Student Evaluation
advertisement

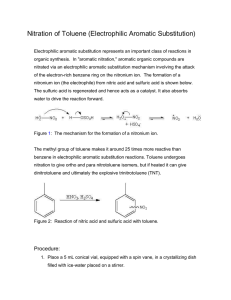
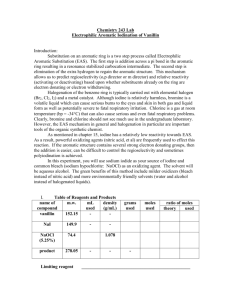
Laboratory Evaluation for A Green, Guided-Inquiry Based Electrophilic Aromatic Substitution for the Organic Chemistry Laboratory Source: Eby, E.; Deal, S. T. J. Chem. Educ., Print 2008, 85, pp 1426-1428. Date: June 2009 Submitted by Kailey Luoma, Josh Camreta, and Devin Stucki, students at Central Oregon Community College in Bend, Oregon. Evaluation The purpose of this lab is to iodinate salicylamide through electophilic substitution. Salicylamide has an activating group (OH) and a deactivating group (CONH2) already attached. Predicting whether the iodination occurs at an ortho/para position or meta position helps reinforce understanding of electrophilic substitution and the characteristics of aromatic molecules. Over all, this lab provides students with several opportunities to develop different laboratory skills including infrared spectrometry, pH testing, vacuum filtration and recrystallization. The solution undergoes several color changes making it possible to visually see chemical changes taking place, which made it appealing to students. This lab provided a very straightforward experiment to determine the directing effects of groups on an aromatic ring upon the addition of iodine. The final placement of iodine on the aromatic ring is identified using infrared spectrometry. The lab procedure seemed very easy to follow and presented directions that helped walk the student through each step, without causing confusion. One problem arose when the solution didn't reach the faint, pale yellow color it was supposed to, but instead remained a bright green. A color change usually signifies a chemical change. Conversely the lack of a complete color change may show that the reaction didn't proceed as was intended. This may have occurred if the amount of sodium hypochlorite solution added was too small to react with all of the salicylamide and sodium iodide. More sodium hypochlorite than the procedure states may need to be added in order to push the reaction to the desired color. In spite of the color differences, the product was determined to be the expected 1,2,4-trisubstituted aromatic molecule. Although this lab uses several toxic materials, like HCl and Sodium hypochlorite, they are used in dilute and small quantities. The toxicity of these chemicals in a dilute state is minimal. There are no major disposal concerns for these materials. This is a good lab for introducing electrophilic aromatic substitution and green chemistry. The procedure is simple, easy to follow, and appears to be effective. It is very useful for reinforcing skills of interpreting infrared spectrograph, as the students have to identify the location of the iodide on the aromatic molecule. 1H NMR would be a very useful tool in identifying this product, but infrared works well also. Experiment: A Green, Guided-Inquiry Based Electrophilic Aromatic Substitution for the Organic Chemistry Laboratory 1 Evaluation Procedure: This evaluation was part of a class assignment for the organic chemistry laboratory. Students were asked to identify, field-test and evaluate a laboratory listed in the GEMs database (http://greenchem.uoregon.edu/gems.html). Activities included out of class meetings, laboratory testing sessions and collaborative development of their evaluations using a Wiki. Laboratories were selected based on their suitability for the Organic curriculum as well as on their ability to effectively introduce green chemistry principles. Evaluations include commentary about the procedure from a student’s perspective. Faculty Advisor and Contact Carol Higginbotham Professor of Chemistry Science Department Central Oregon Community College 2600 NW College Way Bend, OR 97701 Email= chigginbotham@cocc.edu Phone= 541-383-7552 Experiment: A Green, Guided-Inquiry Based Electrophilic Aromatic Substitution for the Organic Chemistry Laboratory 2