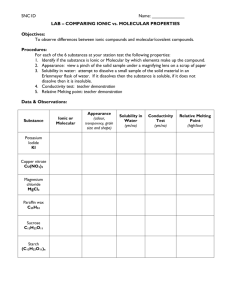
General Chemistry I Laboratory Manual
advertisement

General Chemistry I Laboratory Manual Winter term 2011-12 Lab begins the first week of classes Required Text for CHEM 122 (All sections) You must bring this lab manual, plus safety glasses, to the first lab period. Safety glasses are required, and they are sold at the bookstore. Department of Chemistry & Biochemistry: Laboratory Safety Policy and Rules 4 1. All students will complete the safety training segment prior to starting laboratory work. You must know the location of safety equipment: fire extinguishers, safety showers, eye wash facilities, fire blanket and first aid kit. 2. Approved eye protection (safety glasses/goggles) must be worn at all times in the laboratory. Even if you have completed your lab work, eye protection must remain on in the lab. 3. Stools are not permitted in the laboratory. The only exception to this is near the melt-temp instrumentation in the Organic lab. If stools are moved, they will be moved from the lab. 4. No eating, drinking any liquids (including water), chewing gum or smoking in the laboratory. 5. No bare feet in the laboratory; open-toed sandals are dangerous and are not permitted. Long pants or skirts must be worn. If shorts are worn, you may cover your legs with a knee-length lab coat or apron. Latex gloves are also available for any student who wishes to use them. 6. Long hair must be kept tightly in place. Hair and loose clothing can catch fire easily. 7. Do not enter the lab unless the instructor or supervisor is present. 8. No unauthorized experiments are to be performed. Alterations to an existing lab must be approved by the instructor. 9. Any accident or injury must be reported to the supervisor at once. 10. Chemicals are generally to be kept in designated access areas. Caps are to be replaced promptly on any reagents used in the lab. Use care when transferring or dispersing chemicals. All spills must be cleaned up promptly and completely. 11. If any material is spilled on the skin, wash it off immediately with large volume of water. Notify your instructor. 12. Reading labels and using the exact chemical in its proper concentration is the responsibility of the student. For some reagents, only the instructor is allowed to disperse them (these will be announced in the lab introduction). 13. Do not use cracked or chipped glassware. Dispose of it in a proper manner as indicated by your instructor. 14. Proper clean up and maintaining a well-ordered work space is essential to lab safety. Bench tops and the weighing areas are to be wiped clean. Clean all shared equipment. Pay special attention to the proper cleaning techniques for various pieces of instrumentation. Improper cleaning can damage expensive equipment and render it useless! 15. Instrumentation can only be used after instructor approval or according to any instructions given in the lab introduction. 16. Proper disposal of waste chemicals is the student’s responsibility. If there is a question, ask the supervisor. 17. Read the entire experiment and complete any pre-laboratory assignments before entering the laboratory. These include, for the Organic laboratory, a truncated procedure written in pen in your lab notebook. 18. Inform the instructor immediately of any broken thermometers immediately. If a mercury thermometer is broken, any spills must be cleaned up by the instructor. 19. Any special health factors such as an allergic reaction to a chemical or a pregnancy must be reported to the instructor as soon as possible. 20. Always use common sense in the laboratory. If something is unclear, be sure to ask the instructor before proceeding. Lab Supplement: Tips on using Excel Several of the laboratories require you to analyze your data using the Excel software available in the Zurn Scientific Computing Lab (ZSCL). The most common type of analysis that you will be performing is called a linear regression. This analysis determines the equation of the “best-fit” straight line through your data points. 1. Open the Excel program. 2. Use the topmost cells to label the data columns, this will make it easier for you to keep track of the data. Include units with your data labels. A column can be widened by selecting the top of it and dragging with the mouse. 3. Enter your x-coordinate data into the column on the left. Do not include units in cells with numbers. 4. Enter your y-coordinate data into the column on the right. 5. If the data is formatted incorrectly (such as too many/too few decimal places), use your mouse to select the cells with data that you wish to change. You may select entire columns. Under the Number options, select Number as the category and then enter the correct number of decimal places. 6. To chart, use the mouse to select the entirety of data, do not select the labels. 7. Select Insert, and then Scatter Chart with only markers. 8. The plot of your data should now appear superimposed over your window, if it doesn't look like the correct data was plotted, try the process again. If it does, you should format your chart to appear as you would like it. 9. With the chart selected, you can select the Layout to give a chart title, label the axes, format the axes, change the background and remove/add gridlines. 10. With the chart selected, you can select Design to change the data presented and label the data series in the legend. (To do this, choose Select Data, then select your data and Edit it to provide a name). 11. To perform a linear regression, go to the Layout area, and select Trendline under analysis. Move down to “More Trendline Options” and select “Display Equation on Chart” and the Linear Trend. 12. The equation of your line should now appear on your graph. You can move it to where it is read most easily. 13. Too remove a chart that is incorrect, click on the chart, then select, Edit, Clear, All. 14. When you are done, check with your instructor about either printing or e-mailing your chart and data. Using Equations 1. To use equations in a cell, select the cell within which you want a value to be calculated. 2. Type the formula using other cell labels, preceded by an "=" sign. 3. For more complicated formulas, use parentheses. EXAMPLES a) Suppose I wanted to add together the contents of cell A1 and B3. Type into the cell where the result should be: =A1+B3 b) Suppose I wanted to multiply A1 by B1 and then subtract away the contents of B2. Type: =(A1*B1) – B2 c) Suppose I want to take the average of cells A1 to A5, then divide the result by 3.47. Type: =(AVERAGE(A1:A5)) / 3.47 * EXPERIMENT 1 – Chemical Observations The foundation of chemistry is the relationship between the “macroscopic” world of observable, predictable results and the “abstract” world of molecular behavior. Because there exists such a chasm between everyday observables and the molecular level, this relationship is the source of both fascination and frustration for beginning chemistry students. The following simple experiments are designed to give you practice with forming abstract chemical conclusions based upon empirical, macroscopic observations. It is important to practice recording clear yet concise observations and to think clearly about what these observations imply. Part A: The Blue-Bottle Experiment Procedure 1. Measure approximately 50 ml of 0.500 M NaOH (sodium hydroxide) into a 125-ml flask. 2. Weigh out approximately 3.0 g of dextrose and add it to the same flask. Mix until the dextrose is completely dissolved. 3. Add 3-4 drops of methylene blue. 4. Obtain a stopper for the flask, place it on the flask and shake vigorously. Describe what happens in the space provided below. In your description, be as detailed as possible. Describe what happens to an audience that does not know what you are doing. 5. Set the flask on the lab bench and wait several minutes, describe below any changes in the appearance of the contents in the flask. 6. Repeat the shaking procedure. Again, describe what happens below. Write detailed descriptions of your observations: Initial shaking: ________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _________________________________________________________________________________(1.5) Flask left alone: _______________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _________________________________________________________________________________(1.5) Repeated shaking: _____________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _________________________________________________________________________________(1.5) The blue dye in the solution, methylene blue, will become colorless as it reacts with the dextrose in the solution. The methylene blue is in fact being reduced by the dextrose. This process can be reversed by identifying a chemical compound that will oxidize the methylene blue. What chemical compound can oxidize the methylene blue, which is made available through shaking the bottle in the lab? __________________________________________ (0.5) 1 Part B: The Production of Gases * Procedure 1. Place approximately 50 ml of water into each of two 125-ml flasks. 2. Into the first flask, drop two Alka-Seltzer tablets. Into the second flask, place two Efferdent tablets and cover it with a stopper (the stopper may blow off! Replace it as soon as possible if this occurs). 3. Light a wood splint and place it into the Alka-Seltzer flask (the first one) while maintaining a flame on the wood. Hold the splint in the opening of the flask but do not touch the actual liquid. Describe what happens to the flame in the space provided below. 4. Now turn toward the second (Efferdent) flask. One person swirl the flask while it remains covered while the second person lights a wood splint. 5. Let the wooden splint burn for a few seconds, then blow out the flame to leave as much of the glowing embers as possible. 6. As soon as the flame is blown out (but not before!), the first person should uncover the flask and allow their lab partner to place the glowing embers into the flask. Try to place the embers as close to the largest bubbles as possible without placing it directly into the liquid. You may need to try this several times to see any effects. Describe what happens below. 7. Extinguish the flame and rinse before discarding it. Descriptions of Observations (Alka-Seltzer) __________________________________________ ______________________________________________________________________________ __________________________________________________________________________(1.5) Descriptions of Observations (Efferdent) ____________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ __________________________________________________________________________(1.5) The two chemical reactions that have occurred are listed below. Determine which you believe was due to Alka-Seltzer and which was due to Efferdent and circle your answer for each. (1) (a) 3 Na2CO3(aq) + 2 H3C6H5O7(aq) 3 CO2(g) + 3 H2O(l) + 2 Na3C6H5O7(aq) Alka-Seltzer Efferdent (b) 2 H2O2(aq) O2(g) + 2 H2O(l) Alka-Seltzer Efferdent Explain why. [Hint: consider the gases (g) that are produced by each of these two reactions.]__________________________________________________________________ ______________________________________________________________________________ ___________________________________________________________________________(1) 2 EXPERIMENT 2 – Elements and Compounds Goal: To learn how to name compounds given the molecular formula and to give the molecular formula given a name. Objectives: After completing this experiment, the student should be able to do the following: 1. Given the formula of a compound, write its name. 2. Given the name of a compound, write its formula. 3. Given the symbol of an element, write its name. 4. Given the name of an element, write its symbol. 5. Give the names and symbols of the seven elements that exist as diatomic molecules at room temperature and pressure. 6. Be familiar with the following terms: element, atom, compound, diatomic, element, protons, neutrons, electrons, nucleus, atomic number, molecular compound, ionic compound, ions, anions, cations Background Elements An element is a pure substance that cannot be decomposed by any chemical reaction into simpler substances. As of 2007, there were 117 known elements. The smallest particle of an element that retains all of the properties of the element is an atom of the element. An atom is also the smallest unit of an element that can enter into a chemical reaction. Atoms are made up of protons (mass of 1 atomic mass units (amu), charge of “+1”), neutrons (mass of 1 amu, charge of “0”), and electrons (mass of 0 amu, charge of “-1”). These subatomic particles do not have the properties of elements. Elements are not electrically charged. Every atom of a given element is identical in the number of protons in the atom‟s nucleus. The number of protons in the atom‟s nucleus is referred to as the atomic number (Z) of the element. The nucleus of the atom contains the atom's protons and neutrons. The electrons are separated from the nucleus. Uranium (Z = 92) has the greatest atomic number of the naturally occurring elements. The transuranium elements have atomic numbers greater than uranium. They are made by induced nuclear reactions. The first transuranium element (Z = 93) was synthesized in 1940. The American nuclear chemist Glenn Seaborg, along with his research groups, were the first to synthesize elements 94-101, and his groups may have been the first to synthesize several of the others. Seaborg won the Nobel Prize for chemistry in 1951 for his work concerning the transuranium elements. Element 106, seaborgium, is named for him. 1 Names of elements For the General Chemistry course, you should know the names of the following elements. You may wish to practice now by writing the name next to the elemental symbol: H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Cr Mn Fe Co Ni Cu Zn Br Kr Ag Sn I Xe Ba Au Hg W Pb U Sr Compounds A compound is a substance containing two or more elements chemically combined in definite proportions. Compounds, unlike elements, can be decomposed chemically into simpler substances (simpler compounds or elements). We presently know the chemical formulas and structures of over 13 million compounds, with about 800,000 new compounds being discovered each year. Most of these compounds can be classified as either being molecular or ionic. Molecular compounds are comprised of individual molecules. A molecule is the smallest portion of a molecular compound that retains all of the properties of the molecular compound. Molecules are not electrically charged. Ionic compounds are comprised of ions. An ion is an electrically charged atom or group of atoms. An ionic compound is held together by attractive forces that exist between positively and negatively charged ions. A positively charged ion is called a cation; a negatively charged ion is called an anion. Seven of the elements occur as diatomic molecules at room temperature and pressure. These elements are: Molecular Normal Element Symbol Formula State (room temperature, normal pressure) Hydrogen H H2 colorless gas Nitrogen N N2 colorless gas Oxygen O O2 colorless gas Fluorine F pale yellow gas Cl F2 Cl2 Chlorine Bromine Br Br2 reddish-brown liquid Iodine I I2 purple-black solid yellow/green gas 2 Rules for Naming Compounds 3 types of compounds you will learn how to name in today‟s lab are: I. Molecular compounds (two non-metals). II. Ionic compounds (contain a cation and an anion, often metals combined with non-metals). III. Acids. I. Molecular Compounds (for cases with two non-metals, no true ions present): a. Name the first element present and then the second element. Usually, the first element is located furthest left and/or furthest down on the periodic table. b. The suffix –ide should be used with the second element present. c. Use prefixes with molecular compounds. Use mono-, di-, tri-, tetra-, penta-, hexa-, etc. to indicate the number of atoms of each element. “Mono-” is usually omitted from the firstnamed element. Examples (we will cover all examples together, but practice now on your own!) ICl CCl4 NO2 S2O3 II. Ionic Compounds (contain a cation and an anion): a. Never use prefixes with ionic compounds. b. Common cations and anions are listed on later pages. c. If the cation has only one possible oxidation state „charge‟, simply name the cation and the anion. Examples (remember to practice now!) CaCl2 AlCl3 NH4NO3 BaCrO4 d. If the cation is a transition metal, it often has more than one possible oxidation state (e.g. Fe). Such cations are listed as cations with variable valences. Use Roman numerals with no space between the cation and the numeral to identify metal oxidation state with transition metals. Therefore, the Cu2+ ion would be called copper(II). An older, outdated nomenclature system that you may still come in contact with uses the suffixes –ous and –ic to signify a lower and higher oxidation state, respectively. This system, however, can be unclear when several charges are possible and the Roman numeral system is currently the proper nomenclature method. Examples FeCl3 Fe(NO2)2 3 III. Acids a. Acids usually contain the H+ ion as the cation. If this is the case, you have an acid. b. The name of the acid is based upon the name of the anion. c. Determine the anion in your acid. If the anion ends with –ide, the acid is named by using the hydro– prefix to the anion name and replacing the –ide suffix with –ic. This term is followed by the word acid. If the anion ends in –ate, replace the –ate ending with –ic. This term is followed by the word acid. If the anion ends in –ite, replace the –ite ending with –ous. The term is followed by the word acid. Examples: HNO2 HNO3 HCl HClO4 Note: These rules apply to acids present in aqueous solution. Many acids have other common names when present as a pure compound in the gaseous state. Examples of this are hydrogen chloride gas, HCl(g), hydrogen sulfide gas, H2S(g), and hydrogen iodide gas, HI(g). In this exercise, assume that all acids are present in aqueous solution. 4 Experiment 2 – Pre-lab Assignment * Name: _________________________________ Lab Day and Time: _________________ 1. Circle the elements below that are metals and underline those that are non-metals. (0.5 points) C 2. Mg Fe P H Si K N Zn F a) List three cations that have a charge of +2 (0.3) b) List three anions that have a charge of -1 (0.3) 3. Consider the two compounds FeCl2 and FeCl3. How do the iron ions in these two compounds differ (besides the fact that they form ionic bonds to a different number of chlorides)? (0.5) 4. All of the acids that we consider in this exercise have which element present in their molecular formula? (Note: this element is not necessarily present in all acids, just those that we consider for this lab exercise) (0.4) 5 Cations Cations (1+ charge) Cations (2+ charge) H+ hydrogen Be2+ beryllium Li+ lithium Mg2+ magnesium Na+ sodium Ca2+ calcium K+ potassium Sr2+ strontium Ag+ silver Ba2+ barium Zn2+ zinc NH4+ ammonium Cations (3+ charge) Al3+ aluminum Cations with common variable valences Cr2+ chromium(II) Cr3+ chromium(III) Mn2+ manganese(II) Mn3+ manganese(III) Fe2+ iron(II) Fe3+ iron(III) Co2+ cobalt(II) Co3+ cobalt(III) Cu+ copper(I) Cu2+ copper(II) Sn2+ tin(II) Hg22+ mercury(I) Pb2+ lead(II) Hg2+ Mn4+ manganese(IV) Sn4+ tin(IV) Pb4+ lead(IV) mercury(II) Note: In an older, outdated system that you may encounter, the lower valences use the –ous suffix and the higher valences use the –ic suffix. Some examples are ferrous(Fe2+) and ferric(Fe3+); cuprous(Cu+) and cupric(Cu2+); stannous(Sn2+) and stannic(Sn4+); mercurous(Hg22+) and mercuric(Hg2+). 6 Anions Anions (1– charge) Anions (2– charge) F- fluoride O2- oxide Cl- chloride S2- sulfide Br- bromide CrO42- chromate I- iodide Cr2O72- dichromate H- hydride CO32- carbonate CN- cyanide SO32- sulfite OH- hydroxide SO42- sulfate ClO- hypochlorite S2O32- thiosulfate ClO2- chlorite HPO42- monohydrogen phosphate ClO3- chlorate ClO4- perchlorate MnO4- permanganate Anions (3– charge) C2H3O2- acetate PO43- H2PO4- dihydrogen phosphate NO2- nitrite NO3- nitrate HCO3- hydrogen carbonate (bicarbonate) HSO4- hydrogen sulfate (bisulfate) phosphate 7 * A. Naming Exercises [To be done during lab period] Provide the proper chemical name for the following compounds (0.1 point each) 1. HClO 2. SO2 3. Ca(NO2)2 *4. HI 5. HNO3 6. NO2 7. HClO2 8. FeSO3 9. SnCl4 10. Al2O3 11. Hg2SO4 12. CS2 13. BaCl2 14. Na3PO4 15. KClO3 16. NaH 17. Cu2SO3 18. N2O 19. CuCO3 20. Pb(NO3)2 21. AlPO4 22. Fe2(SO4)3 23. KC2H3O2 24. NH4Cl 25. NaHCO3 26. SnO2 27. KMnO4 28. Na2S2O3 29. CCl4 30. H2SO3 31. SO3 32. Co2(CO3)3 33. Cu2O 34. NaNO2 *35. H2S 36. P2O3 37. ZnCr2O7 38. Hg2SO4 *39. HCN 40. NaCl * Assume these to be in the aqueous form. 8 * B. Naming Exercises [To be done during lab period] Write a chemical formula for the following compounds (0.1 point each) 1. potassium chlorate 2. aluminum oxide 3. magnesium phosphate 4. calcium bromide 5. potassium thiosulfate 6. barium acetate 7. chromium(III) chloride 8. sulfurous acid 9. sodium chromate 10. nitrogen dioxide 11. potassium hydroxide 12. hydrofluoric acid 13. ammonium chloride 14. phosphoric acid 15. hydrogen cyanide 16. potassium fluoride 17. cobalt(II) fluoride 18. sodium hydrogen carbonate (sodium bicarbonate) 19. mercury(I) chloride 20. tin(II) iodide 21. mercury(II) oxide 22. lead(II) chloride 23. iron(II) sulfate 24. sodium hypochlorite 25. silver(I) nitrate 26. copper(II) sulfate 27. manganese(IV) oxide 28. potassium dichromate 29. carbonic acid 30. aluminum sulfate 31. aluminum phosphate 32. potassium sulfate 33. sulfuric acid 34. potassium dihydrogen phosphate 35. chromium(II) chloride 36. potassium permanganate 37. lithium chloride 38. ammonium sulfate 39. zinc(II) chloride 40. chromium(II) nitrite 9 EXPERIMENT 3 – Measurement of Mass and Volume GOAL The goal of this lab is to calculate the density of water as accurately and precisely as possible. OBJECTIVES After completion of this experiment, students should be able to do the following: 1. Define: mass, volume, density, accuracy, precision 2. Given the mass of a sample of a pure substance and its volume, calculate the density of the substance. 3. Given a collection of mass and volume data for sample, construct a graph that would allow the determination of the density of the sample, using all data points. 4. Given the density of a pure substance and its volume, find the mass of the sample. 5. Given the density of a pure substance and its mass, find the volume of the sample. 6. Perform simple algebraic manipulations, keeping track of the significant figures contained in the result. 7. Name four types of volumetric containers and list them in order of accuracy. 8. Distinguish between volumetric (or “transfer”) pipettes and Mohr pipettes. 9. Explain the difference between “To Deliver” and “To Contain.” 10. Define “meniscus.” 11. Describe the best way to read a graduated cylinder or pipette. 12. Distinguish between mass and weight. 13. Explain the difference between an “extensive property” and an “intensive property”. 14. Explain the difference between “accuracy” and “precision”. 15. Know the SI units for base quantities and derived units. INTRODUCTION Is there a difference in the density of aqueous (water-based) solutions based upon what has been added? You will attempt to determine the answer this question by making precise measurements of mass and volume. We will be investigating regular soda pop, which contains dissolved suger in the form of sucrose, and diet soda pop that uses an artificial sweetener. Because of the strong flavor of the sweeteners used, the amount of artifical sweetener used in diet soda is much less than the amount of sugar used in regular soda. The samples we will use are „flat‟, meaning that the dissolved carbon dioxide has been allowed to escape the solution. Many experiments require some type of measurement, and often these are simple measurements of mass and volume. The validity of an experiment will probably depend on the reliability of these measurements. A measurement's reliability is usually considered in terms of its accuracy and precision. The accuracy of a measurement describes how close it is to the true value, whereas the precision describes the reproducibility of the measurement. Reliable results (such as those that would be made in industry, research or hold up in a court of law) often must be made as precisely as possible, within the limitations of the measuring device and technique. This precision does not guarantee, however, that the results are accurate. The outcome is often influenced by systematic errors that are independent of the instrument‟s precision or the scientist‟s technique. In this laboratory, you will be precisely measuring the mass and volume of liquid samples in order to determine their densities. Specifically, you will determine the densities of solutions of water, regular soda pop and diet soda pop. You will need use precise techniques in order to observe differences between the solutions. 1 Balances The lab instructor will provide details concerning the operation of the balances in your laboratory and the precision that you can expect. You should be able to achieve the maximum precision offered by your balance almost immediately. ATTENTION: Always keep these instruments neat, dry, and clean! Transfer and Mohr Pipettes A transfer pipette is calibrated to deliver a specified volume of a liquid. Mohr pipettes allow the user to measure a range of volumes of a liquid. Transfer pipettes will be used in this laboratory. Correct use of pipettes require a good deal of coordinative skill. As with any other skill, practice is mandatory. The precision that you can achieve with a pipette will be dependent on the time you devote to practice. The correct use of a pipette, including an explanation of the markings “TD” and “TC”, which are seen on many pipettes, will be demonstrated by your laboratory instructor. CAUTION: Never use your mouth to draw a liquid into the pipette, even if the liquid is water. Use a suction bulb. PROCEDURE You will be able to practice using a balance and a transfer pipette in order to gain confidence. Next, you will measure the mass of a flask using a digital balance. You will add water to the flask from a filled 10-mL pipette, and then measure the mass of the flask and water. You will repeat this process three more times. After calculating the mass of the water that was delivered each time from the pipette, you will determine the mass of your water sample. Make certain that you report the correct number of significant figures in each calculation. Once you have completed the measurements with water, you will repeat them with regular soda and diet soda. From your data, you will be able to determine the mass of your solutions using graphical techniques. Getting started 1. Obtain about 100 mL of distilled water in a beaker. Also, obtain about 50 mL of diet soda and 50 mL of regular soda in separate, labeled beakers. 2. Obtain a 10-mL pipet, a thermometer, and a 50-mL Erlenmeyer flask with a rubber stopper or cork. 3. Plan on using the same analytical balance and the same pipet throughout the experiment. 2 Using the Balance and Pipette 1. The lab instructor will provide you with instructions for operation of the balances in the weighing room. 2. Practice with your pipette using distilled water until you are comfortable with the technique and confident in its use. 3. Using the thermometer, note the temperature of all three solutions in your beakers. To avoid contamination, be sure to dry the thermometer before moving it between solutions. All temperatures should be equivalent to each other within one degree Celsius. Record the temperature to the nearest degree in part A of the Data and Calculations page. Record also the density of water determined from Table 1. This value will also be reported in Part A on your Report sheet. This is the expected value for the water density that you will determine graphically. 4. Read ahead to determine what data you will need in Part B of the Data and Calculations page. Take the stoppered flask to the weighing room. Measure and record neatly the mass to the correct number of significant figures. 5. Remove the flask from the balance. Go back into the lab and pipette exactly 10.00 mL of the room-temperature water into the flask without touching the flask with your fingers. Replace the stopper to prevent evaporation. Do not pipet liquids in the weighing room. 6. Return to the weighing room with the stoppered flask containing the liquid. Measure and record the combined mass of the liquid and the stoppered flask. (Be neat and have the correct significant figures!) 7. Remove the flask from the balance. Go back into the lab and pipet another 10-mL sample into the flask. Do not pour out the first sample. The volume of liquid in the flask should now be 20.00 mL. Replace the stopper and reweigh. 8. Repeat until 4 samples of water have been delivered to the flask and the final volume is 40.00 mL. 9. Empty, clean and dry the flask. Rinse the pipet with a small amount of the next solution to be analyzed be drawing a few mL into the pipet, swirling and letting drain into the sink. 10. Repeat steps 5-9 with the second, and then the third solution. Report all of your data on the DATA and CALCULATIONS sheet. Table 1. Density (g/mL) of Water at Various Temperatures (C) TEMP. 17 18 19 20 21 DENSITY 0.9988 0.9986 0.9984 0.9982 0.9980 TEMP. 22 23 24 25 26 DENSITY 0.9978 0.9976 0.9973 0.9971 0.9968 TEMP. 27 28 29 30 31 DENSITY 0.9965 0.9962 0.9959 0.9956 0.9953 3 Determination of Density 1. For the most precise determination of density using all of the data you have collected, you will perform a graphical analysis for each of the three solutions. You will construct a graph using Excel on computers in Zurn 203. Because we are expecting a linear relationship between the mass and volume of the solution, a linear regression can be performed on each data set. The resultant slope from this regression analysis will correspond to the density. 2. With the tips for using Excel in the introduction to the lab manual, plot the mass of solution for your data points (y-axis) vs. the total volume of solution added (x-axis). Be sure that the variable you want for the x-axis is in the first column and that the proper number of decimal place are displayed. (Refer to the Excel page in your manual). To plot all three solutions on one graph, use the second, third and fourth columns in Excel to enter the masses of the liquid from the water, regular soda and diet soda, respectively. Include mass = 0.0 and volume = 0.0 as your first data point. Perform a linear regression to determine the slope for each of your data sets, and therefore the density of the solutions. (Remember, you will obtain the mathematical form: y = slope x + b). You should be able to determine density to four significant figures!! 3. Report your density values on the Report Sheet. 4. Observe what happens when a can of regular soda and diet soda are placed into a container of water. (Post-lab question 1 refers to your observation) 4 Experiment 3 – Pre-lab Assignment * (use your general chemistry textbook as a reference) Name: ________________________________ 1. Lab Day and Time: _________________ Explain the difference between “accuracy” and “precision.” _____________________________ _________________________________________________________________________________ _________________________________________________________________________________ _______________________________________________________________________(0.2 pts.) 2. a. Perform the subtraction 18.278 g – 6.118 g. Report the answer with the correct number of significant figures. (0.3) b. Perform the multiplication 2.812 g/ml 0.60 ml. Report the answer to the correct number of significant figures. (0.3) 3. Give the SI units for: (0.2) length ______ time ______ mass ______ volume ______ 4. area ______ temperature ______ Suppose that after you plot mass(g) vs. volume(mL) data from a solution and perform a linear regression, Excel determines the following best-fit line equation: y = 0.9986 x + 0.0023 What is the density of your solution? (0.5) 5 Experiment 3 – Data and Calculations Name: __________________________ * Lab Day and Time: _____________________ A. Determine the density of your water sample from Table 1. Include the correct units! (0.5) Water temperature: __________ Water density from Table 1: ___________ B. As neatly as you possibly can, construct three data tables – one for each solution – below. Each data table should have three columns. These will correspond to the (i) volume added, (ii) the mass measurements that you recorded, and (iii) the calculated mass of liquid present in the flask. All values should be reported with the correct number of decimal places. Label the columns and rows in your table and indicate the units that are used, either directly or in the labels. (2.0) Mass of stoppered 50 ml flask: __________ Solution 1: Distilled Water Solution 2: Regular Soda Pop Solution 3: Diet Soda Pop 6 Experiment 3 – Report Sheet * (Be sure to include all units and the proper number of significant figures!) A. Density of water determined from temperature measurements. ____________________ (0.5) B. Density of solutions determined from graphical analysis. Include a copy of your plot(s) that are wellorganized, labeled and clearly indicate your densities were found. (2.0 ) (i) Density of water: (ii) Density of regular soda pop: (iii) Density of diet soda pop: Include your printed (or saved) graph with your lab report. The Excel data sheet I not needed. One copy is required for each individual. Make sure your name is included. C. Percent error between the graphical value of water density, B(i) and the value determined from temperature considerations, A. To determine this value, find the absolute value of the difference between the two and divide by the expected density. The expected density is that found from temperature considerations. Multiply by 100%. (1.0) % error _________________ 7 Experiment 3 – Post-lab Questions * 1. Do your observations of the regular soda and diet soda placed into water correspond with your results? Why or why not? (0.5) 2. Why is there a difference between the regular soda and the diet soda? (0.5) 3. Calculate the expected volume of water that would be contained in 5.211 g of water at 30 C. The density of water at 30 C is 0.9956 g/mL. (0.5) 4. Using the data in Table 1, would you expect colder or warmer water to be on top of a lake? How does this compare to what you know about ice formation on lakes? This table only goes down to 17C. What must happen to the water density as it approaches its freezing point, 0C? (0.5) 8 EXPERIMENT 4 – Ionic Reactions in Aqueous Solutions Goal: To write and balance Molecular, Total Ionic and Net Ionic equations for common reactions. Objectives After completing this experiment, students should be able to do the following: 1. Define: (a) (c) (e) (g) (i) (k) (m) solubility; insoluble; centrifugation; acid; neutralization; ionic equation; electrolyte. (b) (d) (f) (h) (j) (l) precipitation; molarity; metathesis reaction; base; molecular equation; net ionic equation 2. Be able to write the molecular reaction products for (a) two aqueous ionic compounds undergoing metathesis; (b) common neutralization reactions. 3. Be able to convert molecular equations into ionic and net-ionic equations. 4. Be able to use litmus paper to check the acidity of an aqueous solution. INTRODUCTION Reactions in aqueous solutions have far-reaching importance. These reactions occur in our homes as well as in rivers, lakes, and oceans, in biological systems such as our bodies, and in many industrial applications. Most of these reactions involve ions. You will examine precipitation reactions and reactions of acids and bases. You will have an opportunity to examine certain precipitation reactions and test the solubility rules shown in Table I (see next page). You will also examine a reaction in which a gas is formed and some reactions of acids and bases. You will find that H+ ions from acids cause blue litmus paper to turn red or pink. Similarly you will see that OH- ions from bases cause pink litmus paper to turn blue. Moreover, you will find that the liberation of heat, a signal of a chemical reaction, accompanies the reaction of an acid with a base. A Word About Molarity In this experiment, you will encounter the symbol M. It stands for molarity and has units of moles per liter (mol/L). Molarity is a measure of the concentration of a solution. 1 Table I: Empirical Rules for the Solubilities of Common Ionic Compounds Soluble Compounds Nitrates, NO3- Exceptions None Acetates, C2H3O2- None Halides (Cl-, Br-, I-) Insoluble with compounds of Ag+, Cu+, Hg22+ and Pb2+ Sulfates, SO42– Insoluble with compounds of Ca2+, Sr2+, Ba2+, Ag+ and Pb2+ Insoluble Compounds Sulfides, S2- Exceptions Alkali metal ions and NH4+ compounds Be2+, Mg2+, Ca2+, Sr2+, and Ba2+ Carbonates, CO32- and phosphates, PO43- Alkali metal ions and NH4+ compounds Hydroxides, OH– Alkali metal ions and NH4+ compounds Ca2+, Sr2+, and Ba2+ PROCEDURE 1. Work in pairs. You may split-up the solubility tests between you and your partner. Each person (“Person A” and “Person B” in the procedure) needs 4 test tubes. 2. Obtain several pieces of red and blue litmus paper and 4 small test tubes. 3. Obtain instructions for using the centrifuge in the laboratory. CAUTION! When you use a centrifuge, always wear safety glasses, tie back long hair, balance the weight in the centrifuge rotor, and never leave a running centrifuge unattended. 4. Discard the solutions that you use during this experiment in an inorganic waste container. CAUTION! Sodium hydroxide and ammonia are common laboratory bases. Hydrochloric acid and acetic acid are common laboratory acids. These chemicals can cause burns in addition to ruining your clothes. If you spill any of these solutions on you, immediately rinse the contaminated area thoroughly with tap water. Heavy metal ions such as Ag+, Ba2+, and Pb2+ can be toxic when present in high concentrations. We cannot pour solutions containing heavy metal ions down the sinks in the lab. All solutions containing these ions must be poured into inorganic waste containers in the laboratory. You are encouraged to wear disposable gloves for today’s experiment. 2 Part 1, Testing the Solubility Rules Person A 1. Add 20 drops of 0.1 M NH4NO3 to each of your four test tubes. 2. Now, add 20 drops of 0.1 M NaBr to the first tube, 20 drops of 0.1 M Na 2SO4 to the second, 20 drops of 3 M NaOH to the third, and 20 drops of 0.1 M Na2CO3 to the fourth. Shake each test tube gently. Record your observations, noting the colors of all precipitates. If a precipitate initially forms, then disappears upon shaking, note that observation in your data chart. Do not be discouraged if no precipitates form. 3. Discard the contents of the tubes into the inorganic waste container. 4. Wash the test tubes carefully, and rinse them with distilled water. 5. Repeat Steps 1 through 4, substituting 0.1 M Ba(NO3)2 for the 0.1 M NH4NO3 in all tubes. 6. Repeat Steps 1 through 4, substituting 0.1 M AgNO3 for the 0.1 M NH4NO3 in all tubes. Person B 1. Add 20 drops of 0.1 M Pb(NO3)2 to each of your test tubes. 2. Now, add 20 drops of 0.1 M NaBr to the first tube, 20 drops of 0.1 M Na 2SO4 to the second, 5 drops of 3 M NaOH to the third, and 20 drops of 0.1 M Na2CO3 to the fourth. Shake each test tube gently. Record your observations in your chart, noting the colors of all precipitates. 3. Empty the contents of all four tubes into the designated inorganic waste container in the lab. Rinse the tubes with distilled water from your wash bottle. 4. Now, add 20 drops of 0.1 M Ni(NO3)2 to each of your tubes, and repeat Step 2. For the tube where you combined Ni(NO3)2 and NaOH, perform Step 5. 5. Centrifuge the contents of the test tube that originally contained 0.1 M Ni(NO3)2 and 3 M NaOH. About 1 minute will be required. Decant (pour off) and discard the solution into the inorganic waste container. Hold onto the centrifuge tube containing the precipitate for subsequent use. 3 Part 2, Looking at Acids and Bases Person A 1. Wash all of your test tubes, except the one containing the precipitate formed by the reaction between Ni(NO3)2 and NaOH (this tube was prepared by Person B in step 5). Person B will use this tube for additional analysis. Rinse all of your other tubes with distilled water from your wash bottle. 2. Take your spatula to the bottle containing solid CaCO3. Obtain a pea-sized portion of CaCO3. Place it into one of your test tubes. Add 20 drops of 3 M HCl. Record your observations. If the sample does not completely dissolve, add more HCl. Clean the test tube. 3. Add 20 drops of 3 M HCl to one test tube, 20 drops of 3 M HC2H3O2 (acetic acid) to a second tube, 20 drops of 3 M NH3 to a third tube, and 20 drops of 3 M NaOH to the fourth. 4. Obtain two strips of red litmus paper. Tear each strip in half, giving you four small pieces. One solution at a time, place a clean stirring rod into each solution, and touch the rod to one of the small pieces of red litmus paper. Record your observations. 5. Repeat Step 4 using two strips of blue litmus paper. 6. Save the four solutions of acids and bases from step 3 for Person B. Person B 1. Add 15 drops of 3 M HCl to the centrifuge tube containing the precipitate that was saved from the reaction between Ni(NO3)2 and NaOH. Stir gently using a disposable pipette. If you stir too vigorously, you will break-off the tip of the pipette. Record your results. 2. Place a thermometer into the test tube containing the NH3 (after Person A completed the litmus paper tests in steps 4-6). Add the contents of the test tube containing HCl to the test tube containing NH3. Is heat evolved? Record the results. 3. Place a thermometer into the test tube containing the NaOH. Add the contents of the test tube containing HC2H3O2 to the test tube containing NaOH. Is heat evolved? Record the results. 4 Experiment 4 – Pre-lab Assignment Name: ______________________ 1. * Lab Day and Time: _____________ (a) What is a methathesis reaction? (0.1 point) (b) In this experiment, what type of evidence will you observe that points to the occurrence of a metathesis reaction? (0.1 point) 2. 3. Predict the solubilities of the following substances in water using Table I, found in the theory section of this experiment. These substances are relevant to this experiment. (0.5 point) a. NaNO3: e. AgBr b. NH4Br: f. (NH4)2SO4: c. AgOH: g. Pb(OH)2: d. Ag2CO3: h. PbCO3: Some of the compounds involved in this experiment are common acids or bases that are frequently used in laboratory work. Give names and formulas for 2 common acids 2 common bases that you will encounter in this experiment. (0.3 point) 5 Experiment 4 – Results (Part 1, Testing the Solubility Rules) * Name: ___________________________________ (1 point) NaBr Na2SO4 NaOH Na2CO3 NH4NO3 Ba(NO3)2 AgNO3 Pb(NO3)2 Ni(NO3)2 6 Experiment 4 – Results (Part 2, Looking at Acids and Bases) * A. Reactants Observation HCl + CaCO3(s) ________________________________________________ (0.5) HCl + ppt. from [Ni(NO3)2 + NaOH] ______________________________________ (0.5) B. (0.5) Red Litmus Paper Blue Litmus Paper HCl HC2H3O2 NH3 NaOH C. (0.5) Is heat evolved? HCl + NH3 HC2H3O2 + NaOH 7 Experiment 4 – Post-lab Questions I. * Write balanced molecular, complete ionic, and net ionic equations for only 5 of the precipitations that you observed. Choose only those reactions that formed precipitates! You can write your reactions on below. You must include the charges of all ions and the states, (aq), (s), or (l) for all species for full credit. (0.5 point each) The solubility rules given for this lab are based upon a solubility of 0.01 mol/L or greater. Your observations may or may not match directly with the solubility rules in Table I due to varying concentrations and/or contaminated samples. If you chose to write molecular, ionic, and net ionic equations for a precipitation that doesn’t conform to the solubility rules of Table I, mark alongside the reactions that you write that there is a discrepancy with the solubility rules of Table I. 1. Molecular: __________________________________________________________________________ Complete Ionic: _____________________________________________________________________ Net Ionic: ___________________________________________________________________________ 2. Molecular: __________________________________________________________________________ Complete Ionic: _____________________________________________________________________ Net Ionic: ___________________________________________________________________________ 3. Molecular: __________________________________________________________________________ Complete Ionic: _____________________________________________________________________ Net Ionic: ___________________________________________________________________________ 4. Molecular: __________________________________________________________________________ Complete Ionic: _____________________________________________________________________ Net Ionic: ___________________________________________________________________________ 5. Molecular: __________________________________________________________________________ Complete Ionic: _____________________________________________________________________ Net ionic: ___________________________________________________________________________ 8 * II. These reactions are difficult and may require you to make educated guesses about products. Part A. Write balanced molecular, ionic, and net ionic equations for each reaction of a solid with HCl. (Hint: Look at section 4.3 in your textbook and use your observations) (i) Calcium carbonate and HCl (1 pt) (molecular) CaCO3(s) + HCl(aq) (ionic) (net ionic) (ii) Nickel nitrate and HCl (1 pt) In the first step nickel hydroxide solid was formed in a precipitation reaction. Ni(NO3)2(aq) + 2NaOH(aq) Ni(OH)2(s) + 2NaNO3(aq) Write equations for the acid base reaction that you performed in the second step. (Hint: a common product of acid-base reactions is H2O(l) ) (molecular) Ni(OH)2(s) + HCl(aq) (ionic) (net ionic) Part B. (i) Which color litmus paper do you use to test for an acid? __________________ (0.25) (ii) Which color litmus paper do you use to test for a base? ___________________ (0.25) Part C. Below or on the back of this sheet. write balanced molecular, ionic, and net ionic equations for each neutralization reaction that you tested for heat evolution: (i) HCl and NH 3, (ii) HC2H3O2 and NaOH. Give your best guess for the products of reaction (Hint: an acid-base reaction is one in which an H+ ion is transferred from the acid to the base). (1 pt) 9 Experiment 5 – The Decomposition of Potassium Chlorate Goal: To determine the identity of a product using the ideal gas law. Objectives 1. Use the universal (ideal) gas law for solving problems. 2. Explain what is meant by the term “vapor pressure”. 3. Explain how Dalton’s Law of Partial Pressure is demonstrated in this experiment. 4. Determine the number of moles of gas that was produced in the collection. Introduction Small quantities of molecular oxygen (O2) can be obtained from the thermal decomposition of certain oxides, peroxides, and salts of oxyacids. Some examples of these reactions are 2Ag2O(s) 4Ag(s) + O2(g) 2BaO2(s) 2BaO(s) + O2(g) Concept of the experiment: The thermal decomposition of KClO3 will be studied. You will be able to identify the solid that remains after the decomposition from the quantity of oxygen that is evolved. You will verify this identification by comparing the measured mass of the solid product with a calculated value. There are three possibilities for the solid product that results from the thermal decomposition of KClO3. The solid product could be either KCl (potassium chloride), KClO2 (potassium chlorite) or KClO (potassium hypochlorite). The prelab introduces three possible reactions, and the experimental data will answer which product forms. A sample of KClO3 of known mass in the absence of a catalyst will be heated until the evolution of oxygen is complete. Oxygen will be collected in a flask by the displacement of water. Here, the volume of water displaced equals the volume of O2 gas produced. In order to determine the correct stoichiometry of this reaction, the number of moles of O2 that have been evolved must be obtained. This quantity can be calculated from the rearranged form of the ideal gas law: n PV RT where P refers to the partial pressure of oxygen in the collected gas mixture, V will equal the volume of water displaced, T is the Kelvin temperature of the gas mixture, and R is the gas constant. A commonly used value for R is 0.082056 L·atm mol–1 K–1. If this value for R is used, then P must be expressed in atmospheres and V in liters. Since the oxygen is collected over water, water vapor will also be present in the gas. The experiment is designed so that the sum of the pressures of the oxygen and water vapor will be equal to the atmospheric pressure: PAtmosphere PO 2 PH 2 O CHEM 122 – Experiment 5 1 One can easily measure atmospheric pressure with a barometer. The partial pressure of oxygen in the flask can then be calculated if the vapor pressure of water is known. Table 1 gives the vapor pressure of water at various temperatures. Figure 1 shows the apparatus for this experiment. The sample of KClO3 is placed in the test tube and the Florence flask is filled with water. Some of the water is displaced by oxygen and is pushed into the beaker. The volume of water in the beaker will be identical to the volume of oxygen in the flask. Procedure 1. Obtain and clean the glassware for the apparatus in this experiment. 2. Obtain and record the atmospheric pressure from a barometer supplied by your instructor. 3. The solid product that will be produced in this experiment can be washed into the sink once the reaction is complete. 4. Observe the following safety precaution throughout this experiment: Caution: KClO3 is a very strong oxidizing agent. Make certain to place the lid back on the bottle containing the KClO3 after obtaining the sample. Do not let this substance contact paper or the rubber stopper in the test tube belonging to the apparatus. 5. Assemble the apparatus as shown in Figure 1 and as demonstrated by the instructor. 6. Fill the beaker with approximately 2 inches of water. Fill the Florence flask with tap water, so that the level of the water is about 1 inch below the short glass tube. Open the pinch clamp. 7. Remove the stopper from the test tube. Use a suction bulb to force air through the glass tube until the rubber tube is filled with water. 8. Close the pinch clamp near the end of the tubing where the water will exit. 9. Make sure that the test tube is clean and dry. Take the test tube and a clean, empty, dry 400 mL beaker to the top-loading balances. Tare-off the mass of the empty beaker. Place the test-tube in the beaker. Measure and record the mass of the empty test tube to the nearest 0.01 g. 10. Make sure that the test tube is cool. With the test tube in the beaker, add a small amount of KClO3 into the test tube. Weigh the apparatus. Continue to add KClO3 until about 1.0 g of KClO3 has been added into the test tube. A sample in the mass range of 0.9 g to 1.1 g of KClO3 is fine. 11. Record the exact mass of the KClO3 reactant. 12. Clamp the test-tube to the ring stand and stopper the test tube, as shown in Figure I. 13. Open the clamp. Lift the beaker with your hands until the water level in the beaker is equal to the water level in the Florence flask. When the water levels are equal, have your partner close the pinch clamp. This equalizing process will ensure that the pressure acting on the water in the beaker (atmosphere) is equal to the pressure acting on the water in the flask. 14. Empty the beaker but do not dry it. The volume of the water drops that remain in the beaker will be roughly equal to the volume that will remain after the displaced water is poured into a graduated cylinder for measurement. CHEM 122 – Experiment 5 2 15. Place the glass tube (connected to the hose) back into the beaker. MAKE CERTAIN THE PINCH CLAMP IS OPEN! Caution: If the clamp is not opened at this point, the build-up of gas during heating could cause an explosion, although it is more likely that a stopper would be forced to loosen. Also, make certain that the longer glass rod is not touching the bottom of the Florence flask. This would also result in a closed system and an explosion could result. 16. Heat the test tube as shown in Figure 1. Be cautious at first and brush the flame over the test tube. After a few minutes, heat the test tube more strongly. The solid will melt, oxygen will be evolved, and water from the flask will be displaced into the beaker. A significant volume of water should be displaced due to the reaction. 17. Heat the solid thoroughly until no more gas is evolved. The contents of the test tube will solidify, since the melting point of the product is greater than that of KClO3. 18. Turn off the flame and allow the system to come back to room temperature. Allow five minutes for this process. 19. As in step 14, equalize the water levels (this may require lifting the Florence flask) and close the clamp. 20. Remove the tube from the beaker. Measure the volume of water in the beaker by pouring it into a 100 mL graduated cylinder as many times as necessary. Record the volume. Do not use a beaker to measure the volume of water. 21. Measure the temperature of the water to the nearest degree. We will assume this is the temperature of the gas. Use it to determine the appropriate vapor pressure of water from Table 1. 22. Once cool, obtain the mass of the test tube and its contents. Calculate and record the mass of the product. 23. Repeat steps 6-22 with a second sample of KClO3. 24. Repeat a third trial if deemed necessary to make an accurate determination of the product. Caution: Before you leave the laboratory, make sure that all gas outlets are closed. Add distilled water using a squirt bottle to assure that all KClO3 has reacted. Thoroughly rinse your test tube with tap water. 25. Use the data to determine the reaction that occurred by two different methods. First, the determination can be made based upon the number of moles of oxygen gas that were produced in the reaction. Secondly, the mass of the solid product can be analyzed to determine which reaction occurred. Draw conclusions based on these determinations and error analysis. CHEM 122 – Experiment 5 3 Figure 1: A diagram of the laboratory setup used in this experiment. Fill w/ water using bulb Bulb goes here to force air through, which forces H2O through the second tube (fill it). Pinch clamp (clamp once water is ~ 2 inches in beaker) OPEN during filling w/ water and heating! Fill to ~ 2 inches high, then empty Bunsen burner Florence flask (400 or 600 mL beaker) (NOT touching the bottom!) (Make sure these are the UNCOATED CLAMPS!) Table 1: Vapor Pressure of Water (data from the CRC Handbook of Chemistry and Physics, 49th edition, 1968.) TEMPERATURE (C) 16 17 18 19 20 21 22 23 24 25 VAPOR PRESSURE (mm Hg) 13.6 14.5 15.5 16.5 17.5 18.7 19.8 21.1 22.4 23.8 TEMPERATURE (C) CHEM 122 – Experiment 5 26 27 28 29 30 31 32 33 34 35 VAPOR PRESSURE (mm Hg) 25.2 26.7 28.3 30.0 31.8 33.7 35.7 37.7 39.9 42.2 4 Experiment 5 – Pre-laboratory Assignment Name: ________________________________________ * Lab Day and Time: _____________ 1. Write a balanced chemical equation for each of the three possible reactions that could occur when potassium chlorate (KClO3) is thermally decomposed. One possibility is that the products would be potassium chloride (KCl) and molecular oxygen. A second possibility is that the thermal decomposition yields potassium hypochlorite (KClO) and molecular oxygen. A third possibility is that the reaction products would be potassium chlorite (KClO2) and molecular oxygen. (When complete, record the reactions on the data sheet.) Reaction #1: Reaction #2: Reaction #3: 2. Suppose the atmospheric pressure during the experiment was 751 mmHg. The temperature of the water was found to be 23.0 °C. What is the pressure of the oxygen gas, PO2, that is produced? CHEM 122 – Experiment 5 5 Experiment 5 – Data * Organize all data and calculations in the clearest and neatest way. Be sure to include the proper number of significant figures and units. Include data taken to obtain the mass of KClO3 reacted, the volume of water displaced, the temperature and vapor pressure of water, and the mass of the product. Include at least two trials. Atmospheric pressure: ________ mm Hg Reactions from Pre-lab Reaction #1: KClO3(s) Reaction #2: KClO3(s) Reaction #3: KClO3(s) CHEM 122 – Experiment 5 6 Experiment 5 – Calculations * Method 1: Volumetric Analysis of Gaseous Product Show all calculations for determining which reaction occurred based upon the volumetric analysis of the gas. Use the ideal gas law to determine the moles of oxygen gas produced and compare this to the expected number of moles of oxygen gas produced for each of the three reactions. For each trial, you should have 3 values for the expected number of moles based upon the three possible reactions. Method 2: Gravimetric Analysis of Solid Product Show all calculations for determining which reaction occurred based upon the mass of the solid product. Determine the expected mass of the solid product for each of the three possible reactions. CHEM 122 – Experiment 5 7 Experiment 5 – Post-laboratory Questions * 1. Which of the three potential reactions occurred? Explain your answer based upon your results and an analysis of the major errors in the experiment. The error analysis should correspond to your conclusions with an explanation for why your results may be too high or too low. 2. Suppose a reaction was run that produces hydrogen gas and a sample of H2(g) is collected. As a result of the collection, 145 mL of water is displaced. The pressures of the gas and the atmospheric pressure are equalized using the method described in this experiment. The atmospheric pressure is 745 mm Hg. The temperature of the water and gas are identical at 22 C. How many moles of H2(g) have been evolved? CHEM 122 – Experiment 5 8 EXPERIMENT 6 – Thermochemistry and Hess's Law Goal To determine the enthalpy change for reactions with calorimetry and to use Hess’s Law to calculate H. Objectives After completing this experiment, students should be able to do the following: 1. Define: (a) (c) (e) (g) thermochemistry; heat capacity; endothermic reaction; Hess’s Law. (b) thermochemical equation; (d) specific heat; (f) exothermic reaction; 2. Given the heat capacity of a calorimeter system, the moles of reactants participating in the reaction, and the temperature change for the reaction, find the values for q and H for the reaction. INTRODUCTION The energy changes that accompany chemical reactions are nearly always reflected by the release or absorption of heat. There are many practical and theoretical reasons for studying the quantitative aspects of this phenomenon. These studies are an application of thermochemistry. You will measure the enthalpy change for three reactions. The enthalpy change, H, under our lab conditions is equal to the amount of heat lost or gained during the reactions. First, you will measure the enthalpy change that occurs when solutions of sodium hydroxide (NaOH) and hydrochloric acid (HCl) are mixed. Then you will measure the enthalpy change that occurs when solutions of ammonia (NH3) and hydrochloric acid are mixed. Third, you will measure the enthalpy change that occurs when solutions of sodium hydroxide and ammonium chloride (NH4Cl) are mixed. The chemical reactions that will occur during this experiment are given in the following equations: Rxn. 1 Rxn. 2 Rxn. 3 NaOH(aq) + HCl(aq) NaCl(aq) + H2O(l) NH3(aq) + HCl(aq) NH4Cl(aq) NaOH(aq) + NH4Cl(aq) NaCl(aq) + NH3(aq) + H2O(l) The three chemical reactions that will occur in this experiment are examples of bases reacting with acids. You will learn much more about acids and bases in General Chemistry II. For now, simply recognize which species are the bases and acids in this experiment: Bases: NaOH and NH3 Acids: HCl and NH4Cl The heat evolved or absorbed during these reactions will be measured with a coffee-cup calorimeter. In order to determine the enthalpy change from a measured change in temperature, you will have to utilize the fundamental concepts of calorimetry and thermochemistry. You may or may not have gotten to this point in the lecture portion of the class, but you can refer to the Appendix at the end of this experiment for a step-by-step description and examples of the calculations you will perform in order to determine the enthalpy change for the three reactions, H1, H2, and H3. You should determine these with units of kJ/mol. Once you have determined your individual H values, class data will be pooled in order to 1 determine average H values for each of the three reactions. You will report your values and the class averages on the report sheet. Post-lab question 2 asks you to consider the value for H3 more closely. This question relies on the fact that you can use your values for H1 and H2 to calculate H3 directly. You can do this using the principles of Hess's Law. Note the following relationship among the studied reactions: If Rxn. 1 is combined with the reverse of Rxn. 2, the third equation is generated. This relationship provides the basis for using Hess's law of heat summation. The post-lab question asks you to attempt to predict the enthalpy change for the third reaction by combining the enthalpy changes for the first and second reactions. PROCEDURE 1. Work with a partner. 2. Obtain a coffee-cup calorimeter. The HCl-NaOH, HCl-NH3, and NH4Cl-NaOH Reactions 1. Obtain exactly 50.0 mL of the 3.0 M solution of HCl in a clean, dry graduated cylinder. Pour the liquid into your coffee-cup. Rinse the graduated cylinder with distilled water from your wash bottle. Dry the cylinder. Obtain exactly 50.0 mL of the 3.0 M NaOH using the graduated cylinder. Caution: Acids and bases can cause chemical burns in addition to ruining your clothing. If you spill one of these solutions on you, immediately rinse the contaminated area thoroughly with water. 2. Measure the temperature of the solution in the coffee-cup. Record this temperature as the initial temperature, Ti. 3. Add the NaOH solution to the calorimeter. 4. Immediately, place the top on the calorimeter and begin swirling the cup. Do not stir the cup with the thermometer! 5. Record the temperature to the nearest 0.1 C at the times listed in your data table. 6. Repeat the procedure of the HCl-NaOH reaction for trials involving the following acid base pairs and amounts: Rxn. 2: 50.0 ml of 3.0 M HCl, 50.0 ml of 3.0 M NH3. Rxn. 3: 50.0 ml of 3.0 M NH4Cl, 50 ml of 3.0 M NaOH 7. Using Excel, obtain a scatter plot of the temperature (y-axis) against the time (x-axis) on one set of axes. Print out your plots without performing a linear regression. Manually extrapolate your results to the time of mixing (time = 0 s) on the y-axis. This is done by drawing a straight line through your points and extending it back to t=0. The temperature at which your line crosses the axis is Tf. Record the Tf value for each of the three reactions. 8. Calculate H in units of kJ/mole for each reaction. Use 430 J/°C for the heat capacity of the calorimeter plus contents. See the appendix for an explanation of this calculation. 2 Experiment 6 – Pre-lab Assignment * Name: ______________________________________ Lab Day and Time: _____________ 1. If an exothermic reaction occurred in your calorimeter, would you expect the temperature to increase or decrease? Why? (0.5) 2. A stranger tells you that they had measured H for a reaction and had found a value of –80 kJ and another stranger tells you that H for the same reaction was measured to be –40 kJ/mol. Which piece of information is more useful? Why? (0.5) 3. a. When solutions of two reactants were mixed in a coffee-cup calorimeter, the following temperatures were recorded as a function of time. Perform the following steps in order to determine the final temperature, Tf, from your data. (i) Plot, by hand, the data on one of the available pieces of graph paper. Plot time on the x-axis and temperature on the y-axis. (ii) Determine the point where the solution begins to cool. (iii) Use this point and all of the points afterwards. Draw a straight line through these points (ignoring the first few points where the solution was still heating up). (iv) Extend this straight line back to the y-axis to find Tf. The initial temperature (or Ti), was 24.3C. Include the Ti point on your graph. Make sure you label your axes and put a title on your graph. (0.25) Time (s) 0 30 60 90 120 T (C) 24.3 38.6 39.7 39.5 39.4 Time (s) 150 180 210 240 T (C) 39.3 39.2 39.1 39.0 b. What is the value of T for the above example? (0.25) 3 4 Experiment 6 – Data * (1.5) Enthalpy Changes for the NaOH-HCl, NH3-HCl, and NH4Cl-NaOH Reactions. Concentration of HCl: _______ Concentration of NaOH: _______ Concentration of NH3: _______ Concentration of NH4Cl: _______ NaOH-HCl NH3-HCl NH4Cl-NaOH Volume of acid added (mL) ________ ________ ________ Volume of base added (mL) ________ ________ ________ Initial temperature of solution in calorimeter. TI(C) ________ ________ ________ 20 s ________ ________ ________ 60 s ________ ________ ________ 100 s ________ ________ ________ 140 s ________ ________ ________ 180 s ________ ________ ________ 220 s ________ ________ ________ 260 s ________ ________ ________ 300 s ________ ________ ________ Tf (C) from plot extrapolation ________ ________ ________ T (Tf –TI) ________ ________ Temperatures (C) after: ________ Results (show calculations for all of the following quantities on the next page) Heat evolved or absorbed by reaction (J) ________ ________ ________ Moles of reactant ________ ________ ________ H(kJ/mol) ________ ________ ________ 5 Experiment 6 – Calculations and Report * I. Neatly show all calculations for the heat H in units of kJ/mol. Use the method discussed in the appendix. Find H for each of your three reactions. (3) NaOH and HCl H1 = ____________ NH3 and HCl H2 = ____________ NaOH and NH4Cl H3 = _____________ II. Class data (Include your own.) (1) Reaction 1) NaOH-HCl H (kJ/mol) ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ 2) NH3-HCl H1(average) = ________ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ 3) NaOH-NH4Cl Average H H2(average) =________ ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ H3(average) =________ 6 Experiment 6 – Post-lab Questions * 1. The net ionic equation for the HCl-NaOH reaction is: H+(aq) + OH-(aq) H2O(l) Calculate the H expected for the above reaction using Hf data given in the thermodynamic data tables (usually in an Appendix section) of your chemistry textbook. Compare with your value of H for reaction 1. (1.5) 2. a. Use your class’s average H values for reactions 1 and 2 to calculate (using Hess’s law) the enthalpy change to be expected for the third reaction: NaOH(aq) + NH4Cl(aq) NaCl(aq) + NH3(aq)+ H2O(l) Show your reasoning below (0.75) b. The literature value for reaction 3 is -3.9 kJ/mol. Is the class average experimental value, H3(average) from the previous page, or the result from Hess’s Law most accurate?. Why do you believe this to be the case? Discuss in terms of an error analysis for this laboratory. (0.75) 7 Experiment 6 – Appendix Using a Coffee-Cup Calorimeter Polystyrene coffee cups make excellent calorimeters because of their ability to block the passage of heat. The complete calorimeter consists of two nested 6 ounce cups, a top, and a thermometer. Like any other calorimeter, the coffee-cup calorimeter provides the means to measure the heat flow between a system and its surroundings. The meaning of these words must be understood in terms of our calorimeter. Defining the Surroundings and the System We must begin by defining how much of the surrounding we will be required to consider. Can we limit the surroundings to a small region, or must we consider the entire laboratory? The problem becomes rather simple if we assume that our calorimeter is perfectly insulated. Heat, we assume, will not flow through the walls of the calorimeter. This assumption allows us to restrict the extent of the surroundings. Because heat cannot flow out of or into the calorimeter, we can define the surroundings as the complete calorimeter system. This includes the cups, thermometer, and water that made up the solutions that were mixed together. Any water that is produced in the reaction is part of the system (see next paragraph). The system includes any other substance or substances that are contained in the calorimeter. This definition of a system includes substances that are dissolved in the water, such as the reactants and products of a chemical reaction. It also includes water produced in the reaction. Heat Flow The enthalpy change for a process, H, can be calculated with calorimetry in the following way. The First Law of Thermodynamics states that the amount of energy in the universe is constant. If the amount of work done in a process is zero or can be neglected (such as in this lab) the only form of energy is heat. Thus, the amount of heat in the universe can not increase or decrease due to the reactions that we performed. One way of saying this mathematically is that the heat lost(or gained) by the system plus the heat lost(or gained) by the surroundings is equal to zero. q(system) + q(surroundings) = 0 By convention, heat that is gained is positive and heat that is lost is negative. The equation can be rearranged to give: q(system) = –q(surroundings) The heat lost(or gained) by the system is equal to the enthalpy change of our process, H. All we need to do is find the heat lost(or gained) by the surroundings and change the sign! The heat lost(or gained) by the surroundings is found by multiplying the heat capacity (H.C.) of the surroundings by the temperature change. q(surroundings) = (H.C.) T 8 If we know the heat capacity (H.C.) of the entire calorimeter system (this includes the cup and solvent water), we can easily calculate the heat lost(or gained) by the system, q(system), by measuring the temperature increase of the calorimeter and water. q(system) = –(H.C.)(T) This q(system) is the enthalpy change, H, in units of energy. An Example Suppose the temperature of 50 mL of 1.0 M NaOH in a coffee-cup calorimeter is 25.3 C. When 50 mL of 1.0 M HCOOH (formic acid), whose temperature is also 25.3 C, is added to the calorimeter, an exothermic reaction occurs. This causes the temperature to increase to 30.8 C. The chemical reaction is NaOH + HCOOH ---> NaHCOO + H2O The temperature change of the calorimeter system can be calculated as 30.8 C - 25.3 C = 5.5 C. If the heat capacity of the calorimeter system is 430 J/C, the value for q(system) would be: q(system) = –(5.5 C)(430 J/C) = –2400 J Enthalpy Changes (Hrxn) Enthalpy changes are typically reported in intensive units, such as kJ/mol. This can be calculated by dividing q(system) by the number of moles of the limiting reactant. For the example above: 50.0 mL x 1 L/1000 mL x 1.0 mol/L = 5.0 x 10-2 mol The enthalpy change becomes Hrxn = –2400 J/5.0 x 10-2 mol = –48000 J/mol , or –48 kJ/mol. The reaction was exothermic because H has a negative value, and released heat to the surroundings. Correcting for Imperfect Insulation The equation for heat flow was obtained using the assumption that our calorimeter is perfectly insulated. We must now recognize that this assumption is not warranted, because heat will flow through the walls. After all, hot coffee in a polystyrene coffee cup cools even if the top is covered. Because heat leaks through the walls, we will not be able to observe the highest (or lowest) temperature that could have been achieved in a perfectly insulated calorimeter. However, we need to know that temperature, because it is tf in our equation for heat flow. We will estimate that temperature by plotting temperature as a function of time. We will then extrapolate to the time at which the solutions were mixed. This temperature is Tf. 9 EXPERIMENT 7 – Visible Spectra of the Elements Goal To observe periodic trends and view electronic transitions. Objectives After completion of this experiment, students should be able to do the following: 1. Describe the energy levels that are present in the hydrogen atom. 2. Calculate the energy difference between two energy levels in a hydrogen atom 3. Understand the relationship between light and the electronic structure of elements. 4. Write the ground-state electron configuration of an atom or ion. 5. Given a series of orbital diagrams, determine which electron configurations are allowed and which are not allowed. INTRODUCTION The Hydrogen Spectrum and the Bohr Model The Bohr model of the hydrogen atom postulates that only orbits of certain radii are allowed for electrons. Each of these allowed orbits corresponds to a definite energy that can be found using the equation: 1 En (2.178 10 18 J ) 2 n (Equation 1) In this equation, the variable n corresponds to an integer that designates the energy level of the electron. The lowest possible energy level corresponds to n = 1 and E1 = –2.17810–18 J. As the radii of the possible electron orbits increase, the values of n increase (n = 1, 2, 3, 4, ……), and the the energies associated with the levels increase. A value of n = corresponds to the ionization of the hydrogen atom and E = 0. This is the largest possible energy. (Note: all other energy values are negative!). As electrons move between the allowed energy levels, energy is absorbed or emitted by photons of light. Because the allowed levels have definite energies, only certain energies can be observed due to a transition between levels. Furthermore, any transition between an initial, Ei, and final, Ef, energy level leads to a change in energy: E = Ef – Ei (Equation 2) The quantity E corresponds to the energy of the photon of light that was absorbed or emitted due to the transition. A positive change in energy corresponds to an absorption of a photon whereas a negative change in energy corresponds to an emission of a photon. The key point is that the same energy change will be observed for every photon associated with a particular transition. Every definite change in energy corresponds to a specific frequency, v, and wavelength, , for the photon of light absorbed or emitted, as shown from equation 3 below: E h hc (Equation 3) In this equation, h is Planck’s constant (h = 6.62610–34 Js) and c is the speed of light (c = 2.998108 m/s). Note that the absolute value of ΔE is used to determine the frequency and 1 wavelength of the photon because these are always positive quantities – regardless of whether the photon was absorbed or emitted. The first part of this experiment will give you the opportunity to observe and analyze the visible portion of the hydrogen spectrum. This spectrum provides direct evidence for the existence of electronic energy levels in the hydrogen atom, and was instrumental in the development of the Bohr model. The success of Equation 1 in predicting the location of the lines in the hydrogen spectrum was critical toward the acceptance of quantized energy levels, a significant step in the develop of quantum mechanics. The significance of these ideas lies in the fact that most experimental evidence concerning the structure of molecules and atoms consists of measurements associated with their absorption and emission of light. Flame Analysis The description of the occupied atomic orbitals of a particular atom or ion is called the electron configuration. When the electrons occupy the lowest possible configuration of orbitals, they are said to be in the ground state electron configuration. You should have recently discussed the process of writing the ground state electron configurations for elements and ions in class. If energy is added to an element (as is done when the element is heated in a flame), the electrons can move to higher energy configurations. When this occurs, the atom or ion is said to be in an excited state. In most cases, photons will be emitted quickly as the electrons return to the ground state configuration. This emission of photons can be observed by instrumentation, or with the naked eye if it occurs in the visible portion of the spectrum. Electronic transitions between orbitals can be produced in any element. Due to larger nuclei and multielectron interactions, however, the orbital energies can not be simply calculated as was done with hydrogen. Nevertheless, photons of specific wavelength are emitted from elements when the electrons are excited to higher energies and return to their ground state configurations. When heated in a flame, many alkaline earth metals give off characteristic colors. To observe this phenomenon with the naked eye, the transitions must result in the emission of electromagnetic energy in the visible portion of the spectrum. Evidence for atomic transitions will be investigated by placing solutions containing the alkaline earth ions Mg2+, Ca2+, Sr2+, and Ba2+ directly in a flame. You will also observe the flame present in Na+. PROCEDURE Part I: The hydrogen spectrum 1. Using equation 1, calculate the energies of the first five energy levels of the hydrogen atom. 2. On the page labeled “Hydrogen Energy Levels”, construct an energy diagram by drawing horizontal lines to represent the energies of the first five energy levels of hydrogen. The lines should occur at the appropriate location on the energy scale (y-axis). Be careful! Due to the negative signs on the energy levels and small exponents mistakes are common. You may want to construct your energy diagram in pencil first. At the top of your energy diagram, the energy level corresponding to n = and E = 0 has been done for you (remember - the n =1 level should be the lowest). Once you have your five lines in the correct place on your chart, label them n = 1 through n = 5. Obtain your instructor’s initials. 2 3. Using equation 2, calculate the E for the 10 possible transitions between the first five energy levels. 4. Using equation 3, calculate the 10 possible wavelengths of light that would be produced from transitions between the first five energy levels in hydrogen. 5. Obtain a diffraction grating. By viewing light through the grating, you will be able to observe the separation of the colors that comprise the visible portion of the electromagnetic spectrum. When looking at a light source, you should orient the slit towards the source in a vertical orientation and make sure that you can see the separated colors arranged vertically to the side. 6. View the incandescent light (light bulb) through the grating. Record your observations. 7. View the white fluorescent lights overhead through the diffraction grating. Record your observations. 8. View the hydrogen spectrum tubes through the diffraction grating. 9. You should be able to observe the wavelengths associated with at least three spectral transitions (you may see four). Match the colors observed with 3 of the 10 possible transitions. Use the visible spectrum (found on page 268 of the 6th edition of your textbook) as a guide. Report these in the Results section of your lab report. 10. Record these transitions in the report sheet. For example, a transition from the 2nd energy level to the first would be labeled: n = 2 to n = 1 Part II: Flame analysis You will perform flame tests on the following 1.0 M solutions: (i) Sr(NO3)2 (ii) Mg(NO3)2 (iii) Ca(NO3)2 (iv) Ba(NO3)2 (v) NaCl 1. Work in pairs, share Bunsen burners where possible. 2. Obtain a nichrome wire. Obtain 5 separate test tubes and place 10 drops of the solutions into the test tubes, such that each test tube contains a separate solution. 3. Clean the wire by holding the looped end in a hot flame until it is red hot. Note: To clean precipitate off of the wire, you may also want to dip it in a HCl solution before placing it in the flame. 4. Allow the wire to cool. (Be careful!!) 5. After cooling, dip the wire into the solution. 6. Hold the wire steady in the flame. 7. Look for the first flash of color within 5 seconds. 8. Repeat if necessary. Record your results. 3 Experiment 7 – Pre-lab Assignment Name: _____________________________ 1. * Lab Day and Time: ______________ (a) Which of the following transitions in the hydrogen atom would result in the emission of a photon with the largest amount of energy? (Circle below) (0.25) n = 3 to n = 1 n = 4 to n = 1 (b) Which of the following transitions in the hydrogen atom would result in the emission of a photon with the largest wavelength? (Circle below) (0.25) n = 3 to n = 1 2. n = 4 to n = 1 You will learn to write ground state electron configurations for atoms and ions. By writing these configurations, the orbital location of each electron in an atoms or ion is specified. (a) How many electrons must be accounted for in the strontium atom (Sr)? What about the strontium ion (Sr2+)? (0.50) Sr ________ Sr2+ ________ (b) Write the ground state electron configuration for Sr. (0.25) (c) Write the ground state electron configuration for Sr2+. (0.25) 4 Experiment 7 – Calculations * Energies for the first five energy levels (n = 1) to (n = 5): (0.5 pt) Possible E values for transitions between the first five energy levels (there are 10): (0.5 pt) Possible wavelengths of light that would be observed: (1 pt) 5 Experiment 7 – Hydrogen Energy Levels * (1 pt) Energy (J) 0 n= -5.010-19 -1.010-18 -1.510-18 -2.010-18 -2.510-18 Instructor Initials: ____________ 6 Experiment 7 – Results * (1 pt) Part I: The hydrogen spectrum Qualitative observation of incandescent light: (0.25 pt.) Qualitative observation of fluorescent light: (0.25 pt.) Visible wavelengths and transitions in the hydrogen atom. You should report the exact, calculated wavelength that you believe was observed and the transition responsible. You may use your textbook as a guide for the approximate colors in the visible spectrum. (1 pt.) Wavelength Observed Transition responsible = _________ n = ________ to n = _______ = _________ n = ________ to n = _______ = _________ n = ________ to n = _______ Part II: Flame Analysis (0.5 pt.) Ion Tested Color Observed Intensity Sr2+ Mg2+ Ca2+ Ba2+ Na+ 7 Experiment 7 – Report Sheet and Post-lab 1. * (a) What is the wavelength of the photons that are emitted when an electrons move from the n = 2 to the n = 1 level in the hydrogen atom? (0.5) (b) Why was the n = 2 to n = 1 transition not observed in this laboratory exercise? (0.5) 2. a. Explain why different colors are associated with different elements undergoing flame analysis. (0.5) b. Does the fact that Mg2+ gives no observable color during flame analysis mean that no electron transitions are occurring? Explain. (0.5) 3. 4. The following are ground state electron configurations of four atoms. Identify each atom. Several configurations are those of important exceptions to the standard filling pattern discussed in class. Consult with your instructor about these exceptions if necessary. (0.5) (a) 1s22s22p4 Element: ________ (b) [Kr]5s2 Element: ________ (c) [Ar]4s13d5 Element: ________ (d) [Xe]6s25d1 Element: ________ An excited state of an electron configuration occurs when one or more electrons are present in higher energy orbitals than the ground state configuration. (a) Give the ground state electron configuration for Ba. (0.5) (b) Give an excited state electron configuration for Ba. (Any excited state is acceptable) (0.5) 8 EXPERIMENT 8 – The Empirical Formula of an Oxide Goal You will experimentally determine the empirical formula of an unknown metal oxide. Objectives After completing this experiment, students should be able to do the following: 1. Define: (a) (c) (e) (f) (h) (j) molecular oxygen; molecular nitrogen; Law of Definite Proportions; mole; an “oxide”; empirical formula; (b) (d) (f) (g) (i) (k) atomic oxygen; limiting reactant; molar mass; Avogadro’s number; a “nitride”; an “active” metal. 2. Given the mass of a metal sample prior to reaction with oxygen, and given the mass of the oxide of the metal after complete reaction with oxygen, determine the formula of the oxide and the identity of the metal that was formed in the reaction. INTRODUCTION Antoine Lavoisier (1743-1794) showed that metals and other elements will burn in air because they react and combine chemically with a component of the air. Air is a mixture composed chiefly of molecular nitrogen (N2) and molecular oxygen (O2) in an approximate ratio of 4:1. Air also contains small amounts of other substances. What Happens When an Element is Burned in Air? Molecular oxygen (O2), alone or in air, is a very reactive substance when it is heated. Many elements will react with it. When a metal reacts and combines chemically with molecular oxygen, an oxide (a compound involving the oxide ion, O2-) is formed. Molecular nitrogen (N2), the chief component of air, is a rather unreactive substance, even at a high temperature. Only the more active metals will react and combine chemically with molecular nitrogen during heating. When nitrogen does react with an active metal, a nitride (a compound involving the nitride ion, N3-) is formed. Although the amount of molecular nitrogen in the air is approximately four times the amount of molecular oxygen, more oxide than nitride will be formed when an active metal is burned in air. The reason is the superior reactivity of molecular oxygen. CONCEPT OF THE EXPERIMENT In this experiment, after a known mass of an unknown metal (X) is burned, the product will consist of a metal oxide that forms in a 1:1 stoichiometry (XO) and smaller amounts of a metal nitride (X 3N2). Water will convert the metal nitride to a metal hydroxide [X(OH)2] with the liberation of ammonia (NH3). Heat will cause the conversion of the hydroxide to the oxide with the loss of gaseous water. 1 The end product will consist of two elements, the unknown metal and oxygen. The mass of the metal in the product is the same as the original mass used as a reactant. You can determine the mass of oxygen that is present in the metal oxide product by subtracting the original mass of metal from the mass of the product. Using your data, the known stoichiometry of the oxide and chemical reasoning, you should be able to deduce the identity of the unknown metal. PROCEDURE 1. Obtain a crucible and lid. Rinse and dry them. The crucible should be as clean as possible. 2. Obtain about 0.1 g of the unknown metal. This may correspond to several small pieces of ribbon. Use the top-loading balances in the lab to determine that you have obtained about 0.1 g. 3. Place the empty crucible in a clay triangle on a tripod or ring stand with a ring holder. Place the lid ajar on the crucible. 4. Adjust the height of the burner so that the bottom of the crucible will be in the hottest part of the properly adjusted laboratory burner. 5. Using a hot flame, heat the empty crucible and lid. Heat slowly at first, then apply full heat. The bottom of the crucible should attain a red-hot glow during this time. After one minute, remove the lid with crucible tongs and place the lid on a piece of wire gauze. After another 30 seconds, shut off the burner and allow the crucible to cool to room temperature. To speed up the cooling process, use your tongs to move the crucible onto the wire gauze next to your crucible lid. When the crucible and lid are cool, you should feel no heat when you place one of your fingers about 1/2 inch from the bottom of the crucible. Caution: Avoid burning your fingers! 6. When the crucible and lid are cool, take the crucible and lid to the weighing room. 7. Place a piece of wax weighing paper on the analytical balance. Close all doors of the balance. Tare the mass of the paper. Place your crucible and lid on the paper. Obtain and record the mass of the empty, covered crucible. Record the mass to 0.0001 g on the analytical balance. Return to your lab bench. 8. Fold the metal piece (or cut) and place it such that the entire piece is at the bottom of the crucible. 9. Cover the crucible. Obtain the mass of the crucible, lid and metal, using the same analytical balance as before. 10. Return the crucible to the clay triangle. The lid should be in place but not on too tightly. Place the burner under the crucible and heat the crucible using a hot flame for three minutes. 2 11. Use crucible tongs to carefully lift the lid by a slight amount to allow more air to enter the crucible. Do not open the lid too far, as this will cause the metal to enflame and do not leave the lid off for more than a few seconds. The metal should glow brightly without flames. Flames from the metal will carry part of the solid oxide out of the crucible and into the air as a smoke. Replace the lid quickly. 12. Repeat Step 11 once per minute or so until it appears that all of the metal has reacted. Then, remove the lid and carefully place it on a wire gauze. Heat the crucible and its contents in the open air for seven minutes. 13. Allow the crucible and its contents to cool as you did earlier. The contents should be white or slightly gray. 14. Add 10-15 drops of distilled water directly and evenly on the contents of the crucible. The smell of ammonia may (or may not) be evident at this point. 15. Place the lid back on the crucible so that the lid is slightly ajar. Turn the flame on the Bunsen burner down. Carefully warm the crucible by brushing it with the flame for approximately 1 minute. Overheating will cause loss of sample, thereby ruining the experiment. 16. Once dry, remove the lid and heat the open crucible strongly for ten minutes to convert the metal hydroxide to metal oxide by driving off water vapor. 17. Allow the crucible and its contents to cool to room temperature by placing the crucible on wire gauze on the lab bench. 18. Replace the lid and measure the mass of the covered crucible and its contents. 19. You will likely want to save the crucible and solid until after you have completed the calculations portion of the laboratory. At that point, you can place the solid in the crucible into a trash can. Rinse the crucible and lid. CAUTION: BEFORE LEAVING THE LABORATORY, MAKE SURE THAT YOUR GAS OUTLET AND THOSE OF YOUR NEIGHBORS ARE CLOSED. 3 Experiment 8 – Pre-lab Assignment Name: _____________________________ * Lab Day and Time: _____________ 1. What are the molar masses of atomic oxygen and molecular oxygen? Include units. (0.25) 2. Will the metal or the oxygen be the limiting reactant in the reaction you are performing in this lab? How do you know? (0.5) 3. Suppose that you reacted an unknown metal, X, with oxygen in a (2:1) stoichiometric ratio to give an oxide with formula X2O(s). If you knew that the solid contained 0.242 grams of oxygen and 0.211 g of the metal, what is the identity of the metal? (Hint: find the moles of oxygen and use this to determine the moles of X. You can then find the molar mass of X with the mass and the moles) (0.75) 4 Experiment 8 – Data and Calculations * Organize your data and calculations in the clearest and neatest way that you see fit. Be sure to include the proper number of significant figures and units!! Data Record the mass of the empty crucible, the crucible with contents before reaction, and the crucible with contents after reaction. (0.5 pt) _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _________________________________________________________________________________ Calculations Show clearly how you used the data collected above to calculate useful information toward your objective of identifying the unknown metal. (3 pt) 5 Experiment 8 – Report Sheet * 1. What do you believe to be the identity of the unknown element? Clearly state the reasoning that you used in order to arrive at this conclusion. (1 pt) _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ 2. What errors do you believe have affected your results? Explain how they systematically affected your results (for example – did they cause a result to be too high or too low?). Your discussion of errors should support your reasoning in question #1. (0.5 pt) _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ 3. Write balanced equations for the following reactions. When writing the molecular formula of the metal and its compounds, use the metal identity that you have determined in this lab. Do not ask your laboratory instructor for the products of these reactions….all products are given in the text of the experiment. (0.25 pt each) a. The metal with molecular oxygen: b. The metal with molecular nitrogen: c. The metal nitride with water: d. The decomposition of the metal hydroxide upon heating: 6 Experiment 8 – Post-lab Question 1. * When a 0.192 g sample of phosphorus is burned, 0.440 g of a white oxide of phosphorous is obtained as the product. a. Determine the empirical formula of the oxide product. (1.5 pt) Procedure: Subtract the mass of the phosphorus sample from the mass of the oxide of phosphorus that was the product of the reaction. This will give you the mass of oxygen that is contained in the oxide product. Find the number of moles of phosphorous (P) present in the initial mass of phosphorous sample that reacted, using the atomic mass of phosphorous. Then find the number of moles of oxygen (O) that are contained in the oxide by using the mass of oxygen in the oxide and the atomic mass of oxygen. Then obtain the lowest integer ratio possible for the relative number of moles of phosphorous present to moles of oxygen present. Do not round your numbers too much! b. Write a balanced chemical equation for the reaction of molecular phosphorus (P 4) and molecular oxygen (O2) to produce an oxide product, based on the empirical formula for the oxide product you determined in part “a.” (1 pt) 7 EXPERIMENT 9 – Viewing and Calculating Molecular Properties with Chem3D Objectives by the end of this lab, the student should be able to: 1. Know how to create simple Lewis structures. 2. Recognize and determine shapes of molecules. 3. Create molecules in Chem3D and to calculate their energies. 4. Determine bond lengths and bond angles from molecular displays in Chem3D. INTRODUCTION Not all chemists spend their time doing experimental work, or “wet chemistry”. Many properties of molecules and reactions can be determined using sophisticated computer software and programs developed in research labs. Often the information that the chemists are looking for can be found less dangerously or less expensively with computers than with experiment. This relatively new field of chemistry has been termed computational chemistry. In recent years, the field of computational chemistry has grown at a steady rate. You can now find computational chemists working with pharmaceutical companies designing new drugs, in the biochemical industry modeling DNA replication and protein synthesis, and even designing new materials such as metals and plastics. In addition to the use of computers in computational chemistry, most chemists use some type of software programs that help them „see‟ the molecules that they are working with more clearly. With software now available, chemists can color-code different types of elements, rotate molecules in three-dimensions and build „theoretical‟ molecules to observe their shapes and sizes. The types of programs used by computational chemists are nearly as varied as the types of methods used by their experimental counterparts. Some programs use only quantum mechanical principles to calculate molecular properties whereas others rely on experimentally observed data. The Chem3D program is a widely used program for molecular visualization. It allows the user to build a molecule, rotate it, measure bond lengths and bond angles, and display the molecule in a variety of different ways. In addition, Chem3D allows the user to compute the strain energy, the distribution of charges, and the electron orbitals in a molecule. The energy in Chem3D is based upon the MM2 algorithm, which computes the strain energy based upon experimentally observed parameters and the distances and angles between atoms. PURPOSE You will use the program Chem3D to build and view molecules. For many of the molecules you will be asked to compute the strain energy associated with a particular geometry. You will determine if the Lewis structure that you have postulated does a good job of describing the bonding in the molecule. You will also observe the shapes of the molecules that you have built. These exercises will allow you to see how molecular visualization software is used. In addition, you will gain a better understanding of molecular shapes and bonding patterns. 1 Common Utilities in Chem3D To open Chem3D Ultra, go to the Start Menu, choose the Chemistry selection and then the ChemOffice selection. We will be running ChemBio3D Ultra 11.0. Upon opening, you can select 'OK' for any options then maximize your window. There are several ways to use the software to achieve the desired results. Creating an atom – Go to the letter A on the menu at top. Click in the window and a box will appear. Type in the name of the atom that you want in upper-case. (For example, type C hit "enter" and a carbon atom will appear). The atoms will be automatically 'rectified'. This means that they will have all of their possible bonds filled with white-colored hydrogen atoms. Selecting an atom – The central atom will first appear yellow. This indicates that this atom has been "selected". To de-select an atom, choose the arrow from the far left of the top menu. Then click anywhere in your window that does not contain an atom. Rotating a molecule – Select the Rotate tool (the circle with an arrow inside) from the top menu. Click anywhere in the window and hold down the left mouse button. While holding down on the button, move the mouse to rotate. To rotate about a specific axis, move your mouse to the edges of the Chem3D window and various options should appear. Changing an atom – Go to the letter A on the menu at top. Click on the atom you want to change (to select it) and a box will appear. Type in the symbol of the atom, hit "Enter" and it will change. Deleting an atom – Go to the eraser at top, and then click on the atom you want to delete. If you wish to delete a hydrogen, you will have to turn off the rectification, which is in general not a good idea. Do not do this without permission! Selecting a bond – From the menu at top, select the arrow. Point to a bond on your model and the bond length will be displayed. Click on the bond to select the bond and it (along with the two atoms it contains) will turn yellow. Changing the bond order – To create a double or triple bond, first select a bond as described above. You can then right-click your mouse Set Bond Order. Breaking a bond – Select a bond as described above. Select Break a Bond. View a bond angle – First, you must select three atoms. Select the arrow from the menu at top. Select the first atom. While holding down the 'Shift' key, select the second and third atoms. You can then release the shift key. When three atoms are selected, point to one of them and the bond angle will be given. Optimizing a molecule – To give the optimum structure of a molecule, the program will attempt to find a structure with the lowest energy. Go Calculations from the top menu and the select MM2 and Minimize Energy. It is not necessary to change any of the run parameters, So choose Run. Alternatively, you can just select the "MM2" button from the menu. The result from the minimization will appear below the Chem3D window. The Total energy will signify the strain energy in units of kcal/mole. Clear Screen – Choose Edit from top menu, then Select All, then Delete from keyboard. Magnify or Reduce – Choose the button with a large and small arrow on it from the menu. Adjust the magnification with your mouse. Experiment with Settings – Go to View from above, and then choose Model Display. 2 Experiment 9 – Pre-lab Assignment For each of the molecules listed in Part 1, draw the best possible Lewis structure in the space provided. You can use the space below to practice so your report will remain neat. 3 Experiment 9 * You should have (or you will have before you begin) a Lewis structure for each of the molecules below. When you get to lab, build each of the molecules using the Chem3D program. When stated, optimize the geometry of the structure. Each molecule is worth 1 point. PART 1 1. CH4 (Methane) Lewis Structure a) What is the carbon-hydrogen bond length? (Report in units of Ångstroms) b) What is the hydrogen–carbon–hydrogen bond angle? c) The methane molecule is said to have tetrahedral geometry. Are all of the H-C-H bond angles the same in the methane molecule? 2. CF2Cl2 (A chlorofluorocarbon, molecules of this type are destructive to the ozone layer) Lewis Structure a) Minimize the structure using the MM2 procedure. How does the C–F bond length compare to the C–Cl bond length? How do these compare to the C–H bond length in question 1? b) Are all of the bond angles exactly the same in this molecule? Why or why not? 4 * 3. CH3CH2OH (Ethanol, try bonding together first as C–C–O, then add hydrogen atoms) Lewis structure a) Run an optimization. The pink circles on your screen represent lone pairs of electrons. They can be selected and hidden if you like. What is the carbon-oxygen bond length? b) Are the two carbon atoms and the oxygen linear (do they lie along one line)? Explain why or why not by describing the geometry around the central carbon atom. 4. H2CO (formaldehyde) Lewis structure a) Are their double bonds in this molecule? b) Minimize the structure. How does the C–O bond length compare with the C–O bond length from question 3? Why is this so? c) What is the H–C–O bond angle? d) How would you describe the shape around the carbon atom? 5 5. H2C=CH2 Lewis structure * a) Run a minimization. What is the C–C bond length? Are the four hydrogen atoms all in one plane or not? b) How many atoms or lone electron pairs (termed electron domains) are around each carbon atom? What is the shape around each carbon atom? c) Change the C–C bond to a single bond, resulting in the C2H6 molecule. Minimize this structure. Did the bond length become longer or shorter? d) Answer the questions in part (b) again for your new structure. How do your answers change? 6. C3H6 ring (Put the three carbons in a ring, each carbon should have two hydrogen atoms) Lewis structure a) Minimize the structure. What is the C–C–C bond angle? This program computes a value that it calls the strain energy, which is a measure of how the geometry and atomic interactions differ from standardized values. The strain energy is given at along the bottom of your window in units of kcal/mol. The more positive the strain energy, the less stable the structure is compared to one with a standard geometry and zero intramolecular interactions. What is the strain energy of this molecule? 6 b) To break the ring, break one of the C-C cyclopropane bonds. Do this and then make one of the bonds a double bond. This gives another isomer of C3H6. Minimize the energy of the structure. How does the strain energy compare to the ring structure? Why might this be so? 7. C6H6 (Benzene, the six carbon atoms form a ring with one hydrogen bonded to each) Lewis structure a) Run an optimization. Do all of the atoms lie in the same plane? b) What are the C–C bond lengths? Are they all the same? Is this consistent with your Lewis structure? c) If your model is not consistent with the Lewis structure , what might be an explanation and how should benzene be drawn? This example illustrates the limits of the Lewis structure model in many circumstances. The structure that you see is a combination of several resonance structures. 7 Part 2 * These questions may be a bit more challenging. Be sure to discuss with your partner and other classmates. Try your best to find the answer to these questions without consulting the instructor. Each problem is 1 point. 1) Build a structure that has eight carbon atoms connected together. You can branch off your chain of carbons, but do not include a ring. Now add one oxygen atom to one of your carbons and one nitrogen atom to another. Build this structure without any double bonds. Minimize the energy. Draw the structure of your molecule below (you do not need to include the H-atoms in your drawing). What is the strain energy of your molecule? Compare the strain energy of your molecule with a classmate‟s molecule. Who do you think has the more stable structure? 2) Build at least two molecules with the molecular formula H4C2O, and with bond connectivity of C–C–O. (Hint: one has a C=C bond and the other has a C=O bond). Draw their Lewis structures below. Minimize the energy in each. Circle the one that has the lowest strain energy. How much lower in energy is it? Can you think of a reason why one might be lower than the other? 3) The following are types of molecules that you will see in organic chemistry. Build and determine the strain energy of each type of molecule. (Hint: The way the structures are written is common in organic chemistry. Try drawing Lewis structures without hydrogen first, the lines are given to guide you) O O H Strain Energy: C HC OH _________ H3C NH2 _________ N CH3 H _________ Note: ChemBio3D is free to download for anyone with a Mercyhurst e-mail!! You can build molecules at home! To Download, go to: http://sitelicense.cambridgesoft.com/sitelicense.cfm?sid=764 8