total Reflectance) sample holder of the Thermo-Nicolet FT-IR. Set the press setting to 10 mm and then run an IR spectrum. Expand the % transmittance by clicking and holding the left mouse button down on the spectrum baseline. Print out the IR spectrum. ESTERIFICATION OF VANILLIN The esterification of vanillin, 4-hydroxy-3-methoxybenzaldehyde (1) to the ester (4) occurs rapidly at room temperature with acetic anhydride (3) in sodium hydroxide. O H H O O C C C O CH3 O C (3) H3C CH3 O CH3 NaOH O O OH O C (4) (1) CH3 Mechanism? Procedure: 1. Place 0.60 grams of vanillin (1) in 10 mL of 10% NaOH in a 125 mL Erlenmeyer flask. Swirl the flask to dissolve the vanillin in the NaOH solution. 2. Add 12.0 grams of ice and 2.0 mL of acetic anhydride (3) [CAUTION: AVOID CONTACT WITH SKIN!] to the reaction mixture. Swirl the solution and a white precipitate will form immediately. If a white precipitate does not form, add an additional 1mL of acetic anhydride. 3. Stopper the flask and swirl the reaction mixture. Allow the reaction mixture to stand for 15 minutes. Swirl the reaction mixture every 5 minutes. 4. Collect the white precipitate by vacuum filtration and wash the product two times with 2 mL of deionized water. Leave the aspirator on and allow the product to air dry in the funnel for 5 minutes. Analysis of Product 1. Determine the melting point of the ester product (4). The literature melting point of the ester product (4) is 77-79 C. 2. Calculate the % yield of the ester product (4). 3. Run an infrared spectrum (IR) of the product (4). See the Reduction of Vanillin Experiment for the procedure for running an IR on a solid sample. Question: This question MUST be answered before you leave lab today! You may collaborate with any one in your problem set group. 1. Propose a mechanism for the formation of 4-hydroxy-3-methoxybenzy-alcohol (2) from vanillin (1). See Reduction of Vanillin Handout. 2. Propose a mechanism for the formation of the ester product (4) from vanillin (1). 3. Propose a synthesis for the compound below. Start with phenol, acetic anhydride, and methane. O CH3 C O H2C O CH3