Borohydride Reduction of Vanillin
advertisement

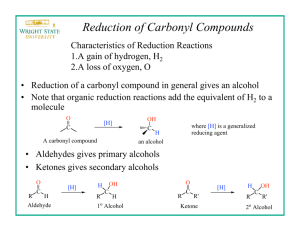
BOROHYDRIDE REDUCTION OF VANILLIN (12/22/2009) Metal Hydride Reductions Prior to the late 1940s, the reduction of the carbon-oxygen double bond in carbonyl compounds to an alcohol was very difficult to accomplish. However, during the late 1940s, two solid reducing agents, lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4), were introduced that conveniently, efficiently, and rapidly reduced carbonyl compounds to the corresponding alcohols. Lithium aluminum hydride is the more reactive of the two, and is capable of reducing practically all carbonyl functions and a number of other functional groups. Reducible functions include aldehydes, ketones, carboxylic acids, esters, amides, acyl halides, nitriles, epoxides, nitro groups, and alkyl halides. But unlike catalytic hydrogenation, lithium aluminum hydride will not reduce carbon-carbon multiple bonds under normal conditions. This high reactivity of lithium aluminum hydride limits its usefulness with polyfunctional compounds because the reduction of one functional group in the presence of another is extremely difficult, if not impossible, to accomplish. Lithium aluminum hydride reacts violently with water and any compound that contains -OH, -SH, or -NH2 functions to liberate hydrogen gas. Thus lithium aluminum hydride can be used only in aprotic solvents (e.g. diethyl ether or tetrahydrofuran) under completely anhydrous conditions. In addition, lithium aluminum hydride is expensive and difficult to handle safely; it has been known to ignite upon being ground in a mortar, or even when being removed from its container with a metal spatula! In contrast to lithium aluminum hydride, sodium borohydride is a much milder, and a more selective, reducing agent that is relatively safe to handle. Furthermore, sodium borohydride reacts so slowly with hydroxylic solvents that reductions with it may be conducted in aqueous or alcoholic solvents. Of all the functional groups reduced by lithium aluminum hydride, only aldehydes, ketones, and acyl halides are reduced by sodium borohydride. Because of its greater selectivity, sodium borohydride is capable of reducing aldehydes or ketones that contain other functional groups. For example, keto ester 1 is reduced to the corresponding hydroxy ester 2 by sodium borohydride. CH3 O O COCH3 COCH3 CH3 C O CH OH 1 2 Reductions with sodium borohydride are usually conducted in water or an alcohol (methanol, ethanol, or 2-propanol). Borohydride is not stable in these solvents at low pH; it decomposes at a rate of about 4.5% per hour at neutral pH and 25 oC. When reductions are conducted in aqueous solution, dilute sodium hydroxide is used as the solvent. If acidic functions are present, they must be neutralized before reduction is attempted, or else they may cause rapid decomposition of the borohydride. The stability of borohydride in alcoholic solvents increases in the order: methanol < ethanol < 2propanol. When choosing an alcoholic solvent for a borohydride reduction, not only must the stability of the borohydride be considered, but also the ease of recovery of the product from the solvent (e.g. NaBH4 is more stable in 2-propanol than in methanol, but 2-propanol is much more difficult to remove than methanol). Methanol or ethanol is the preferred solvent when reaction times are short (approx. 30 min), but 2-propanol is preferred for longer reaction times. With regard to mechanism, sodium borohydride reductions can be considered as nucleophilic additions of hydride ion (H-) to the carbonyl function, even though no free hydride ions are believed to be present. Experimental evidence indicates that a solvent molecule bonds to the boron atom as it transfers a hydride to the carbonyl carbon, while another solvent molecule transfers a proton to the carbonyl oxygen (see mechanism shown below). All four hydrides on the boron are usable, and the stoichiometry for the reaction is four moles of aldehyde or ketone reduced per one mole of borohydride. Usually a 10-20% molar excess of borohydride is used to compensate for any decomposition of the borohydride by the solvent. RO H O C H BH3 H OR H RO HOCH H3B OR ROH HOCH H3BOR The normal procedure for reduction with borohydride calls for the addition of a solution of the aldehyde or ketone in the reaction solvent to a solution of sodium borohydride. The addition is preformed at such a rate that the temperature remains below 25 oC. The amount of solvent is not critical as long as it is sufficient to keep both reactants in solution. Upon completion of the reaction, the excess borohydride is destroyed by slowly acidifying the reaction mixture to about pH 6 with 3-6 M HCl. Product isolation may be accomplished by filtration or extraction, depending upon the nature of the product (i.e. solid or liquid) and of the solvent (i.e. water or alcohol). When an alcohol has been used as the solvent, the alcohol is usually evaporated and the residue extracted with an organic solvent such as diethyl ether. Reduction of Vanillin After reading the section above, devise a procedure for the reduction of vanillin (25 mmol) to vanillyl alcohol with sodium borohydride. Your procedure should be detailed enough that someone with the appropriate background could perform the reaction successfully. The procedure should include (1) the amounts of reactants and solvent needed, (2) a description of the reaction conditions and steps to be followed, and (3) a description of how you plan to isolate and purify the product. Submit an outline of your procedure to your TA for approval two weeks BEFORE YOU ARE TO BEGIN THE EXPERIMENT. CHO CH2OH OCH3 OCH3 OH OH vanillin vanillyl alcohol NOTE: Acidification of the reaction mixture must be done carefully because vanillyl alcohol is sensitive to strongly acidic conditions. In strongly acidic solutions, especially if above room temperature, vanillyl alcohol will react to form intractable materials. Reaction and Physical Data CH2OH CHO + 4 NaBH4 + 4 H2O OCH3 O + 4 Na B(OH) 4 OCH3 O Physical data ---------------------------------------------------------------------------------------------Compound M.W. mp(oC) bp(oC) ---------------------------------------------------------------------------------------------Vanillin 152.15 80 - 81 285 * Vanillyl alcohol 154.17 113 - 115 dec ** 37.83 400 dec --NaBH4 --------------------------------------------------------------------------------------------*Vanillyl alcohol is soluble in cold alcohol or diethyl ether and soluble in hot water, benzene, or ethyl acetate. **Caution: avoid breathing or skin contact with NaBH dust and keep 4 NaBH4 away from concentrated acids. Name:______________________________________ Section:_________ Date:____________ POSTLAB EXERCISE: BOROHYDRIDE REDUCTION OF VANILLIN (12/22/2009) >> Due the lab following the completion of the experimental portion of the notebook (30pts. TOTAL). Please answer questions on this form. Attach sample calculations and copies of any TLC plates, GC traces, and/or spectra which you interpret (on the spectra, please!).<< PRODUCT INFORMATION (15pts) %Yield (5pts): Melting Point (5pts): Appearance (describe) (5pts): QUESTIONS (15pts) 1. (4pts) If you were going to repeat this experiment, what changes would you make in the procedure to improve the isolation, purification, and yield of the vanillyl alcohol. 2. (5pts) Acetone is a better solvent for organic compounds than ethanol. Would acetone be a suitable solvent for conducting sodium borohydride reductions of aldehydes that are insoluble in ethanol? 3. (6pts) A chemist tried to reduce 0.20 mole of ketone 3 using 0.060 mole of NaBH4 in ethanol to produce alcohol 4, but when he worked up the reaction he obtained only a 20% yield of alcohol 4. Briefly explain. OH O CH3 CO2H C 3 CH3 CO2H CH 4