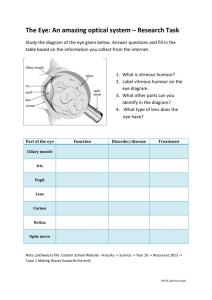
PDF Edition - Review of Optometry
advertisement

RCCL APRIL 2015 REVIEW OF CORNEA & CONTACT LENSES SPECIAL ISSUE CONTACT LENS COMPLICATIONS Expert advice on diagnosing, treating and—most of all—preventing problems. • Bringing Clarity to CLARE • Is That Corneal Infiltrate Sterile or Infectious? EARN 1 CE CREDIT • Special Care Keeps Specialty Lens Wearers Safe • Five Steps to Increase Contact Lens Adherence • Anti-VEGF for Corneal Neovascularization ! SAFETY STRATEGIES • Troubleshooting GP Lens Complications ALSO • Dry Eye: See It Through Their Eyes Supplement to 001_0515_rccl_cover.indd 1 3/27/15 3:46 PM BIOFINITY MULTIFOCAL LENSES AVAILABLE UP TO -10.00D An easy fit for you and your presbyopic patients. CooperVision Biofinity® multifocal lenses combine a high-performing 3rd generation material with a streamlined fitting process. Now even your most challenging presbyopic patients can enjoy the freedom of all-distance clarity and lasting comfort. Biofinity® multifocal Balanced Progressive™ Technology enhances vision near, far and intermediate. It also allows for an individualized fitting for each wearer and each eye. Dominant eye lens Non-Dominant eye lens Distance vision Spherical central zone Near vision Spherical central zone Intermediate vision Progressive zone Intermediate vision Progressive zone Near vision Spherical zone Lens edge Biofinity & Biofinity XR Distance vision Spherical zone Lens edge Biofinity toric Biofinity multifocal Download your Biofinity multifocal 3-step fitting guide at coopervision.com/fitting-guide ©2014 CooperVision, Inc. 1315 12/14 RO0115_Cooper Biofinity.indd 1 12/22/14 11:43 AM contents Review of Cornea & Contact Lenses | April 2015 departments 4 News Review Sclerals May Affect Corneal Nerves; Age and Astigmatic VA: No Link 6 My Perspective Getting Serious About OSD By Joseph P. Shovlin, OD 7 Lens Care Insights The Great Silicone Cover-Up By Christine W. Sindt, OD 8 Derail Dropouts Five Steps to Increase Contact Lens Adherence By Mile Brujic, OD, and Jason R. Miller, OD, MBA 10 Pharma Science & Practice Anti-VEGF in the Anterior Segment By Elyse L. Chaglasian, OD, and Tammy P. Than, OD, MS 30 GP Expert Troubleshooting GP Complications By Stephanie L. Woo, OD 32 Corneal Consult Managing Acute Corneal Hydrops in Keratoconus By James Thimons, OD 34 features 12 16 20 24 Dry Eye: See It Through Their Eyes Patient questionnaires quantify the subjective experience of the disease. Though vital for research, are they worth using in your practice? By Aliza Martin, Associate Editor Bringing Clarity to CLARE Understanding and knowing how to treat this common contact lens complication can benefit both your patients and your practice. By Lindsay A. Sicks, OD Special Care Keeps Specialty Lens Wearers Safe Contact lens care is a vital step to the continued safety and health of the contact lens patient. So how does lens care differ in the case of sclerals and other specialty lenses? By Susan J. Gromacki, OD, MS CE — Is that Corneal Infiltrate Sterile or Infectious? Out of the Box Differentiating between the two requires close observation and analysis. Here’s a results-oriented approach. By Jeffrey Sonsino, OD, and Shachar Tauber, MD Are You a Mentalist? By Gary Gerber, OD Cover design by Matt Egger ©iStock.com/Jobsonhealthcare /ReviewofCorneaAndContactLenses 003_RCCL0415_TOC.indd 3 #rcclmag REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 3 3/27/15 3:40 PM News Review Sclerals May Affect Corneal Nerves E xtended wear of fluid-filled scleral contact lenses may change corneal nerve function in patients with certain diseases, according to research published in the April 2015 Cornea.1 Researchers measured tear production, central corneal sensation, sub-basal nerve density and tortuosity, and stromal nerve thickness of 20 patients from the Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) treatment program. Patients were divided into two groups—distorted corneas (DC) or ocular surface disease (OSD)—and evaluated before and after 60 days of wear for a minimum of eight hours per day. Researchers found basal tear production significantly decreased and corneal sensation increased in patients with DC following long-term wear of the PROSE prosthetic device. In contrast, tear production and corneal sensation did not change in patients with OSD. This difference, the researchers say, may be because patients with DC have a healthier ocular surface; thus, the intact lacrimal functional unit (LFU) “responds to the constant saline exposure by reducing the basal tear production and increasing corneal sensation, which are possible signs of improvement in corneal disease.” In contrast, “patients with OSD did not have similar alterations in LFU function possibly because of ongoing inflammatory processes disrupting the LFU.” No significant change in subbasal nerve density and tortuosity or stromal nerve thickness was observed in either patient group. • Corneal crosslinking may accelerate epithelialization and reduce length and severity of necessary treatment in moderate bacterial keratitis, according to a study in the April 2015 Cornea.1 Researchers separated 32 bacterial keratitis patients into two groups. The control group was treated using standard medical therapy (i.e., lubrication, fortified cefazolin (50mg/mL) every hour, and systemic doxycycline every 12 hours following loading doses of fortified cefazolin and gentamicin) and the case group was treated with CXL and standard medical therapy. No statistically significant difference was noted between the two groups one day following treatment, but researchers noted the epithelial defects and the area of infiltrates were both smaller in the CXL group compared to the control group by day seven following the beginning of treatment. 1. Wang Y, Kornberg DL, St. Clair RM, et al. Corneal nerve structure and function after longterm wear of fluid-filled scleral lens. Cornea. 2015 April;34(4):427-32. • Tobramycin can help prevent secondary corneal infections in patients wearing therapeutic soft contact lenses, says new research published in the March 2015 Eye & Contact Lens.1 Researchers cultured 40 therapeutic soft lenses of patients being treated for recurrent corneal erosion following a two-week wearing period. During wear time, patients were treated four times per day with topical tobramycin 3% and topical sodium hyaluronate 0.1%. Upon culturing, however, nine of the 40 lenses yielded positive cultures, with Staphylococcus epidermidis identified as the predominant organism. Methicillin-sensitive coagulase-negative staphylococci, methicillin-resistant coagulase-negative staphylococci, Enterobacter gergoviae and Citrobacter freundii were also isolated. No clinical signs of infectious keratitis were found. Age and Astigmatic VA: No Link A ge has no significant influence on visual acuity in the presence of defocus and astigmatic blur, reports a study published in the March 2015 Optometry and Vision Science.1 Researchers dilated the right eyes of 22 participants—12 young adults and 10 older adults—using cyclopentolate 1.0%, then provided each with artificial pupils mounted on the back of a trial lens. To evaluate visual acuity, researchers simulated 13 blur conditions using five spherical lens conditions and two cross-spherical lenses at four negative cylinder axes. In each instance, participants were asked to read lines of decreasing size of high-contrast letters based on the Bailey-Lovie 4 IN BRIEF chart through the center of the artificial pupil. Following visual acuity measurements, aberrations were also measured. Researchers found no significant differences in visual acuity between the two age groups, disproving their hypothesis regarding the older group experiencing less decrease in visual acuity with blur; accordingly, they reported no need to test their second hypothesis that variations between the two age groups is explained by differences in higher-order aberrations. However, they suggested further study with more participants may yield a different outcome. 1. Mathur A, Suheimat M, Atchison DA. Pilot Study: Effect of Age on Visual Acuity with Defocus and Astigmatism. Optom Vis Sci. 2015 Mar;92(3):267-71. 1. Bamdad S, Makelhosseini H, Khosravi A. Ultraviolet A/Riboflavin Collagen Crosslinking for Treatment of Moderate Bacterial Corneal Ulcers. Cornea. 2015 Apr;34(4):402-6. 1. Park YM, Kwon HJ, Lee JS. Microbiological Study of Therapeutic Soft Contact Lenses Used in the Treatment of Recurrent Corneal Erosion Syndrome. Eye Contact Lens. 2015 Mar;41(2):84-6. Advertiser Index Bausch + Lomb .................... Page 5 CooperVision ......Cover 2, Cover 3 Menicon .............................. Cover 4 REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 004_rccl0415_news.indd 4 3/27/15 4:01 PM Advertorial Bausch + Lomb ULTRA® Contact Lenses with MoistureSeal® Technology A real no-brainer for my patients and my practice by Dean Nolan OD Private Practice Lawton, Oklahoma n my own practice my goal is to provide contact lens patients with the lens that is best for them. These days, that means finding a lens that not only offers the best comfort and performance, but also offers excellent value. In a relatively short time, the recently launched Bausch + Lomb ULTRA® contact lens has become my “go to” lens in the monthly replacement category. I For many of my patients there’s a kind of “Ah hah” moment on lens insertion; they find that with Bausch + Lomb ULTRA® contact lenses they truly don’t feel the lens on their eye. When it comes to lens selection, I encourage patients not to decide too quickly but instead to take a couple of days in making up their minds. What I’m finding is that even patients who have been refit in the last year or so and are very happy with their current lens typically voice a desire to go with the Bausch + Lomb ULTRA® contact lenses once they’ve tried them. I take time to explain to patients what’s behind the exceptional comfort and performance that Bausch + Lomb ULTRA® contact lenses offer, starting with oxygen transmissibility. I explain to patients that the cornea needs oxygen to stay healthy, particularly for the long wearing cycles and sustained visual demands of today’s digital device users. With a Dk/t of 163, the Bausch + Lomb ULTRA® contact lens has the highest oxygen transmissibility among the leading monthly replacement lenses.1 Surprisingly, the lens also has a low modulus, running counter to the long-held presumption that an increase in Dk/t also meant an increase in modulus. In fact, the Bausch + Lomb ULTRA® contact lens also has the lowest modulus among the leading monthly replacement lenses.1 As a third important component, the Bausch + Lomb ULTRA® lens also has high water content (46%), so it’s also an extremely wettable lens.1 These physical properties are summarized in the table. Lastly, the addition of aspheric optics combine to offer a lens with best in class performance that my patients deserve. Over the years, I have developed a reputation for offering my patients the very best in cutting edge lens technology. I tell patients to come back at no charge if the contact lenses they are wearing 1 'DWDRQ¿OH%DXVFK/RPE,QFRUSRUDWHG5RFKHVWHU1< 'LVWULEXWHGE\%DXVFK/RPED'LYLVLRQRI9DOHDQW3KDUPDFHXWLFDOV1RUWK$PHULFD//&%ULGJHZDWHU1- %DXVFK/RPE,QFRUSRUDWHG%DXVFK/RPE8/75$DQG0RLVWXUH6HDODUHWUDGHPDUNVRI%DXVFK/RPE ,QFRUSRUDWHGRULWVDIILOLDWHV$OORWKHUEUDQGSURGXFWQDPHVRUORJRVDUHWUDGHPDUNVRIWKHLUUHVSHFWLYHRZQHUV RCCL0415_BL Ultra Adv.indd 1 are not the absolute best they have ever worn. It’s been over seven years since we have really had anything new to offer our patients in monthly replacement contact lenses, so I find it very exciting to be able to recommend an innovative, best in class lens that represents a great value. An important note: the level of innovation Bausch + Lomb ULTRA® contact lenses bring to the monthly replacement category does not come with an inherently expensive price tag: they are very affordable to the patient. Beyond that, a $60 rebate is offered to patients who order a 4-box annual supply of lenses; in effect, they get the last box for free, effectively reducing the price per box—pretty exciting for such a lens. Comparison chart showing physical properties among leading replacement lenses. High Dk/t, low modulus, high water content and aspheric optics combine to give excellent overall performance.1 BRAND Dk/t MODULUS 163 70 46% ACUVUE OASYS 147 73 38% AIR OPTIX AQUA 138 102 33% Biofinity 160 82 46%* Bausch + Lomb ULTRA® contact lenses WATER CONTENT ASPHERIC OPTICS 0HDVXUHGZDWHUFRQWHQW about the author: Dean Nolan, OD has practiced in his hometown of Lawton, Oklahoma since being among one of the first graduates from Northeastern Oklahoma College of Optometry in Tahlequah, and is a proud member of the Oklahoma Optometry Association. 86=86D SPONSORED BY 3/23/15 2:22 PM My Perspective By Joseph P. Shovlin, OD Getting Serious About OSD We have made huge strides in understanding dry eye disease, with more on the way. H ave you noticed the new catch phrase, “wellness of the ocular surface”? It heralds a change in thinking that emphasizes routine screening and maintenance in all patients. Indeed, the most important part of an initial diagnostic exam for lens wearers is an accurate assessment of the ocular surface, as many contact lens-related problems can be blamed on an unstable tear film, lid disease or overall poor ocular surface health. Fortunately, we’re now armed with ways to assess and treat ocular wellness. When I started in practice three decades ago, artificial tears were the mainstay of ocular surface treatment, and sometimes the only option for combating an issue. Our inability to accurately diagnose the problem was also a major impediment—for example, until recently dry eye was mostly attributed solely to aqueous deficiency. Now, however, we know that’s not the case. Dry eye is a complex disease with many interactions and cascades. In the last four decades, researchers like James McCulley, MD, and others have refined a classification scheme based on research data. For example, his work on lid disease has led to the reclassification of posterior blepharitis into three broad categories: hypersecretory MGD (also called meibomian seborrhea), hyposecretory MGD (either primary or obstructive) and turbid hypersecretory MGD. Other research has revealed as many as 50% of all patients with blepharitis co-present with dry eye, likely because the detergent effect on the lipid layer 6 alters epithelial cell membranes, leading to cell death and inflammation—a possible contribution to an aqueous-deficient dry eye. POINT OF CARE OPTIONS New in-office testing options have also changed how we handle this broad disease category by reducing diagnosis time, improving patient education and acting as a metric to assess treatment effectiveness. In addition to TearLab’s osmolarity test, newer procedures include the following: • InflammaDry (RPS) provides an assay of the proteolytic enzyme matrix metalloproteinase 9 (MMP9). A marker for inflammation, MMP-9 is a measure of epithelial cell stress and is complementary to measuring tear osmolarity; its real value, however, is in identifying risk and treatable problems that respond to steroid and immunomodulator therapy. We know that if the test is negative for MMP-9, the patient’s issue is not dry eye-related. The opposite, however, is not true: a positive response (i.e., >40ng/mL) doesn’t serve as confirmation of dry eye disease since there are many different conditions with an elevated MMP-9 value. • The TearScan MicroAssay offers two diagnostic tests: one detects tear film lactoferrin content to assess lacrimal gland function and the other quantifies IgE to gauge the allergic component of ocular inflammation. It has a relatively good sensitivity in detecting an aqueousdeficient dry eye. • One in 10 dry eye patients have Sjögren’s syndrome. Unfortunately, detection of conventional or tra- ditional biomarkers for SS-A (Ro) and SS-B (La) is only about 70% sensitive in confirming a diagnosis. Early diagnosis of Sjögren’s is critical, as its morbidity is troubling and its link to lymphoma—including non-Hodgkins lymphoma—is well established. Now, other novel biomarkers such as salivary protein-1, carbonic anhydrase-6, and parotid secretory protein, instead offer extremely sensitive measures of early Sjögren’s. They can be identified using the Sjö test from Nicox. Ongoing R&D involving secretagogues, IL-1 blocking anti-inflammatories, LFA-1 antagonists, selective glucocorticoid receptor agonists and even androgen modulation promise more treatment breakthroughs coming down the pike. In the face of all these new developments, however, let us not forget that decades ago our knowledge and techniques, while crude, still helped our patients achieve some symptomatic relief. Assessing the tear film, looking for debris and meniscus height, staining the cornea and conjunctiva and looking for lid pathology helped shape our diagnostic decisions and treatment plans then, and still have relevance today. In closing, thank you to our pioneers for paving the way and asking the right questions that led to the products we have today. I’m certain anyone looking back decades from now will be as equally amazed at the progress made combating ocular surface disease, particularly dry eye disease. We look forward to the future work that helps assess the “vital signs” of ocular wellness that’s sure to come. Stay tuned! RCCL REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 006_RCCL0415_MP.indd 6 3/27/15 3:40 PM Lens Care Insights By Christine W. Sindt, OD The Great Silicone Cover Up A new lens surfacing option improves wettability and lubricity. How does it work? I t can be argued that the most frustrating aspect of contact lens practice isn’t determining the fit or the power, but rather dealing with a nonwetting lens—it decreases clarity and comfort and affects a patient’s overall lens wearing experience. Silicone-based materials are inherently hydrophobic, so any exposed silicone in a lens has the potential to be non-wetting. Additionally, in some patients, excessive lipids in the tear film may deposit onto the lens to create a foggy, hydrophobic surface. This issue can be reduced by dispensing low-silicone-content contact lenses; however, such lenses also impede oxygen transmission, increasing the chance for corneal complications. Other solutions include switching lid hygiene regimens and the most common method, treating the lens with plasma. THE 4th STATE OF MATTER Plasma—ionized gas with an approximately equal number of positively and negatively charged particles—is neither completely a gas nor a liquid but has properties similar to both. It’s created by forming a vacuum in a reaction chamber, then refilling with a low-pressure gas such as oxygen.1 During contact lens treatment, high-energy oxygen plasma bombards the lens surface, transferring energy from the plasma to it. This also cleans and oxidizes the surface by creating reactive species (i.e., free radicals, ions, electrons, short-wavelength photons and unstable oxygen species) that react with water, altering the lens surface to a hydrophilic state. This effect occurs to depths of several hundred angstroms to 10µm without any change to the bulk properties of the lens material. Note that while plasma treatment is superior to other options, the hydrophilic result may last for only a few minutes to several months.1 A NEWCOMER Hydra-PEG (Ocular Dynamics) is a polyethylene glycol (PEG)-based polymer mixture that is covalently (permanently) bonded to the surface of the Before (left) and after (right) Hydra-PEG treatment. contact lens, effectively creating a wetting surface comfort in patients suffering from on the underlying lens material and contact lens-induced dry eye.8 separating it from the ocular surface Note: although it is a permanent and tear film. PEG has been used in coating, Hydra-PEG has only been ocular lubricants for decades and tested out to three months of simuis known to improve lens surface lated rubbing/cleaning cycles. Ocuwettability, which improves tear lar Dynamics currently recommends breakup time, increases lubricity hydrogen peroxide-based cleaners, and reduces protein and lipid depobut reports it is also in the process sition. Hydra-PEG can be applied of testing multipurpose solutions for to hydrogel, silicone hydrogel or GP compatibility. lenses. Thus far, my clinic experience During application, the first step with this product has been positive: of lens surface preparation is either I have found it creates a wettable, the addition of a functional activaclear and comfortable surface, even tor to the monomer mix or a short in the most challenging of condiplasma surface treatment. Once tions, such as lagophthalmos and active, the lenses are then soaked significant ocular surface disease. I in the Hydra-PEG polymers during anticipate Hydra-PEG will provide the extraction/hydration step, or the another useful option in most conHydra-PEG polymers are added to tact lens practices. the blister pack during the autoclave 1. William Hoffman. Personal communication. process.2 In either case, once the 2008. active lens is placed in the PEG poly- 2. Ocular Dynamics. Hydra-PEG Manufacturing. Available at: www.oculardynamics. mers, the Hydra-PEG permanently com/#!manufacturing/c11sc. Accessed March 25, 2015. bonds to the lens surface. 3. Measured via Captive Bubble Contact Angle. In ex-vivo tests, Hydra-PEG Ocular Dynamics data on file, 2014 4. Measured via Water Breakup Time. Ocular was shown to improve wettability, Dynamics data on file, 2014 surface water retention, lubricity 5. Measured via Digital Friction Evaluation. Ocular Dynamics data on file, 2014 and deposit resistance.3-6 These lens 6. Measured via Radiolabeled Lysozyme Deposisurface properties have been corretion Assay. Ocular Dynamics data on file, 2014 lated with contact lens comfort in a 7. Jones, L. et al. “The TFOS International on Contact Lens Discomfort: Report number of studies.7 When applied to Workshop of the Contact Lens Materials, Design, and Care the Acuvue Oasys lens in a random- Subcommittee”. IOVS, October 2013, Vol. 54 8. Caroline, P. “Hydra-PEG: A Solution to Contact ized controlled trial, Hydra-PEG Lens Discomfort?” Poster presented at Global Specialty Lens Symposium 2015. surfaced lenses demonstrated good RCCL REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 007_RCCL0415_LCI.indd 7 7 3/27/15 3:41 PM Derail Dropouts By Mile Brujic, OD, and Jason Miller, OD, MBA Five Steps to Increase Contact Lens Adherence Getting patients to comply with lens wear and care guidelines is a well-known battle. Here are some points to make it a little easier. R ecent advances in contact lens technology have given us a host of new lens options in the realms of presbyopic, toric and single vision designs. Positive improvements in contact lens care systems also continue to provide additional benefits to those who use them. Together, these developments have increased our arsenal of options to re-engage with those contact lens wearers who may have dropped out in the past. However, other reasons for contact lens drop out still remain. When a lens wearer abuses their modality or care system, complications—although rare—can arise as a result of non-adherence to practitioner recommendations, a common problem across all health conditions, especially contact lens care regimens.1 This month, we share five highly effective ways to better emphasize the importance of adherence to your contact lens patients. By increasing and tailoring our efforts, we improve our patient’s chances of successfully wearing contact lenses, which will ultimately help keep them in lenses long-term. Be aware of how your patients are caring for their lenses. How often do contact lens wearers walk into our practices without any idea of which solution or rewetting drops—if any— they are using? What about the 1. 8 condition of the patient’s contact lens case? Certainly, migrating patients to a daily disposable contact lens will help eliminate these potential issues, as they obviate the need for care solutions and lens cases. However, the patient may still be using drops that you are unaware of to alleviate comfort issues. So, how do you find out what exactly your patients are doing to care for their lenses? The answer is fairly simple: ask them to bring in their contact lens case, solution and any other care products, as well as any drops they may be using. Seeing them firsthand gives us the opportunity to educate patients on proper lens care if needed and gives us the opportunity to intervene with appropriate clinical solutions if necessary, including refitting them into a daily disposable lens or suggesting an alternate product. Educate patients on how to appropriately care for their lenses. While proper lens care is common knowledge for the eye care practitioner, many patients are not as educated regarding its importance and influence. Surprisingly, even the most seasoned contact lens wearers may not know how to appropriately care for their lenses. Try asking your contact lens patients to explain their process for applying and removing their contact lenses. How do you educate these 2. patients? Devise a consistent conversation to have with all contact lens patients and modify it according to each patient’s individual needs. Explaining the importance of adherence and correcting patient-specific errors means they are more likely to adhere to your guidelines. As an example, you can say, “I want you to be able to consistently wear your contact lenses comfortably and in a healthy way. This can best be achieved by following our recommendations on cleaning and caring for your contact lenses.” Next, follow this with the actual steps necessary to care for the lenses, and consider a discussion of a daily disposable lens use. If the patient has issues or complications, show them. Sometimes, patients will present with symptoms caused by contact lens abuse, such as GPC on the superior tarsal plate from lens overwear when deposits occur on the lens surface and interact with the lid while blinking.2 This response leads to the clinically evident giant papillae and, often, excessive mucus production. In these instances, contact lens wearers typically seek symptomatic relief—and delivering it will likely create a more compliant lens wearer.3 But what about changes that are less symptomatic, but still important to teach patients about? In these cases, we often use slit lamp imaging systems as an educational 3. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 008_RCCL0415_DD.indd 8 3/27/15 3:42 PM RCCL REVIEW OF CORNEA & CONTACT LENSES 11 Campus Blvd., Suite 100 Newtown Square, PA 19073 Telephone (610) 492-1000 Fax (610) 492-1049 Editorial inquiries (610) 492-1003 Advertising inquiries (610) 492-1011 E-mail rccl@jobson.com tool. For example, the patient who is sleeping in hydrogel lenses, but who is asymptomatic, may have significant corneal neovascularization that can be imaged and demonstrated. Or, someone who is keeping their lenses in for longer than prescribed who presents with significant deposits on the lens surface might benefit from seeing those deposits up close. In either case, imaging of these scenarios helps the patient understand their condition and hopefully, the need for more compliant wear. Give them the tools they need. As practitioners, we have heard almost every single excuse in the book for why our patients are noncompliant with their replacement schedules. We have made it a point to make it as easy as possible for patients to remember to replace their contact lenses. So, regardless of the modality, be sure to give them the best tools possible to help them remember their replacement schedule. Obviously, daily disposable lenses are the easiest modality to replace. It’s one of the main reasons these lenses are associated with such a high level of adherence.4 For patients wearing two-week or monthly disposable lenses, however, we need to guide them to select a day or two days, depending on the modality, as their designated replacement day. Depending on your practice, you may also have the means to provide patients with contact lens cases, solution and other accoutrement to help with adherence. 4. 008_RCCL0415_DD.indd 9 Don’t assume non-adherence is the reason. A patient wearing contact lenses who comes in with a corneal infiltrative response is often immediately assumed to be someone who has abused their contact lenses. While this is often the case, take caution to consider other clinical entities that may present similarly. For example, a point-of-care test such as AdenoPlus can be used to rule out adenoviral keratoconjunctivitis in a contact lens wearer presenting with an acute red eye and corneal infiltrates.5 Clinically, we will often pigeon-hole these patients as contact lens abusers when in fact they are contact lens wearers who simply have another etiology responsible for the cause of their red eye. It is well understood that adherence in health care is a constant challenge. But by incorporating these strategies, we can help influence contact lens adherence in a positive way, reduce complications and ultimately help those patients who may discontinue lens wear continue wearing their lenses successfully. 5. EDITORIAL STAFF EDITOR-IN-CHIEF Jack Persico jpersico@jobson.com ASSOCIATE EDITOR Aliza Martin amartin@jobson.com CLINICAL EDITOR Joseph P. Shovlin, OD, jpshovlin@gmail.com EXECUTIVE EDITOR Arthur B. Epstein, OD, artepstein@artepstein.com ASSOCIATE CLINICAL EDITOR Christine W. Sindt, OD, christine-sindt@uiowa.edu CONSULTING EDITOR Milton M. Hom, OD, eyemage@mminternet.com SENIOR ART/PRODUCTION DIRECTOR Joe Morris jmorris@jhihealth.com GRAPHIC DESIGNER Matt Egger megger@jhihealth.com AD PRODUCTION MANAGER Scott Tobin stobin@jhihealth.com BUSINESS STAFF PUBLISHER James Henne jhenne@jobson.com REGIONAL SALES MANAGER Michele Barrett mbarrett@jobson.com REGIONAL SALES MANAGER Michael Hoster mhoster@jobson.com VICE PRESIDENT OPERATIONS Casey Foster cfoster@jobson.com EDITORIAL BOARD Mark B. Abelson, MD James V. Aquavella, MD Edward S. Bennett, OD Aaron Bronner, OD Brian Chou, OD S. Barry Eiden, OD Gary Gerber, OD Susan Gromacki, OD Brien Holden, PhD Bruce Koffler, MD Pete Kollbaum, OD, PhD Jeffrey Charles Krohn, OD Kenneth A. Lebow, OD Kelly Nichols, OD Robert Ryan, OD Jack Schaeffer, OD Kirk Smick, OD Barry Weissman, OD RCCL 1. Claydon BE, Efron N. Non-compliance in contact lens wear. Ophthalmic Physiol Opt. 1994 Oct:14(4):356-64.2. Elhers WH, Donshik PC. Giant papillary conjunctivitis. Curr Opin Allergy Clin Immunol. 2008 Oct;8(5):445-9. 3. Chigbu D. The management of allergic eye diseases in primary eye care. Cont Lens Anterior Eye. 2009 Dec;32(6):260-72. 4. Dumbleton K, Woods C, Jones L, et al. Patient and practitioner compliance with silicone hydrogel and daily disposable lens replacement in the United States. Eye Contact Lens. 2009 Jul;35(4):164-71. 5. Sambursky R, Trattler W, Tauber S, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013 Jan;131(1):17-22. REVIEW BOARD Kenneth Daniels, OD Desmond Fonn, Dip Optom M Optom Robert M. Grohe, OD Patricia Keech, OD Jerry Legerton, OD Charles B. Slonim, MD Mary Jo Stiegemeier, OD Loretta B. Szczotka, OD Michael A. Ward, FCLSA Barry M. Weiner, OD 3/27/15 3:42 PM Pharma Science & Practice By Elyse L. Chaglasian, OD, and Tammy Than, MS, OD Anti-VEGF in the Anterior Segment Corneal neovascularization from contact lens overwear and other hypoxic events may respond to this posterior segment therapy. O ne of the hallmarks of the cornea is its avascular, transparent nature, which is a result of the precise composition and arrangement of its constituent parts. A variety of affronts—including infection, inflammation, ischemia, degeneration and loss of the stem cell barrier—can lead to the loss of this avascularity in the form of corneal neovascularization.1,2, Over 1.4 million patients develop corneal neovascularization each year, with up to 12% of cases associated with subsequent decreased acuity as immature and abnormal vessels invade from the limbal vascular plexus, causing scarring, edema and inflammation.1,3 This invasion occurs when the habitually precise balance between pro- and anti-angiogenic factors is disturbed by an excess of pro-angiogenic factors.4 While a number of constituents promote new vessel proliferation, vascular endothelial growth factor (VEGF) is one of the key regulators of this process and, as such, has become an important target for medical therapy.5 Anti-VEGF treatments, a mainstay of therapy for retinal conditions, also hold promise for corneal applications and may play a particularly auspicious role in graft survival after penetrating keratoplasty.2 A BETTER WAY? Conventional therapy for corneal neovascularization includes steroids, thought to suppress activation and 10 Vessel encroachment into the cornea in a case of neovascularization due to hypoxic stress. Might this be a patient who would benefit from anti-VEGF? migration of macrophages, mast cells, cytokines and other inflammatory cells promoting angiogenesis.2,6,7 Steroids are also often used in combination with oral matrix metalloproteinase (MMP) inhibitors such as doxycycline in an effort to regress abnormal corneal vasculature. These techniques are limited in efficacy, however, and lead to well-known side effects of topical steroids including cataracts and glaucoma. Nonsteroidal antiinflammatory medications, photodynamic therapy, laser photocoagulation, fine needle diathermy and conjunctival, limbal and amniotic membrane transplantation have also been used with varying success.2,6 Topical anti-VEGF therapy for corneal neovascularization has been investigated off-label using both bevacizumab and ranibizumab, which are monoclonal antibodies against VEGF and traditionally used for retinal indications.2,8,9 Both target VEGF-A, leading to inhibition of abnormal blood vessel formation and decreased vascular permeability. Bevacizumab has been used more often in off-label indications and in studies as a result of its increased affordability. Bevacizumab was originally approved for metastatic colorectal cancer, but has long been used in ophthalmology for off-label therapy of wet AMD, proliferative diabetic retinopathy and iris rubeosis.2,10 Therapy with anti-VEGF medications has been studied both in subconjunctival and topical use and has shown promise in the treatment of herpetic keratitis, recurrent pterygium, corneal transplant rejection and Stevens-Johnson syndrome.11 Multiple studies confirm the effectiveness of topical bevacizumab in reducing corneal neovascularization in experimental animal models, and human use has shown significant reductions in abnormal vasculature, even in patients recalcitrant to traditional anti-inflammatory therapies.2,6 Early treatment appears REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 010_RCCL0415_PSP.indd 10 3/27/15 3:43 PM more efficacious in both animal and human models, with both topical and subconjunctival therapy.6,12,13 Chronic vascular conditions tend not to respond as well to therapy as active or acute angiogenesis.6,12 Clinically, corneal neovascularization can be seen after infectious, inflammatory and traumatic events.1,2 Inflammatory stress tips the balance of growth and inhibitory factors in favor of angiogenesis and leads to the growth of new, abnormal vasculature.4 The same can be seen under hypoxic conditions, such as contact lens overwear.2 The resulting neovascularization may be deep, stromal or may present as superficial vascular pannus, depending on the ocular insult.2,14 Deep and stromal neovascularization may be associated with interstitial and disciform changes seen in herpetic keratitis, while superficial changes are typically associated with ocular surface disease.2 Other bacterial, viral, protozoan and fungal antigens may also induce a keratitis that can lead to subsequent neovascularization. Trauma (including chemical burns), ischemia (i.e., limbal stem cell deficiency), and inflammatory conditions may also promote the abnormal vasculature.2,11,15 Autoimmune diseases (e.g., Stevens-Johnson syndrome, graft rejection and cicatricial pemphigoid) and corneal degenerations (e.g., pterygium and Terrien’s marginal degeneration) have further been implicated in corneal neovascularization.2,11,15 Perhaps the most widespread application, however, lies in corneal transplantation, where recipient neovascularization before transplantation doubles the FROM VEGF TO VESSELS While there are a number of iterations of VEGF found throughout the human body, VEGF-A is one of the key regulators of hemangiogenesis in ocular tissues.2 It is secreted by corneal epithelial and endothelial cells, vascular endothelial cells and fibroblasts and microphages found in scar tissue; importantly, this process is exacerbated in inflamed and vascularized corneas.6 Once released, it promotes vascular endothelial cell proliferation, migration and tube formation, and may also play a secondary role in inflammation.1,6 Circulating VEGF exerts its influence by binding to tyrosine kinase receptors, which leads to a signaling cascade promoting cell division and proliferation of vessels.2 risk of graft rejection, and where increases in graft survival after antiVEGF therapy have been demonstrated in animal models.2 GROWTH OPPORTUNITY Clearly, there is a role for antiVEGF therapy in corneal neovascularization, and its potential anterior segment indications are plentiful. Areas for further research include determining the ideal administration, route, dosage and formulation, and whether a targeted combination therapy for multiple growth factors is necessary to completely regress vasculature in these patients. While large, randomized studies are required to firmly establish the safety and breadth of corneal indications, it seems clear that antiVEGF therapy will have an increasingly significant role in the management of corneal neovascularization patients moving forward, and that corneal specialists will have a more efficacious tool at their disposal when treating this potentially blinding condition. The authors would like to acknowledge Stephanie Fromstein, OD, for her invaluable contributions to this article. RCCL 1. Chang JH, Gabison EE, Kato T et al. Corneal neovascularization. Curr Opin Ophthamol. 2001; 12: 242-249. 2. Chang JH, Garg NG, Lunde E et al. Corneal neovascularization: an anti-VEGF therapy review. Surv Opthalmol. 2012 ; 57(5): 415-429. 3. Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998; 43: 245-269. 4. Kvanta A. Ocular angiogenesis: the role of growth factors. Acta Opthalmol Scand. 2006.; 84: 282-288. 5. Gan L, Fagerholm P, Palmblad J. Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 I the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand. 2004; 82: 557-563. 6. Papathanassiou M, Theodoropoulou S, Analitis A et al. Vascular endothelial growth factor inhibitors for the treatment of corneal neovascularization: a meta-analysis. Cornea. 2013; 32(4): 435-444. 7. Phillips K, Arffa R, Cintron C et al. Effects of prednisolone and medroxyprogesterone on corneal wound healing, ulceration and neovascularization. Arch Opthalmol. 1983; 101: 640-643. 8. Avila MP, Farah ME, Santos A et al. Three-year safety and visual acuity results of epimacular 90strontium/90yttrium brachytherapy with bevacizumab for the treatment of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Retina. 2011; 32(1): 10-18. 9. Krebs I, Lie S, Stolba U et al. Efficacy of intravitreal bevacizumab (Avastin) therapy for early and advanced neovascular age-related macular degeneration. Acta Opthalmol. 2009; 87: 611-617. 10. Cheng SF, Dastjerdi MH, Okanobo A et al. Short-term topical bevacizumab in the treatment of stable corneal neovascularization. Am J Ophthalmol. 2012; 154: 940-948. 11. Ambati B. Corneal applications for anti-VEGF agents. Adv Oc Car. 2011: 24-25. 12. Papathanassiou M, Theodossiadis PG, Liarakos VS et al. Inhibition of corneal neovascularization by subconjunctival bevacizumab in an animal model. Am J Opthalmol. 2008; 145: 424-431. 13. Stephenson M. Anti-VEGF for CNV: questions remain. Rev Ophthalmol. 2011. Published online 14. Ellenberg D, Azar DT, Hallak JA et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010; 29: 208-248. 15. Bock F, Konig Y, Kruse F et al. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 281-284. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 010_RCCL0415_PSP.indd 11 11 3/27/15 3:43 PM DRY EYE: See It Through Their Eyes Patient questionnaires quantify the subjective experience of the disease. Though vital for research, are they worth using in your practice? D ry eye is easily one of the most common diseases worldwide, encompassing a wide range of ocular surface alterations with different etiologies and pathophysiologies.1,2 In recent years, eye care practitioners have made great advances in objectively measuring dry eye with precision, using high-tech tools to quantify tear osmolarity, inflammatory cytokines and Sjögren’s biomarkers in addition to familiar clinical evaluation tools like the Schirmer’s test and tear film breakup time. Methods to assign an objective number or severity score to dry eye have flourished. But despite this success, the subjective component of the disease— how it feels for patients—remains for the most part poorly documented. Thus, it’s no surprise a number of patient evaluation questionnaires exist as part of an ongoing movement to quantify patient symptomatology. Dry eye questionnaires are commonly used in clinical research to screen participants, grade disease severity and assess the effects of treatments. They vary in length, focus and extent of validation, and often involve a number of rating scales that are combined to produce a total raw score (see “Comparing Notes,” p. 14).13 So, how does one determine which to use in a busy 12 By Aliza Martin, Associate Editor clinical practice, especially given that such questionnaires typically measure patients against a pre-established clinical diagnosis of dry eye? This article discusses some of the more popular questionnaires available and examines their relevance in the context of evaluating patients in a clinical practice setting. EXPERT TESTIMONY In 2007, the International Dry Eye WorkShop published a report on the epidemiology of dry eye, which evaluated the practicality of a number of dry eye questionnaires.17 Requirements for consideration included that the questionnaire had been used in randomized clinical trials (RCTs) or epidemiologic studies, had passed psychometric testing and been deemed suitable for evaluating general, non-disease specific dry eye populations.17 Fourteen questionnaires met the criteria, including five discussed here. The committee identified characteristics that designate a questionnaire as suitable for use in epidemiologic studies and RCTs. First, it must be able to detect and measure changes in symptoms with effective treatment or disease progression.17 The recall period must also be specified and the ability to set a threshold of disease severity as an inclusion criterion should be present.17 Additionally, because of the possibility of dry eye symptoms worsening over the course of the day, there must be a single, set time for administering dry eye examinations and the questionnaire.17 The subcommittee recommended adding a better definition of clinically meaningful changes in scores as well as a better concept of the “worst” symptom and a question on visual function with respect to dry eye.17 Also, more research on the relationship between frequency and severity of dry eye symptoms as a means to better identify a clinically meaningful change in symptoms is warranted.17 WHAT DO YOU USE? Granted, dry eye questionnaires are commonly used in clinical research as a means to grade disease severity and assess treatment effects, all within a controlled environment with a pre-selected population segment. But are they useful in clinical practice, where many different patients present who have not been pre-sorted and who may fall within a range of disease severity and treatment types and stages? A validated questionnaire, says Arthur B. Epstein, OD, provides a good starting point to evaluate the patient’s unique experience and gather information about the specifics of the disease. Dr. Epstein is director of clinical research at REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 012_RCCL0415_F1ANDF2.indd 12 3/27/15 4:28 PM Photo: Mile Brujic, OD Does reliance on dry eye signs, like the corneal staining above, overshadow the role of the patient's symptomatic experiences and quality-of-life issues? Phoenix Eye Care and runs a dry eye clinic at the practice. He uses dry eye questionnaires to evaluate every patient who walks in. “I use questionnaires for documentation and especially for progress evaluations,” he says. “While both are important, my personal bias is to measure outcomes by a reduction in patients’ symptoms even more than a reduction in physical signs. Questionnaires provide a standardized way of assessing how well the patient is doing and if they are responding to therapy.” Dr. Epstein uses both the OSDI and SPEED questionnaires, and says each has its own benefits. “SPEED is quicker, but OSDI provides a bit more information. As odd as it sounds, I haven’t totally settled on either, but I make sure we use the same one we used previously to monitor change” in a specific patient. Overall, he adds, the usefulness of the different dry eye questionnaires varies depending on the patient. “For example, the CLDEQ is optimized for lens wearers, and the DEQS focuses more on quality-oflife issues. Some are research tools and less useful in clinical practice.” Al Kabat, OD, and Whitney Hauser, OD, of Southern College of Optometry’s TearWell Advanced MMP-9 testing,” he says. “We can Dry Eye Treatment Center in Mem- also assess treatment response by phis, also use the OSDI and SPEED following the patient’s symptomatic questionnaires for similar purposes. improvement on the questionnaire.” Because both are validated and used in the practice together, they DIY EFFORTS act as a good system of checks and Some practitioners choose to create balances. “We use the questiontheir own dry eye questionnaire. naires to first quantify the patient’s In addition to using the SPEED symptoms as a finite entity, and questionnaire, Paul M. Karpecki, then track the patient’s progress as OD, of Koffler Vision Group in we perform or initiate specific treat- Kentucky, also uses a custom one of ment regimens,” Dr. Kabat says. his own making (shown below). “Occasionally, patients are in“The second is a much more fluenced by how they feel on a extensive questionnaire about dry particular day, and the surveys eye disease that is administered on a provide a more global view,” adds clipboard when the patient is placed Dr. Hauser. “Dry eye care, unlike in the exam lane waiting on the many other eye diseases, is driven doctor,” he says. Contrary to the by symptom relief, and the surveys SPEED questionnaire, which is used give a measureable indication of on every patient, the custom form is improvement.” only used for new patients referred specifically for dry eye disease evalThe responses from the dry uations. “The SPEED questionnaire eye questionnaires do have a big initiates a potential dry eye patient influence on treatment decisions, workup,” says Dr. Karpecki. “The Dr. Kabat says. “For a severely extensive custom questionnaire symptomatic patient, we are more actually predicts potential diseases apt to initiate aggressive therapy ranging from anterior blepharitis to even in lieu of significant findings. Likewise, se,, for a patient with less symptomology, we might be more m mology, conservative vattive in our treatment algorithm.” hm m.” Eric Donnenfeld,, MD, a Long Island ophop phthalmologist loggist who specialeccializes in catacattaract and d refracr tive surgery, geery, uses OSDI SD DI and SPEED to o guide diagnostic stic testing. Dry eye questionnaires can also “All patients tieents who be custom-made, such as this one from have positive osiitive findings Paul M. Karpecki, p , OD. To download a copy py on the questionnaire ti i of it suitable for use in your practice, look for this article at www.reviewofcontactlenses.com. undergo osmolarity and REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 012_RCCL0415_F1ANDF2.indd 13 13 3/27/15 3:43 PM DRY EYE: SEE IT THROUGH THEIR EYES Comparing Notes: Selected Dry Eye Evaluation Forms The options for documenting the patient’s experience of dry eye range from simple one-page sheets with three key components to a complete soup-to-nuts account of their case history. Here’s a quick overview of several popular ones. To find links to these forms, snap a picture of the QR code on the right with your smartphone or visit www.reviewofcontactlenses.com. • McMonnies Questionnaire. Arguably the first modern dry eye questionnaire, the McMonnies is comprised of 14 items that focus on established risk factors for dry eye including age, sex, contact lens wear, medication use and certain systemic and ocular factors.5 The questionnaire was intended to both determine the presence of dry eye and identify individuals at risk for developing the disease. Several studies validating the McMonnies questionnaire as a means to screen patients for dry eye disease exist.6,7 A separate study evaluating the psychometric properties—reliability, validity and accuracy—of it reported poor internal consistency, moderate test-retest reliability and fair concurrent validity and accuracy.8 The McMonnies questionnaire appears on p. 15. • Dry Eye Questionnaire (DEQ) and Contact Lens Dry Eye Questionnaire (CLDEQ). Both versions of the DEQ include categorical scales to measure prevalence, frequency, diurnal severity and intrusiveness of common ocular surface symptoms in a typical date of a one-week recall period. Participants are asked to indicate “never, infrequent, frequent or constant” with regard to frequency and intensity of comfort, dryness, visual changes, soreness and irritation, grittiness and scratchiness, burning and stinging, foreign body sensation, light sensitivity and itching.9 The two questionnaires also include questions on the perceived time of day that symptoms worsen, how much the symptoms affect daily activities, computer use, use of systemic and ocular medications, and presence of allergies.9 The DEQ has been successfully evaluated for its use in measuring the frequency and intensity of symptoms of ocular irritation in patients with aqueous tear deficient dry eye.10 • Ocular Surface Disease Index (OSDI). Developed by the Outcomes Research Group at Allergan, the OSDI questionnaire is a self-administered 12-question scale designed to assess a range of ocular surface symptoms, their severity and impact on visual function in a one-week recall period.11 Currently, the OSDI is one of only two validated dry eye questionnaires to include quality-of-life measures for clinical use.12 • Subjective Evaluation of Symptom of Dryness (SESoD). The SESoD is a three-item questionnaire created by Allergan to evaluate a patient’s perception of ocular discomfort related to dryness. Together with the DEQ, McMonnies and OSDI, the SESoD has been shown to exhibit unidimensionality—that is, it is comprised of questions that measure specific metrics simply and linearly to yield straightforward values.13 For example, income is a unidimensional variable; socioeconomic status, which includes income, occupation and education, is a multidimensional variable. • Impact of Dry Eye on Everyday Life (IDEEL). The 57-question IDEEL survey from Alcon assesses the effect of dry eye with respect to three primary modules: dry eye symptom bother, impact on daily life (comprising impact on daily activities, emotional state and work) and treatment satisfaction (comprising patient attitude towards treatment effectiveness and treatment-related bother/inconvenience).14 Together with the OSDI questionnaire, the IDEEL survey comprises a small category of dry eye questionnaires that include quality-of-life measures for clinical use.12 A psychometric analysis performed as part of a validation study involving 210 subjects—130 with nonSjögren's keratoconjunctivitis sicca, 32 with Sjögren's syndrome and 48 controls—found IDEEL to exhibit good consistency and reliability.14 Strong correlation between IDEEL and the Dry Eye Questionnaire was also noted.14 • Standard Patient Evaluation of Eye Dryness (SPEED). The SPEED questionnaire is a four-question survey developed by TearScience to assess frequency and severity of patient dry eye symptoms. In particular, it monitors diurnal and longer-term symptom changes over the course of three months.15 The SPEED questionnaire has been shown to exhibit good validity, unidimensionality, objectivity and consistency when compared with the DEQ, McMonnies questionnaire, OSDI and SESoD.15 • Dry Eye-Related Quality-of-Life Score Questionnaire (DEQS). The DEQS is a 15-item questionnaire created to assess the presence of dry eye symptoms and their severity, and the effects of these symptoms on aspects of patients’ everyday lives, including psychological and social aspects.16 A psychometric analysis found the study had good internal consistency, test-retest reliability, discriminant validity and responsiveness to change; thus, the test is valid and reliable for evaluating the multifaceted effect of dry eye disease on a patient’s daily life.16 14 REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 012_RCCL0415_F1ANDF2.indd 14 3/27/15 3:43 PM McMonnies Dry Eye Questionnaire Please answer the following by underlining the responses most appropriate to you: Female / Male (0) Age: less than 25 years years(M2/F6) (M1/F3) / 25-45 years / more than 45 Currently wearing: no contact lenses / hard contact lenses / soft contact lenses 1. Have you ever had drops prescribed or other treatment for dry eyes? Yes(6) / No(0) / Uncertain(0) 2. Do you ever experience any of the following eye symptoms? 1. Soreness 2. Scratchiness 3. Dryness 4. Grittiness 5. Burning 3. How often do your eyes have these symptoms? Never(0) / Sometimes(1) / Often(4) / Constantly(8) 4. Are your eyes unusually sensitive to cigarette smoke, smog, air conditioning, or central heating? Yes(4) / No(0) / Sometimes(2) 5. Do your eyes become very red and irritated when swimming? Not applicable(0) / Yes(2) / No(0) / Sometimes(1) 6. Are your eyes dry and irritated the day after drinking alcohol? Not applicable(0) / Yes(4) / No(0) / Sometimes(2) 7. Do you take: antihistamine tablets(2) or use antihistamine eye drops(2), diuretics (fluid tablets)(2), sleeping tablets(1), tranquillizers(1), oral contraceptives(1), medication for duodenal ulcer(1), digestive problems(1), high blood pressure(1), antidepressants(1) or ___________________? (Write in any medication you are taking that is not listed.) 8. Do you suffer from arthritis? Yes(2) / No(0) / Uncertain(0) 9. Do you experience dryness of the nose, mouth, throat, chest or vagina? Never(0)/ Sometimes(1) / Often(2) / Constantly(4) 10. Do you suffer from thyroid abnormality? Yes(2) / No(0) / Uncertain(0) 11. Are you known to sleep with your eyes partly open? Yes(2) / No(0) / Sometimes(1) 12. Do you have eye irritation as you wake from sleep? Yes(2) / No(0) / Sometimes(1) Scores: Normal (< 10) Marginal dry eye (10 - 20) Pathological dry eye (>20) From: McMonnies C, Ho A: Patient history in screening for dry eye conditions. J Am Optom Assoc 1987, 58(4):296–301. dry eye to allergic conjunctivitis. It also triggers various treatment options.” Also, its short length allows patients to feel like they are making the best use of their time. Dr. Karpecki created a custom questionnaire because he felt he “needed more information and didn’t want to have to rely on my memory to ask the right questions of the patient.” It’s a culmination of his 20 years’ experience running a dry eye clinic plus information from research papers and dry eye studies. Another option is to adapt an existing questionnaire or two. John D. Sheppard, MD, president of Virginia Eye Consultants, uses both the OSDI and SPEED questionnaires, but says “both ask a little less than we’d like to differentiate the different types of ocular surface disease. We all think about dry eye, which is ubiquitous, but also extremely common are MGD and blepharitis as well as ocular allergy.” Patients who present at Dr. Sheppard’s practice take a modified version of the SPEED questionnaire. “A good supplemental question to ask on the SPEED is, ‘Do your eyes itch?’ Answer choices include: Infrequently; frequently; all the time; it’s driving me crazy. Itching is an important symptom that overlaps between the three most common ocular surface conditions but focuses most on ocular allergy,” Dr. Sheppard says. “Another question that seems to help with blepharitis is, ‘Are your eyelids red?’ with the same frequency qualifiers. Also, ‘Are your eyes burning?’ Burning seems to be something that helps with identifying blepharitis. You can also ask patients about crusting and matting on their lids as well.” THE IDEAL What might the ideal dry eye questionnaire look like? Dr. Epstein says SPEED comes closest. “It’s free, it’s quick, it’s repeatable and it should be used consistently with all dry eye patients. It is also an excellent tool for uncovering dry eye among patients who are ‘silent sufferers’ and don’t realize they have a problem that can be effectively—or more effectively—managed.” Dr. Kabat agrees about the basic principles of an ideal questionnaire and that the SPEED is one such example, but also offers a more general set of characteristics. “If the questionnaire takes more than three minutes for the patient to complete, then it is impractical. If it takes more than one minute to score, then it is impractical. If it cannot be administered and scored by a technician or assistant, then it is impractical,” he says. “For the physician, there should be no more of a time commitment than glancing at the number and assessing its value relative to the scale.” (Continued on p. 19) REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 012_RCCL0415_F1ANDF2.indd 15 15 3/27/15 3:43 PM Bringing Clarity TO CLARE Understanding and knowing how to treat this common contact lens complication can benefit both your patients and your practice. By Lindsay A. Sicks, OD T he clinical entity known as contact lens-induced acute red eye, or CLARE, is an inflammatory reaction of the cornea and conjunctiva associated with overnight contact lens wear. It is also commonly referred to as acute red eye or tight lens syndrome. Often, the patient will present to your practice wearing dark sunglasses or clutching a box of tissues in an effort to cope with their symptoms. While treatment is relatively straightforward, episodes of this condition can recur; thus, our job as clinicians is not only to treat the condition in its acute stage, but also to educate the patient and give them the tools to return to lens wear in the healthiest possible manner. SIGNS AND SYMPTOMS CLARE is typically characterized by sudden onset of unilateral eye pain, photophobia, epiphora and ocular irritation. Accompanying slit lamp signs include diffuse conjunctival and limbal hyperemia, as well as the presence of multiple corneal epithelial and subepithelial infiltrates. The infiltrative reaction is generally located in the corneal periphery and mid-periphery; when sodium fluorescein stain is instilled in the 16 eye, the infiltrative areas do not typically exhibit overlying punctate staining, indicating minimal epithelial involvement.3,4 In more severe cases of CLARE, corneal edema or anterior uveitis may also be present, although these signs are not common.1,2 Visual acuity is usually unaffected. It is prudent to ask patients presenting with CLARE symptoms about any recent illnesses, including symptoms of the common cold such as headache, fatigue and runny nose. Often, upper respiratory tract infections are associated with gram-negative organisms like Haemophilus influenza.1,2 One study found that patients who were colonized with H. influenzae were more than 100 times as likely to have had a CLARE or infiltrative response than those subjects who were not colonized with this bacterium.5 CASE HISTORY AND EVALUATION Typically, the most reliable way to accurately diagnose CLARE is with a complete case history and assessment of the symptoms mentioned above. By definition, CLARE is associated with sleeping while wearing contact lenses.2,3 This can be anything from a short afternoon nap to a full night of extended wear—the fact that the eye is closed for an extended period of time is key to our diagnosis. So, consider asking all your contact lens patients how many times per week they sleep or nap in their lenses as part of your routine history sequence. Knowledge of the patient’s habitual lens type and wearing schedule may also have some value in our diagnostic considerations. Conventionally, CLARE is associated with tight fit or poor movement of extended-wear, low oxygen permeability, high water content hydrogel lenses. However, note that CLARE can also be caused by extended wear of silicone hydrogel lenses, which have significantly risen in market share in the United States in the last decade.1,6 CLARE has been reported to occur in 34% of continuous wear hydrogel lens patients and less than 1% of silicone hydrogel extended wear patients.7-9 Reports have also linked CLARE to ABOUT THE AUTHOR Dr. Sicks is an assistant professor at Illinois College of Optometry in Chicago. She is involved in the contact lens didactic curriculum and also serves as a clinical attending physician in the Illinois Eye Institute’s Cornea Center for Clinical Excellence. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 012_RCCL0415_F1ANDF2.indd 16 3/27/15 3:44 PM extended wear gas permeable (GP) lenses, high oxygen permeability silicone elastomer lenses and overwear of daily disposable soft contact lenses.10 In the absence of a lens fit evaluation, history questions regarding hours per day of lens wear and difficulty with lens removal at the end of the day may assist in diagnosis. If you are able to assess the lens on-eye, pay special attention to lens movement and push-up test results. Note, however, that there are reported cases of CLARE occurring with well-fit contact lenses showing adequate movement.10,11 cells, and then infiltration of the injured tissue by polymorphonuclear leukocytes and other cells. This collection of inflammatory cells within the cornea forms what we call an infiltrate. The result is CLARE and its associated signs of conjunctival hyperemia and corneal epithelial and subepithelial infiltrates.7 DIFFERENTIAL DIAGNOSIS In a case that may be CLARE or another corneal infiltrative event (CIE), the most important element to consider is whether the presenting condition is infectious or non-infectious. moderate to severe pain symptoms that worsen with lens removal. Anterior chamber cells and flare and mucopurulent discharge are more common in MK than CLARE and CLPU.3 A positive bacterial culture or the presence of tear film exudate can also help make an MK diagnosis. CLARE can also appear similar to conditions like contact lensinduced peripheral ulcer (CLPU) and infiltrative keratitis (IK).3 However, while CLARE typically presents with multiple small focal and diffuse infiltrates that do not stain with fluorescein, CLPUs are characterized as single circular ETIOLOGY While the etiology of CLARE is not completely understood, it is generally classified as an inflammatory event of the cornea and conjunctiva. General risk factors include wear of high water content lenses, wear of tight fitting lenses and history of a recent upper respiratory tract infection.2 One commonly cited cause of CLARE is coloniAcute contact lens-associated red eye presentation in a 28-year-old Indian male. zation of the lens surHe noted associated blurry vision, foreign body sensation and photophobia. face with gram-negative After a 10-day course of tobramycin/dexamethasone suspension QID and preservative-free artificial tears every hour for relief, he reported a significant bacteria, specifically H. improvement in symptoms. (Case and photo courtesy of Kelli Theisen, OD.) influenzae, Pseudomonas aeruginosa and Serratia Due to its sight-threatening pomarcescens.12 An inflammatory focal infiltrates up to 2mm in response is triggered by endotoxtential if left untreated, microbial diameter that pick up fluoresins released by the breakdown keratitis (MK) should be high on cein stain. IK is associated with of bacterial cell walls. The conthe list of differentials in any conStaphylococcal hypersensitivity dition is worsened in the tight tact lens wearer presenting with a and may occur in one or both eyes lens environment because of lens red eye. To differentiate MK from showing multiple small infiltrates dehydration, minimal lens moveother CIEs, look for a discrete with or without corneal staining. ment, decreased tear exchange and area of fluorescein staining, typiA careful history and slit lamp hypoxia.1,7,10,12,13 cally greater than 1mm diameter examination can help guide your In the inflammatory process, and often located in the central diagnosis. limbal vasodilation occurs, folcornea. There may also be lid The remaining CIEs are catlowed by release of white blood edema, a reactive ptosis, and more egorized as asymptomatic and REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 012_RCCL0415_F1ANDF2.indd 17 17 3/27/15 3:44 PM BRINGING CLARITY TO CLARE clinically insignificant.3 Asymptomatic infiltrative keratitis (AIK) and asymptomatic infiltrates (AI) are simply differentiated from CLARE in that they are seen on physical exam but carry no entering complaints. Other differentials to consider include: chlamydial conjunctivitis, trachoma, adenoviral infection, epidemic keratoconjunctivitis, Staphylococcal marginal keratitis, Thygeson's superficial punctate keratitis and herpes simplex keratitis.14 TREATMENT AND MANAGEMENT Management of CLARE always begins with discontinuation of contact lens wear. Beyond that, the condition is often self-limiting and may not require therapeutic intervention—in many cases, palliative treatment with artificial tears will suffice. However, we often prescribe additional therapeutic options to promote healing and improve patient comfort. Depending on severity, the infiltrates can take days to weeks following cessation of lens wear to heal. Since many of the signs and symptoms of CLARE mimic those of microbial keratitis, it is prudent to instill sodium fluorescein and assess the corneal integrity for any epithelial disruption. Typically, there is minimal to no epithelial disruption with CLARE; however, if there is corneal staining present in association with an infiltrate, the diagnosis no longer clear-cut and the lesion becomes suspicious for MK. In such cases, conservative management warrants using a topical antibiotic for at least the first 48 hours of treatment. Some practitioners may prefer to address both the inflammation and risk of infection as quickly as possible by prescribing a combination 18 topical antibiotic/steroid from the start. Recommended follow-up is daily until signs of improvement are shown. In cases where the photophobia is particularly symptomatic, or where there is an accompanying anterior uveitis component, application of a topical cycloplegic agent is warranted for at least the first 24 hours. Topical and oral NSAIDs are also effective adjunct treatment options to quell the discomfort. If cells and flare persist, consider addition of a topical steroid to the regimen. After complete healing, patients can resume lens wear using a fresh lens right out of the vial or blister pack. Consider changing lens fit, material, modality and/ or replacement schedule prior to resuming lens wear to reduce potential for reoccurrence. For example, if the habitual lens was a tight fit, try selecting different base curve or diameter to improve movement and centration. If the patient has a history of lens abuse or overwear, switch them to a daily disposable lens design instead. Also, consider refitting patients into GP lenses—patients with a history of soft lens complications often adapt well to GP lenses and appreciate the benefits they provide. It is important to note that recurrence of inflammatory complications can happen in 50% to 70% of wearers who resume hydrogel extended wear after resolution of their initial episode of CLARE.15 Additionally, patients who have had a CLARE episode retain higher levels of limbal injection, bulbar injection and conjunctival staining afterwards compared with controls.9 Careful slit lamp examinations and shorter intervals between contact lens appointments following a CLARE episode may be the best practice to follow based on your clinical judgment. Above all, patient education plays an important role in preventing corneal infiltrative events such as CLARE. Stressing the importance of appropriate lens replacement, wear and care schedules to all of your contact lens patients can promote better patient adherence to our recommendations. Patients should be advised to stop wearing their lenses while ill and when lens wear is uncomfortable or painful, particularly while their eyes are closed. For patients who have had a CLARE episode, emphasizing the risk of recurrence as well as a review of symptoms to look out for may also be helpful. Be sure to also provide an easy way for patients to contact your office in case of an emergent issue so that they end up in the best hands possible should another complication occur. RCCL Coding for CLARE Currently, there is no exact match in ICD-9 nomenclature for CLARE, and it does not appear that ICD-10 will have any additional entries that are more appropriate. As such, one should continue to report CLARE using a symptom code appropriate to the chief complaint, such as those for eye pain, redness of the eyes or epiphora. If there is an accompanying corneal infiltrate, additional codes for central and peripheral corneal opacity would also be appropriate. Other applicable options may include anterior uveitis, viral conjunctivitis or corneal edema codes, depending on the case. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 012_RCCL0415_F1ANDF2.indd 18 3/27/15 3:44 PM 1. Dumbleton K, Jones L. Extended and Continuous Wear. in Clinical Manual of Contact Lenses. E. Bennett and V. Henry, Eds. Williams and Wilkins. 2008:410-443. 2. Stapleton F, Keay L, Jalbert I and Cole N. The epidemiology of contact lens related infiltrates. Optom Vis Sci. 2007;84(4):257-272. 3. Sweeney DF, Jalbert I, Covey M, et al. Clinical characterization of corneal infiltrative events observed with soft contact lens wear. Cornea. 2003;22(5):435-442. 4. Sankaridurg PM, Holden BA, Jalbert I. Adverse events and infections: which ones and how many? In e. Sweeney D, Silicone Hydrogels: Continuous Wear Contact Lenses (pp. 217-274). Oxford: Butterworth-Heinemann. 5. Sankaridurg PR, Willcox MD, et al. Haemophilus influenzae adherent to contact lenses associated with production of acute ocular inflammation. J Clin Microbiol. 1996;34(10):2426-2431. 6. Nichols JJ. Annual Report: Contact Lenses 2013. Contact Lens Spectrum;29(January 2014):22-28. 7. Zantos SG, Holden BA. Ocular Changes Associated with Continuous Wear of Contact Lenses. The Australian Journal of Optometry. 1978;61(12):418-26. 8. Nilsson, S. Seven-day extended wear and 30day continuous wear of high oxygen transmissibility soft silicone hydrogel contact lenses: a randomized 1-year study of 504 patients. CLAO J. 2001;27(3):125-36. 9. Stapleton F, Keay L, Jalbert I, Cole N. Altered Conjunctival Response After Contact Lens–Related Corneal Inflammation. Cornea. 2003;22(5):443-7. 10. Sankaridurg PM, Vuppala N, Sreedharan A, et al. Gram negative bacteria and contact lens induced acute red eye. Indian J Ophthalmol, 1996;44(1):29-32. 11. Crook, T. Corneal infiltrates with red eye related to duration of extended wear. J Am Optom Assoc. 1985;56(9):698-700. 12. Holden BA, La Hood D, Grant T, et al. Gramnegative bacteria can induce contact lens related acute red eye (CLARE) responses. CLAO J. 1996;22(1):47-52. 13. Binder PS. The physiologic effects of extended wear soft contact lenses. Ophthalmology. 1980;87(8):745-9. 14. Robboy MW, Comstock TL, Kalsow CM. Contact Lens-Associated Corneal Infiltrates. CLAO J 2003;29(3):146-54. 15. Sweeney DF, Grant T, Chong MS, et al. Recurrence of acute inflammatory conditions with hydrogel extended wear. Invest Opthalmol Vis Sc 34:S1008. DRY EYE: SEE IT THROUGH THEIR EYES (Continued from p. 15) Additionally, Dr. Kabat says, it should assess symptom impact on lifestyle and provide a metric for quantifying symptom severity, and should have the ability to be used “as a screening tool for all patients in a practice with interest in dry eye management, or as part of the data/ history collection in a specialty dry eye practice.” The ideal dry eye questionnaire should also cover certain symptoms. “Key symptoms must be included such as blurred or transient blurred vision, dryness/grittiness, irritation, burning and watering,” Dr. Karpecki says, and also include severity, frequency and which eye drops are currently being used. Dr. Donnenfeld adds, “We want to know the patient’s ability to function at normal tasks.” Dr. Sheppard envisions the development of something more technologically advanced. “I would have a questionnaire that the patient could fill out at home in a reproducible format that we could then plug in digitally when they walk into the office with essentially no effort on the part of the technician,” he says. “The information would then appear as a global score on the chart, with maybe a bar graph read-out that tells us this is aqueous deficiency, this is lipid deficiency, this is blepharitis, this is allergy.” Historical data could then portray the progression or resolution of important complaints in one readout, he says. Ultimately, the choice of which— if any—dry eye questionnaire depends on practitioner preference. But no matter what, “providing surveys to patients about their symptoms demonstrates a sense of empathy for their condition that many practitioners fail to do,” Dr. Hauser says. “Often, dry eye patients feel as if they are relegated to an afterthought by their doctors. The patients recognize that their activities of daily living have been inhibited, if not devastated, by ocular surface disease, and they appreciate the attention to their plight.” RCCL 1. Gayton JL. Etiology, prevalence and treatment of dry eye disease. Clin Opthalmol. 2009; 3:405-12. www.ncbi.nlm.nih.gov/pmc/articles/ PMC2720680/ 2. Savini G, Prabhawasat P, Kojima T, et al. The challenge of dry eye diagnosis. Clin Ophthalmol. 2008 Mar;2(1):31-55. 3. Bjerrum KB. Test and symptoms in keratoconjunctivitis sicca and their correlation. Acta Ophthalmol Scand 1996;74:436–41. 4. Hay EM, Pal TB, et al. Weak association between subjective symptoms of and objective testing for dry eyes and dry mouth: results from a population based study. Ann Reum Dis 1998;57:20–4. 5. McMonnies CW. Key questions in a dry eye history. J Am Otpom Assoc. 1986 Jul;57(7):512-7. 6. McMonnies CW, Ho A. Patient history in screening for dry eye conditions. J Am Optom Assoc. 1987;58:296-301. 7. McMonnies CW, Ho A. Responses to a dry eye questionnaire from a normal population. J Am Optom Assoc. 1987:58:588-591. 8. Nichols KK, Nichols JJ, Mitchell GL. The reliability and validity of McMonnies Dry Eye Index. Cornea. 2004 May;23(4):365-71. 9. Begley C, Chalmers RL, Mitchell GL et al. Characterization of Ocular Surface Symptoms from Optometric Practices in North America. Cornea 2001;20(6):610-18. 10. Begley CG, Caffery B, Chalmers RL, et al. Use of the Dry Eye Questionnaire to Measure Symptoms of Ocular Irritation in Patients with Aqueous Tear Deficient Dry Eye. Cornea 2002;21(7):664-70. 11. Walt JG, Rowe MM, Stern KL. Evaluating the functional impact of dry eye: the Ocular Surface Disease Index [abstract]. Drug Inf J. 1997;31:1436. 12. Grubbs JR Jr, Tolleson-Rinehart S, Huynh K, Davis RM. A review of quality of life measures in dry eye questionnaires. Cornea. 2014 Feb;33(2):215-8. 13. Simpson TL, Situ P, Jones LW, et al.. Dry eye symptoms assessed by four questionnaires. Optom Vis Sci. 2008;85:692–699. 14. Espindle D, Simpson T, Nelson J, et al. Development and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patientreported outcomes (PRO) measure for the assessment of the burden of dry eye on patients. Health Qual Life Outcomes. 2011 Dec 8;9:111. 15. Ngo W, Situ P, Keir N, et al. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32(9):1204-10. 16. Sakane Y, Yamaguchi M, Yokoi N, et al. Development and validation of the Dry Eye-Related Quality-of-Life Score questionnaire. JAMA Ophthalmol 2013 Oct:131(10):1331-8. 17. The Epidemiology of Dry Eye Disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). The Ocular Surface. April 2007;5(2):93-107. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 012_RCCL0415_F1ANDF2.indd 19 19 3/27/15 3:44 PM SPECIAL CARE Keeps Specialty Lens Wearers Contact lens care is a vital step to the continued safety and health of the contact lens patient. So how does lens care differ in the case of sclerals and other specialty lenses? Safe By Susan J. Gromacki, OD, MS W SCLERAL CONTACT LENSES Currently, scleral gas permeable (GP) contact lenses represent the fastest growing segment of the specialty contact lens industry.1 Already invaluable for treating patients with keratoconus and other corneal irregularities, scleral lenses are now also being worn by healthy patients who require simple refractive correction. Inherently larger than corneal GP lenses, sclerals are designed to vault the cornea and rest on the sclera. As such, they must be filled with solution prior to application to prevent air bubbles from forming underneath the lens (Figure 1), which can compromise comfort, vision and corneal health (i.e., compression of the epithelium 20 Photo: Greg DeNaeyer, OD e and our patients are fortunate to live in an age where we have a variety of contact lens options designed to improve vision and comfort, and promote ocular surface health. These specialty lenses are truly different and, as such, require special care. Fig. 1. Scleral contact lens with insertion bubble. and differential oxygen levels). In order to prevent this solution from spilling during application, patients should be instructed to keep their head down, parallel to the ground. In this position, the patient should open both eyelids wide (scleral lenses average about 16.0mm in diameter), gently place the lens on the conjunctiva and then close the eyelids.2 ABOUT THE AUTHOR Dr. Gromacki is a Fellow of the American Academy of Optometry and a Diplomate in the Cornea, Contact Lens, and Refractive Technologies section. She has written extensively and lectured internationally on the topics of cornea and contact lenses and serves as the Director of the Contact Lens Service at a subspecialty group practice in Maryland. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 020_RCCL0415_F3.indd 20 3/27/15 3:44 PM Note a scleral lens’s thickness (approximately 0.3mm), diameter and depth can affect its center of gravity, making it more difficult to balance the lens on one finger compared with a soft or corneal GP lens. As such, a number of methods may be utilized to assist in holding the lens for proper insertion: • The “tripod method.” After forming a tripod with the thumb, index finger and middle finger, rest the lens in the center of the three digits for application. • A large DMV or suction cup. As a recommendation, cut a small slice off the bottom or order the suction cup fenestrated so that it will be easier to remove from the lens following placement on the ocular surface. • A #8 O-ring. Available from GP lens manufacturers or at many hardware stores. Before application, place the ring on the tip of the index finger and place the lens on top of the ring.3 • Ezi Scleral Lens Applicator (QCase). A ring-like device equipped with a bowl on which to balance the scleral lens during application. • See Green Lens Inserter (Dalsey Adaptives). A device equipped with a permanent standing suction cup and green light to help focus the patient’s gaze during application (Figure 2). According to the manufacturer, the device is particularly suited for patients who struggle with manual dexterity, are monocular and cannot see the lens, or need to hold their eyelids.4 FILLING THE LENS Scleral lenses provide minimal tear exchange, meaning the solution placed in the bowl of the lens prior to application remains in direct contact with the cornea during most of the lens-wearing day; thus, it is critical to use a nonpreserved solution to prevent preservatives from inducing allergic or hypersensitivity reactions.5,6 Scleral lenses are commonly filled with unit-dose sodium chloride 0.9% inhalation/irrigation solution, which can be obtained in 3mm or 5mm vials from a pharmacy or online. Note that although it is a non-prescription item, some pharmacies may still require a prescription. I provide a preprinted, signed medical prescription to all of my scleral lens patients. This has two benefits: it tells the pharmacist that the solution is for scleral contact lenses (thus saving us both a phone call) and it increases the likelihood that the patient’s medical insurance will cover the expense.5,6 Scleral patients suffering from dry eye and those whose lenses exhibit areas of touch or minimal clearance may benefit from filling their lenses with unit-dose artificial tears instead, which provide extra lubrication and corneal protection. Keep in mind, however, that only the clear Fig. 2. The See Green Lens Inserter with stand (Dalsey Adaptives). brands, not the milky Enzymatic Cleaners Patients wearing GP, soft or hybrid lenses who are prone to heavy protein deposition can use an enzymatic cleaner once per week or more. or viscous ones, will work without compromising visual clarity. Also, try to avoid formulations with HPGUAR; while a fantastic wetting agent, it has the potential to gel underneath the lens.7 Manufacturers are also increasingly recommending against use of larger (e.g., 4 oz.) bottles of nonpreserved saline because they often contain buffers, which can contribute to debris or mucin buildup underneath the lens.8,9 Patients are also less likely to comply with discarding a larger bottle should it become contaminated. Note that all of the options currently available for filling scleral lenses are considered off-label by the US Food and Drug Administration. That being said, research may one day produce a solution that is more biocompatible and similar to the tear film, but for now, unitdose nonpreserved saline is the best option we have at this time.5,6 CLEANING SCLERAL LENSES Scleral contact lenses are simply large GP lenses, so any solutions approved to clean and disinfect corneal GP lenses can be used for scleral lenses. However, because there is less tear flow under the edge of a scleral lens compared with a corneal GP lens, additional care should be taken to ensure that the lens surface is both clean and free of pathogens. I recommend a separate daily cleaner for all scleral lenses, regardless of whether they’re plasma-treated or not. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 020_RCCL0415_F3.indd 21 21 3/27/15 3:45 PM SPECIAL CARE KEEPS SPECIALTY LENS WEARERS SAFE Examples of daily cleaners suitable for GP lenses include: Boston Cleaner (Bausch + Lomb), Boston Advance Cleaner (Bausch + Lomb), Optifree Daily Cleaner (Alcon), or Optimum by Lobob ‘Extra Strength Cleaner’ (Lobob). The latter, or an isopropyl alcohol-based cleaner approved for GP lenses, may be preferable for cleaning high Dk materials, which may scratch more easily with more abrasive cleaners. I also follow the FDA’s recommendation to rinse with saline, rather than tap water, to remove all cleaner from the lens, due to the fact that all water contains some levels of bacteria, fungi and amoebae.11 Lens disinfection should be performed with a GP conditioning/disinfection solution such as Boston Advance Comfort Formula Conditioning Solution (Bausch + Lomb) or Boston Conditioning Solution (Bausch + Lomb) or with Generic Solutions, Specific Problems According to a recent study, the main determinant (38%) of which type of contact lenses were fitted and purchased was price.14 If lens price has such an effect on the final selection when the doctor is the primary decision-maker, imagine its considerable impact when patients are making purchasing decisions on their own, such as when selecting a solution. Every year or so, a retailer entertains bids on which company will produce and package its private-label solution. When the contract expires, the formulation may likely change. Because a retailer generally accepts the lowest bidder, companies typically do not place their premium solutions in generic bottles. So, because expiration dates are typically 18 to 36 months into the future, there could be two different chemical formulations residing in two of the same bottles sitting side-by-side on the store shelf. Likewise, a retailer can label two bottles of the same formulation differently— each to mimic popular brands.15,16 The bottom line: older formulations were the state-of-the art 10 or 15 years ago, and most work well for most patients. But since then, we have learned more about material/solution interaction; the private label solution may not be what’s most compatible for the patient.17,18 Research has also demonstrated a statistically higher rate of such ocular complications with patients who use private label compared with name-brand solutions.19 Additionally, FDA recommended in 2010 that all multipurpose solutions carry “rub and rinse” instructions, and the care systems launched since then have complied. However, some mass retailers’ packages still contain the words “no rub.” It has been common knowledge for several years that a cleaner lens is a healthier lens, and research has proven that digitally rubbing a lens is more effective than not at removing the deposits that can lead to inflammation or infection.20 We have made great advances in solutions over the past few years—including improved cleaning and disinfection, less toxicity, and increased comfort and wettability—and the patient who buys generic isn't benefiting from that technology.16 22 a GP multipurpose solution such as Boston Simplus Multi-Action Solution (Bausch + Lomb), Menicon Unique pH (Menicon), Optifree GP (Alcon), or Optimum C/D/S (Lobob). In addition, the Menicon Deluxe Care System (with Progent) is now approved for home use. Many scleral lens fitters advise sensitive patients to rinse the lens with nonpreserved saline prior to application. While this removes any residual solution—including its preservatives—left over from the disinfection process, it can also diminish wettability. Hydrogen peroxide solutions like PeroxiClear (Bausch + Lomb) or Clear Care (Alcon) are good preservativefree alternatives; however, these solutions are FDA-approved for GP lenses only if they are digitally rubbed prior to disinfection. If necessary, larger cases that accommodate diameters up to 30mm can be obtained from online stores like the Dry Eye Shop. The catalytic neutralization disc is not included with purchase, however, so one needs to be transferred from the smaller case prior to use.12 HYBRID LENSES Hybrid contact lenses are comprised of a GP center surrounded by a hydrophilic skirt. Examples include the Duette and UltraHealth (SynergEyes). Manufacturer guidelines advise patients who wear these lenses to digitally rub the lens, front and back, with a daily cleaner approved for silicone hydrogel soft lenses, then rinse off the cleaner with nonpreserved saline. For disinfection, manufacturer guidelines recommend Clear Care (Alcon), BioTrue (Bausch + Lomb), Renu fresh (Bausch + Lomb) or Complete Easy Rub (Abbott Medical Optics). It should be noted that UltraHealth, due to its vaulted REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 020_RCCL0415_F3.indd 22 3/27/15 3:45 PM Fig. 3. Acceptable NovaKone (Alden Optical) fit on a keratoconic eye, enhanced with high molecular weight fluorescein. design, needs to be filled with nonpreserved saline or artificial tears prior to insertion, then inserted with the head kept down similar to inserting a scleral lens. For keratoconus patients who wear the KC and ClearKone (SynergEyes) hybrid lenses, preservative-based care systems should be avoided. Hybrid lens manufacturer guidelines recommend Clear Care or Oxysept Ultracare (Abbott Medical Optics). For all hybrid lenses, a digital rubbing step is required, as they have a six-month replacement schedule. Since they contain a soft skirt, gas permeable solutions are contraindicated. SOFT LENSES FOR KERATOCONUS There are now excellent soft contact lens designs used specifically to treat keratoconus. Because these lenses are custom-produced and last up to three months, a digital rubbing step is typically recommended. (Of specific note, Bausch + Lomb recommends rubbing its KeraSoft IC lens in between the fingers, rather than in the palm of the hand.13) Because soft lenses for keratoconus tend to be thicker than disposable soft contact lenses, most manufacturers recommend using nonpreserved disinfectants because of the potential for absorption into the lens matrix. Alden Optical recommends hydrogen peroxide systems for its NovaKone lens (Figure 3), while Bausch + Lomb recommends use of either multipurpose or hydrogen peroxide with its KeraSoft IC. However, if multipurpose solution is used, B+L suggests rinsing it off with sterile rinsing solution prior to application.13 CONCLUSION The fitting of scleral and other specialty contact lenses requires great diligence and attention to detail on the part of the practitioner. However, even the best scleral lens fit can be compromised by poor lens care on the part of the patient. This part of the equation is just as critical to contact lens success. For further information on contact lens care, please consult each lens and/or material manufacturer for its specific care recommendations. RCCL 1. Nichols, J. 2014 annual report: Contact lenses 2014. Contact Lens Spectrum. 2015;30(1):2227. 2. Gromacki SJ. Handling and care of scleral GP contact lenses, Part 1. Contact Lens Spectrum. 2011;27(10):27. 3. Gromacki SJ. Scleral GP contact lens insertion, removal, and care. [Webinar.] Gas Permeable Lens Institute, March 2014. Available at: www.gpli.info/videos/webinar-2014-03.htm. 4. Dalsey Adaptives. The See-Green Lens Inserter. Available at: www.dalseyadaptives.com. Accessed February 2015. 5. Gromacki SJ. Handling and care of scleral GP contact lenses, Part 2. Contact Lens Spectrum. 2012;27(1):19. 6. Gromacki SJ. Scleral GP lens preparation: The latest standard of care. Contact Lens Spectrum. 2013;28(11):25. 7. Smythe M. Personal communication, April 10, 2014. 8. Imavasu M, Hori Y, Cavanagh HD. Effects of multipurpose contact lens care solutions and their ingredients on membrane-associated mucins of human corneal epithelial cells. Eye Contact Lens. 2010 Nov;36(3):361-6. 9. Gorbet MB, Tanti NC, Jones L, Sheardown H. Corneal epithelial cell biocompatibility to silicone hydrogel and conventional hydrogel contact lens packing solutions. Mol Vis 2010 Feb 19;16:272-82. 10. US Food and Drug Administration. Medical Devices. Available at: www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ HomeHealthandConsumer/ConsumerProducts/ ContactLenses/. Accessed February 2015. 11. Ward M. General Session #9: Contact Lens Care. Lecture at The Global Specialty Lens Symposium, January 24, 2015; Las Vegas, Nevada. 12. The Dry Eye Shop. Lens Cases. Available at: www.dryeyeshop.com/lens-cases-c95.aspx. Accessed February 2015. 13. A&R Optical. Patient Instruction/Wearer’s Guide for Intelliwave3/KeraSoft IC. Available at: www.artoptical.com/files/documents/ resources/Intelliwave_Patient_Instruction_-_ Efrofilcon_A_1.pdf. Accessed February 2015. 14. Ichijima H, Shimamoto S, Ariwaka Y, et al. Compliance study of contact lens wearing in Japan, part 1: Internet survey of actual circumstances of lens use. Eye & CL 2014;40(3):169174. 15. Ferris State University. Private Label Lens Care Guide. Available at: www.ferris.edu/ HTMLS/colleges/michopt/vision-researchinstitute/PDFs/contact-lens-solutions.pdf. Accessed February 2015. 16. Gromacki SJ. The truth about generics. Contact Lens Spectrum. 2005;20(12):24. 17. Green JA, Phillips KS, Hitchins VM, et al. Material Properties That Predict Preservative Uptake for Silicone Hydrogel Contact Lenses. Eye Contact Lens. 2012 Nov;38(6):350-357. 18. Andrasko G, Ryen K. Corneal staining and comfort observed with traditional and silicone hydrogel lenses with multipurpose solution combinations. Optom. 2008; 79: 444-454. 19. Forister JY, Forister EF, Yeung KK, et al. Prevalence of contact lens-related complications: UCLA contact lens study. Eye Contact Lens. 2009;35(4):174-80. 20. Schnider C: Clinical performance and effect of care regimen on surface deposition of galyfilcon A contact lenses. [Electronic abstract 055102.] Optom Vis Sci. 2005; 82. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 020_RCCL0415_F3.indd 23 23 3/27/15 3:45 PM 1 CE Credit (COPE Approval Pending) IS THAT CORNEAL INFILTRATE STERILE OR INFECTIOUS? Differentiating between the two requires close observation and analysis. Here’s a results-oriented approach. By Jeffrey Sonsino, OD, and Shachar Tauber, MD D oes this sound familiar? “Doctor, I don’t understand what’s wrong. I have been wearing my contact lenses overnight for years and this has never happened before.” Upon close examination, you note the presence of small, grayish aggregates in the corneal epithelium. The diagnosis? Corneal infiltrates. But how do you tell if they are sterile or infectious—harmless, or a potential serious problem? As practitioners, we have seen any number of contact lens-related complications walk through our doors. Corneal infiltrates and ulcers are two such examples that have long been an unfortunate reality of patient care. In the 19th century, treatment of corneal ulcers included chemical cauterization with silver nitrate. While we’ve come a long way since those days in the care we provide, when such adverse events occur, differentiating infectious and sterile infiltrates is still no easy task. BREAKING IT DOWN Corneal infiltrates result from the penetration of white blood cells into the corneal tissue as part of the body’s inflammatory response 24 to the presence of bacterial toxins, enzymes and byproducts.1-4 A corneal ulcer, by comparison, is an epithelial defect with underlying inflammation (which typically leads to necrosis of corneal tissue). Infiltrates and ulcers are similar in that they both involve disruption of the corneal epithelium; indeed, a staining infiltrate may be the beginning of a corneal ulcer. The difference, however, is that while corneal infiltrates are not sight-threatening, corneal ulcers involve active tissue damage caused either by infectious or non-infectious etiologies. Infectious ulcers are caused by fungus, virus, or parasites like Acanthamoeba) or, most commonly, bacteria.5 Alternately, noninfectious ulcers result from autoimmunity, neurotrophic keratitis, allergy (e.g., shield ulcers), inflammation from blepharitis or chemical burns, or idiopathic conditions (e.g., Mooren’s ulcer). In the case of microbial insult, the damage typically results in an excavation of the corneal stroma, which triggers an anterior chamber response of flare with or without cells (Figures 1, 2 and 3). For this reason, the terms microbial keratitis and bacterial ulcer are sometimes used interchangeably. Four subtypes of contact lensrelated corneal infiltrates exist: microbial keratitis, contact lensinduced peripheral ulcer (CLPU), contact lens-induced acute red eye (CLARE) and infiltrative keratitis. While the etiology of these subtypes is multifactorial, research shows significant overlap between their clinical presentations, suggesting it is not possible to clinically differentiate between them; rather, they should be considered as stages of a single disease spectrum.6 ABOUT THE AUTHORS Dr. Sonsino is a partner in a specialty contact lens and anterior segment practice in Nashville, Tenn. He is a diplomate in the cornea, contact lens, and refractive therapies section of the AAO, a council member of the cornea and contact lens section of the AOA, a fellow of the Scleral Lens Education Society and an advisory board member of the GPLI. Dr. Sonsino is boardcertified by the ABO. Dr. Tauber is an ophthalmologist at Mercy Clinic Eye Specialists and Surgery Center. He is a fellow of the American Academy of Ophthalmology and a member of the American Society of Cataract and Refractive Surgery and International Ocular Surface Society. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 024_RCCL0415_F4_CE.indd 24 3/27/15 4:02 PM Fig. 1. Slit beam evaluation of a corneal ulcer. Deviation of the beam shows corneal excavation. THE “TWO CORNEAS” When determining whether the corneal infiltrate is infectious or sterile, one helpful method is to divide the cornea into two distinct regions. Consider that the central cornea encompasses the 6mm of the cornea apex whereas the peripheral cornea is a 2mm to 4mm doughnut, with the limbus as its posterior border. Based upon the close approximation of the peripheral cornea to the limbus (with its preponderance of stem cells and vascularity), investigators believe the immune response to be more active in this region of the cornea. Quantification of the nerve fibers shows a densely innervated cornea and a five to six times lower innervated peripheral cornea.7 Higher mitotic activity has also been demonstrated in the peripheral cornea.8 These observations indicate the two distinct regions of the cornea have key anatomic, physiologic and pathologic differences, allowing for the generalization that infiltrates in the periphery of the cornea are non-infectious while infiltrates in the central 6mm of the cornea may have an infectious etiology.9 ALWAYS TAKE NOTES As with any medical concern that presents to the clinic, careful history taking can lead the eye care practitioner to identify the proper diagnosis and resultant treatment that gives the patient the best possible outcome with the least risk. • Contact lenses. Contact lens use is one identified risk factor for the development of corneal infiltrates, as evidence shows continuous wear of contact lenses increases the risk of ocular complications. However, it is not the primary cause of corneal infiltrates; rather, it is simply one of several contributors. Other risk factors for corneal infiltrate development in continuous wear contact lens patients include age (i.e., between 18 and Release Date: April 2015 Expiration Date: April 1, 2018 Goal Statement: This course reviews the etiology, contributing factors and clinical presentations of sterile and infectious corneal infiltrates. Key points in ocular examination and treatment will also be discussed. Faculty/Editorial Board: Jeffrey Sonsino, OD, and Shachar Tauber, MD Credit Statement: COPE approval for 1 hour of continuing education credit is pending for this course. Check with your state licensing board to see if this counts toward your 29 years) and a history of smoking, corneal scarring, contact lens acute red eye or corneal infiltrates.10 Of the different corneal infiltrate types, microbial keratitis is the most severe complication of contact lens wear. In the 1980s and 1990s, risk of microbial keratitis was found to be four cases per 10,000 lens wearers per year for daily wear and 20 cases per 10,000 wearers per year for extended wear.11,12 Pseudomonas aeruginosa has been identified as the most common bacterial source of microbial keratitis in contact lens wearers.13 • Corneal trauma. Risk of microbial keratitis also increases any time there is a history of corneal trauma or foreign body presence due to the possibility of incomplete removal. This is especially true when the foreign body is vegetative matter, which is more likely to be contaminated by pathogens. Corneal trauma may also include iatrogenic etiologies, such as retained or broken sutures in penetrating keratoplasty patients. • History of surgery. Because anterior segment surgery compromises epithelial barrier function, any corneal surgery carries a risk of resultant infiltrative keratitis and infectious ulcer. In particular, infiltrative keratitis is associated with astigmatic keratectomy, penetrating keratoplasty, DSAEK, pterygium removal, trabeculectomy, LASIK CE requirements for relicensure. Joint-Sponsorship Statement: This continuing education course is joint-sponsored by the Pennsylvania College of Optometry. Disclosure Statement: Dr. Sonsino consults for SynergEyes, Bausch + Lomb, Alcon, Visionary Optics and Optovue, has received grant support from Visioneering, and owns stock in LVR Technology. Dr. Tauber consults for AMO and Allergan, has received grant support from the Department of Defense, and owns stock in Calhoun Vision and Ocugenics. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 024_RCCL0415_F4_CE.indd 25 25 3/27/15 4:02 PM IS THAT CORNEAL INFILTRATE STERILE OR INFECTIOUS? Fig. 2. Sodium fluorescein evaluation of a corneal ulcer. The hyperfluorescence is attributed to staining of the mucous plug. and cataract surgery.14-17 It is not clear if the relative risk is greater with regards to a particular corneal surgery. • Ocular surface disease. Sterile peripheral (i.e., marginal) corneal infiltrates may result from a compromised ocular surface. Staphlococcal blooms in the lids spill bacterial byproducts onto the cornea, which triggers a hypersensitivity reaction that is theorized to lead to infiltrates.18,19 Often small and multiple in nature, these infiltrates are typically positioned roughly 1mm from the limbus. Marginal infiltrates may be asymptomatic, or may be accompanied by conjunctival injection. Pathogenesis includes bacterial, allergic or autoimmune etiologies. In the case of severe blepharitis and meibomian gland dysfunction (MGD), the bacterial blooms within the meibomian glands can produce a hypersensitivity reaction, leading to peripheral, non-staining subepithelial infiltrates (SEIs). The body responds to the to antigen presented by this presence of Staphlococcal bacteria in the lids by recruiting white blood cells to the area as part of an antibody response. For this reason, the SEIs associated with 26 Staphlococcal marginal keratitis are typically in the 2 o’clock and 10 o’clock regions and the 4 o’clock and 8 o’clock regions, contiguous with the upper and lower lids. Similarly, ocular rosacea can lead to MGD. However, with severe rosacea, potential outcomes include marginal infiltrates, chronic conjunctivitis, sterile ulceration, corneal neovascularization and corneal scarring (Figures 4 and 5). It is also critical to rule out herpes simplex keratitis (HSK), which may resemble the infiltrates seen in Staphlococcal marginal keratitis. HSK lesions, however, are typically harder to treat and appear with deeper stromal inflammation.20 • Allergic conjunctivitis. All eye care practitioners are familiar with the signs and symptoms of Fig. 3. Contact lens-related microbial keratitis. Ulcer filled with mucous plug. seasonal allergic conjunctivitis, atopic conjunctivitis and papillary conjunctivitis. Perhaps less commonly encountered, however, is a variant of allergic conjunctivitis found most often in young boys. Vernal conjunctivitis is a severe bilateral condition characterized by photophobia, chemosis, sticky discharge, eosinophils at the limbus (i.e., Horner-Trantas dots) and shield ulcers.21 Secondary bacterial keratitis typically results from 10% of shield ulcers.22 • Medications. Contamination of ocular medications has been implicated in numerous case studies of corneal ulceration; the pathogen may originate in topical medication dropper tips or within the medications themselves.23,24 LOOK FOR THE SIGNS The next step after collecting a complete patient history is to conduct an ocular examination, which can help determine whether an infiltrate is sterile or infectious. The following are all key elements of a physical examination that can help with proper diagnosis: • Does the patient report symptoms of pain, photophobia or loss of vision? What about corneal sensation? Pain out of proportion with the signs points to Acanthamoeba, while loss of corneal sensation (i.e., lack of pain upon history or with the corneal wisp test) signals HSK. • Do you observe blepharitis, nasolacrimal duct obstruction, poor or incomplete blink or lagophthalmos, entropion/ectropion or conjunctival injection? Is the conjunctival injection localized or diffuse? How about circumlimbal? Be sure to grade the injection (i.e., 1-4+). What about corneal foreign bodies? • Is there discharge and, if so, what are its characteristics? REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 024_RCCL0415_F4_CE.indd 26 3/27/15 4:02 PM Fig. 4. Severe ocular rosacea. Visible on the lid is tyalosis, scalloped lid margin and telangiectasia, which signals severe inflammation within the meibomian glands. • What is the location, depth and size of the infiltrate(s)? Do you observe stromal loss? • Is there visible loss of corneal endothelium? What about plaque or pigment on the endothelium? • What is the status of the patient’s corneal graft, if they have one? • Do you observe any stromal haze and edema? • Do you observe anterior chamber reaction, cells/flare or hypopyon? • Are there vitreous cells present? Note, a corneal ulcer will rarely lead to endophthalmitis with vitreous cells present. THE CULTURAL REVOLUTION In the last 20 years, a major shift in thought regarding the need to obtain corneal material to identify offending organisms and determine sensitivity to antibiotics has occurred. Today, broad-spectrum fluoroquinolones are readily available as the primary treatment for a corneal infiltrate believed to be infectious. Thus, the majority of community-acquired cases of bacterial keratitis are typically resolved with empiric therapy and managed without smears or cultures. However, such tests are indicated in certain cases, including those that involve a corneal infiltrate that is central, large and extends to the mid to deep stroma, particularly with significant thinning of the cornea or scleral extension; those chronic in nature or unresponsive to broadspectrum antibiotics; and those that present with atypical clinical features suggestive of fungal, amoebic or mycobacterial keratitis.25 Smears and cultures may also be helpful in cases with an unusual history, such as trauma caused by vegetable matter or if the patient wore contact lenses while in a hot tub.25 Additional specialized studies can help identify atypical organisms, for example in sight-threatening or severe keratitis of suspected microbial origin.25 However, the American Academy of Ophthalmology—noting that the hypopyon that occurs in eyes with bacterial keratitis is usually sterile—recommends aqueous or vitreous taps should not be performed unless there is a high suspicion of microbial endophthalmitis, such as following an intraocular surgery, perforating trauma or sepsis.25 When obtaining corneal material, proper technique is critical to identify the causative organism and select the proper antibiotic, antiviral, antifungal or antiprotozoal medications. The process of obtaining corneal material should first involve the instillation of a topical anesthetic agent (note that tetracaine should be avoided due to its antimicrobial effect) followed by the use of a heat-sterilized platinum spatula, blade, jeweler’s forceps or other similar sterile instrument to obtain scrapings of material from the advancing borders of the infected area of the cornea. Culture yield may be improved by avoiding anesthetics with preservatives. A thiol or thioglycollate brothmoistened dacron/calcium alginate or sterile cotton swab can also be used to obtain material.25 However, solid as well as liquid plating media is always recommended.26 If treatment is refractory and cultures do not yield results, it is advisable to halt antibiotics in order to isolate the exact pathogen for further treatment. It is also important to consider culturing contact lenses, contact lens cases and contact lens solutions if appropriate and available. TREATMENT OPTIONS Topical antibiotics are the first-line therapy for suspected or cultureproven bacterial keratitis; however, the selection depends on severity. A peripheral infiltrate associated with lid margin disease may be appropriately managed with inexpensive early fluoroquinolones such as ofloxacin, ciprofloxacin, azithromycin or a polymixin-bacitracin ointment, while central or more aggressive infiltrates warrant use of fourth-generation fluoroquinolones such as gatifloxacin, moxifloxacin or levofloxacin. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 024_RCCL0415_F4_CE.indd 27 27 3/27/15 4:02 PM IS THAT CORNEAL INFILTRATE STERILE OR INFECTIOUS? Fig. 5. Corneal scarring with severe ocular rosacea. For more serious keratitis, the use of besifloxacin has recently been advocated. This new fluoroquinolone has high MIC values for many common ophthalmic pathogens and a unique compound containing polycarbophil, edetate disodium dihydrate and sodium chloride, which allows for greater ocular surface contact time. Additionally, its position as the only ophthalmic fluoroquinolone not used systemically makes it unique by reducing the risk of antibiotic resistance. In severe situations with risk for perforation, failure to respond to monotherapy or central aggressive ulcers with the potential for vision loss, the use of combination fortified-antibiotic therapy should be considered. This should be formulated by a compounding pharmacy that is a member of the Pharmacy Compounding Accreditation Board. Note that methicillin-resistant Staphylococcus aureus (MRSA) has been isolated with increasing frequency from patients with bacterial keratitis. Fluoroquinolones are generally poorly effective against MRSA ocular isolates; however, vancomycin has demonstrated some success.27 In cases of severe ulcer, consider more complete coverage with combination therapy.28 Systemic antibiotics are rarely needed, but may be considered in severe cases where the infectious process has moved to adjacent tissues (e.g., the sclera) or when there 28 is impending or frank perforation of the cornea. Research shows oral tetracycline controls the anticollagenase activity commonly seen in necrotizing infections such as Pseudomonas.29 Systemic therapy is necessary in gonococcal keratitis because of the extremely aggressive nature of this organism (corneal penetration in 24 hours with inadequate treatment). For this reason, the CDC recommends immediate hospitalization with IV antibiotics for adult gonococcal infection. Treating viral, fungal and amoebic keratitis may be challenging and is beyond the scope of this article. Regardless, the eye care provider who has clinical suspicion or laboratory data supporting any of these infectious processes should be fluent in their current treatment options or have available the appropriate consultants to offer prompt referral. Determining whether an infiltrate is sterile or infectious is not an easy task for even the best clinicians. However, with careful history taking, physical examination, differential diagnosis and proper treatment, patients have the best chance of making the best of a bad situation. RCCL 1. Josephson JE, Caffery BE. Infiltrative keratitis in hydrogel lens wearers. Int Contact Lens Clin. 1979;6:223–41. 2. Szczotka-Flynn L, Lass JH, Sethi A, et al. Risk factors for corneal infiltrative events during continuous wear of silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5421-30. 3. Pearlman E, Johnson A, Adhikary G, et al. Toll-like receptors at the ocular surface. Ocul Surf. 2008;6:108–16. 4. Sweeney DF, Naduvilath TJ. Are inflammatory events a marker for an increased risk of microbial keratitis? Eye Contact Lens. 2007 Nov;33(6 Pt 2):383-7; discussion 399-400. 5. Online Merck Manual. Corneal Ulcer. Available at: www.merckmanuals.com/professional/eye_ disorders/corneal_disorders/corneal_ulcer.html. Accessed March 10, 2015. 6. Efron N, Morgan PB. Can subtypes of contact lens-associated corneal infiltrative events be clinically differentiated? Cornea. 2006 Jun;25(5):540-4. 7. Müller, Vrensen, Pels, et al. Architecture of Human Corneal Nerves. Invest Ophthalmol Vis Sci. April 1997;38(5):985-94. 8. Lemp MA, Mathers WD. Corneal Epithelial Cell Movement in Humans. Eye. 1989;3:438-45. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 024_RCCL0415_F4_CE.indd 28 9. Mah FS. Corneal infiltrates merit care: Differentiating sterile and infectious conditions key to diagnosis, treatment. Ophthalmology Times. 2010 Oct;35(19):42. 10. McNally JJ, Chalmers RL, McKenney CD, Robirds S. Risk factors for corneal infiltrative events with 30-night continuous wear of silicone hydrogel lenses. Eye Contact Lens. 2003 Jan;29(1 Suppl):S153-6; discussion S166, S192-4. 11. Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321:779–83. 12. Cheng KH, Leung SL, et al. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354:181–5. 13. Mondino BJ, Weissman BA, Farb MD, et al. Corneal ulcers associated with daily-wear and extended-wear contact lenses. Am J Ophthalmol. 1986;102:58–65. 14. Adrean SD, Cochrane R, Reilly CD, Mannis MJ. Infectious keratitis after astigmatic keratotomy in penetrating keratoplasty: review of three cases. Cornea. 2005 Jul;24(5):626-8. 15. Moon SW, Kim YH, Lee SC, Lee MA, Jin KH. Bilateral peripheral infiltrative keratitis after LASIK. Korean J Ophthalmol. 2007 Sep;21(3):172-4. 16. Villarrubia A, Cano-Ortiz A. Candida keratitis after Descemet stripping with automated endothelial keratoplasty. Eur J Ophthalmol. 2014 Nov-Dec;24(6):964-7. 17. Akpek EK, Demetriades AM, Gottsch JD. Peripheral ulcerative keratitis after clear corneal cataract extraction(1). J Cataract Refract Surg. 2000 Sep;26(9):1424-7. 18. Mondino BJ. Inflammatory diseases of the peripheral cornea. Ophthalmol 1998;95:463-12. 19. Mondino BJ, Lahedi AK, Adamu SA. Ocular immunity to Staphylococcus aureus. Invest Ophthalmol Vis Sci 1987;28:560. 20. One Network. Herpes Simplex Virus. Available at: one.aao.org/focalpointssnippetdetail.aspx?id=356f0d13-8853-410f-ac6b7ff5e0257800. Accessed March 25, 2015. 21. Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye. 2004;18:345–51. 22. Reddy JC, Basu S, Saboo US, et al. Management, clinical outcomes, and complications of shield ulcers in vernal keratoconjunctivitis. Am J Ophthalmol. 2013 Mar;155(3):550-9. 23. Donzis PB. Corneal ulcer associated with contamination of aerosol saline spray tip. Am J Ophthalmol. 1997 Sep;124(3):394-5. 24. Schein OD, Wasson PJ, et al. Microbial keratitis associated with contaminated ocular medications. Am J Ophthalmol. 1988 Apr 15;105(4):361-5. 25. American Academy of Ophthalmology Cornea/External Disease Panel. Bacterial keratitis. Limited revision. San Francisco (CA): American Academy of Ophthalmology (AAO); 2011. 26. Bhadange Y, Sharma S, Das S, Sahu SK. Role of liquid culture media in the laboratory diagnosis of microbial keratitis. Am J Ophthalmol. 2013 Oct;156(4):745-51 27. Eiferman RA, O'Neill KP, Morrison NA. Methicillin-resistant Staphylococcus aureus corneal ulcers. Ann Ophthalmol. 1991 Nov;23(11):414-5.) 28. Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol 2008;145:951-8. 29. Ralph RA. Tetracyclines and the treatment of corneal stromal ulceration: a review. Cornea. 2000 May;19(3):274-7. REVIEW OF CORNEA & CONTACT LENSES | JANUARY 2015 28 3/27/15 4:02 PM CE TEST ~ APRIL 2015 EXAMINATION ANSWER SHEET Is That Corneal Infiltrate Sterile or Infectious? 1. a. b. c. d. All of the following are likely symptoms or signs of corneal infection EXCEPT: Pain and photophobia. Discharge and foreign body sensation. Anterior chamber reaction that includes cell and flare. Lack of debris at the tear menuscus. 2. There are many reasons to culture corneal ulcers. Which of the following is NOT one of them? a. Culturing would be a helpful medico-legal component of your record in case the ulcer doesn’t respond to empiric treatment. b. Culturing reveals sensitivities of the organism(s). c. Because no single agent is generally effective for all infections. d. Because ineffectively treated organisms are difficult to isolate. 3. Currently, the most effective/complete treatment plan for resistant bacterial infection is: a. Vancomycin and ceftazidime. b. Tobramycin. c. Gentamycin and Ocuflox. d. Ocuflox and erythromycin. 4. Which of the following statements is true regarding corneal infiltrates? a. Sterile infiltrates are often single in number and are found closer to the visual axis. b. Corneal infiltrates at least histologically present in all infections. c. Large, central infiltrates with overlying stains are probably sterile. d. Corneal infiltrates associated with microbial keratitis generally produce little to no pain and photophobia. 5. a. b. c. d. The most common cause of perennial allergic conjunctivitis is: Ragweed or tree pollen. Summer grasses. Home allergens, such as dust mites and animal dander. Multipurpose disinfecting solutions used in contact lens care. 6. Which of the following is not true regarding the use of systemic antibiotics? a. They often provide a more effective dose to the cornea than topicals alone. b. They have a lower chance of producing an allergic response. c. They are often ineffective when the infection has scleral extension. d. Tetracycline may aid in patients with an impending corneal melt. 7. a. b. c. d. Risk for infiltrative keratitis is increased with all of the following EXCEPT: Poor compliance/hygiene. Smoking. History of corneal scarring and CLARE. Age 39 to 40. Valid for credit through April 1, 2018 Online: This exam can also be taken online at www.reviewofcontactlenses.com. Upon passing the exam, you can view your results immediately. You can also view your test history at any time from the website. Directions: Select one answer for each question in the exam and completely darken the appropriate circle. A minimum score of 70% is required to earn credit. Mail to: Jobson Optometric CE, Canal Street Station, PO Box 488 New York, NY 10013 Payment: Remit $20 with this exam. Make check payable to Jobson Medical Information LLC. Credit: COPE approval for 1 hour of CE credit is pending for this course. Sponsorship: Joint-sponsored by the Pennsylvania College of Optometry Processing: There is an eight-to-10 week processing time for this exam. Answers to CE exam: A B C 1. A B C 2. A B C 3. A B C 4. A B C 5. 6. 7. 8. 9. 10. D D D D D A B C D A B C D A B C D A B C D A B C D Evaluation questions (1 = Excellent, 2 = Very Good, 3 = Good, 4 = Fair, 5 = Poor) Rate the effectiveness of how well the activity: 1 2 3 4 5 11. Met the goal statement: 12. Related to your practice needs: 1 2 3 4 5 13. Will help improve patient care: 1 2 3 4 5 1 2 3 4 5 14. Avoided commercial bias/influence: 15. How do you rate the overall quality of the material? 1 2 3 4 5 16. Your knowledge of the subject increased: Greatly Somewhat Little 17. The difficulty of the course was: Complex Appropriate Basic 18. How long did it take to complete this course? _________________________ 19. Comments on this course: _________________________________________ ___________________________________________________________________ 20. Suggested topics for future CE articles: ______________________________ ___________________________________________________________________ Identifying information (please print clearly): First Name Last Name Email 8. The incidence rate for microbial keratitis has been estimated to range from what to what per 10,000 extended-wear lens patients per year: a. 18 to 20. b. 4 to 5. c. 34 to 38. d. 1 to 2. The following is your: Home Address Business Address Business Name Address City 9. Which of the following is NOT a risk for microbial keratitis? a. Wearing contact lenses overnight. b. History of corneal trauma or foreign body. c. Prior corneal surgery. d. Daily use of aspirin. 10. Which of the following is true regarding monotherapy use in presumed microbial keratitis? a. A fourth-generation fluoroquinolone is probably the best overall option and can be used alone for deep central ulcers. b. Besivance is likely the best option for MRSA infections. c. Monotherapy should be reserved for use in central infiltrates only. d. Erythromycin is generally the best treatment option pending culture results. State ZIP Telephone # - - Fax # - - By submitting this answer sheet, I certify that I have read the lesson in its entirety and completed the self-assessment exam personally based on the material presented. I have not obtained the answers to this exam by fraudulent or improper means. Signature: ________________________________________ Date: _____________ Please retain a copy for your records. LESSON 111206, RO-RCCL-0415 REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 024_RCCL0415_F4_CE.indd 29 29 3/27/15 4:02 PM The GP Expert By Stephanie L. Woo, OD Troubleshooting GP Complications Many GP lens problems are easily resolved once identified. C ontact lenses, whether rigid or soft, are a foreign object on the ocular surface. They interact with the tear film, cornea, bulbar conjunctiva and palpebral conjunctiva; not surprisingly, complications may arise on any of these ocular structures as a result of contact. Additionally, the tear film is separated into a pre- and post- contact lens film with different thicknesses, which can initiate a variety of issues.1 3 AND 9 O’CLOCK STAINING A common complication with corneal GP lenses is 3 and 9 o’clock staining, which is a result of epithelial punctate staining of the peripheral cornea near the edge of the GP lens.2 These so-called 3 and 9 o’clock areas may not be adequately resurfaced with tears after a blink, thus resulting in small desiccated regions. At first the staining is mild, but over time the epithelial defects may become larger and denser. In severe cases, those portions of the cornea may become vascularized and even form an opacity. When treating, be sure to check the edge of the contact lens first. If you have a modification unit within the office, you can alter the edge of the lens to be rounded or a “plus” shape to help with edge-cornea interaction.3 If your patient has a particularly high plus or high minus lens, be sure to use lenticular lens designs to improve the edge of the lens.4 Inferiorly centered GP lenses are another major cause of 3 and 9 30 dirt, dust or another small object adheres to the ocular surface and ends up underneath the lens—can be an extremely frustrating issue for both soft and GP lens wears alike. Even the smallest foreign body can result in ocular surface damage, as with every blink, the trapped object scratches the delicate epithelium. This can lead to track marks, which are both very uncomfortable and potentially painful for the patient (Figure 1). Lens edge design plays a major role regarding foreign body entrapment; for example, if the edge is poorly finished or has unpolished secondary curves, this can lead to more occurrences.5 Thus, rolling the edge and polishing the secondary curves can help reduce foreign body entrapment. Be sure to also check the edges for chipping, as it can also increase risk of foreign body entrapment.5 If the problem persists, consider switching to a larger diameter Fig. 1. Foreign body entrapment under lens, such as a scleral lens, to GP lens shows track marks on the corneal epithelium. reduce or eliminate this issue. 3 and 9 o’clock staining, though going larger is also an option. A SPECTACLE BLUR scleral lens will resolve the issue Every once in a while, a patient completely because of the fluid comes in who complains that layer between the cornea and the when they remove their contacts contact lens, which will act as a and put on their glasses, their lubricating cushion between the vision is blurred. One reason this lens and ocular surface. Switching so-called spectacle blur may octhe patient to a hydrogel material cur is from the accumulation of may also be an answer. fluid within the epithelial cells. When contact lenses are removed, FOREIGN BODIES the swelling of these cells slowly Foreign body tracking—when decreases, and the spectacle blur o’clock staining, so improving the centration of the GP lens is key.3 Additionally, selecting a GP lens material with good wettability may help to decrease peripheral erosion by reducing friction between the lens and ocular surface. Artificial tears and gels can also help increase lubrication, but will need to be used consistently to prevent further complications. If all other modifications fail, try altering the diameter of the lens. Smaller diameters may decrease REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 030_RCCL0415_gpe.indd 30 3/27/15 3:45 PM what to expect usually eases patients so they do not get upset or anxious. LENS ADHESION Lens adhesion is the most common complication of extended wear GP lenses.7,8 Upon examination, the lens will be immobile with mucous/lipid deposits present Fig. 2. Topography of a poorly fitting tangent streak underneath. When GP lens caused corneal steepening. fluorescein is inusually resolves after an hour.6 stilled, none will be seen under the Spectacle blur can also result lens, indicating no tear exchange from changes in corneal curvature or lens movement. When the lens that is not related to edema. This is removed, an arcuate ring patis due to mechanical molding of the cornea (resulting from lens fit) or prolonged metabolic stress. This issue is particularly common in patients who wear flat-fitting or hybrid lenses. In the case of corneal molding, when the fit of the contact lens is poor, the lens reshapes the cornea, thus resulting in spectacle blur (Figure 2). Spectacle blur is Fig. 3. Corneal topography after discontinuing GP lens wear for usually not a large issue, unless several months. the primary cause is because the tern is visible around the edge of contact lens fit is inappropriate. the lens.7 Patients usually do not For example, in more extreme have any complaints with lens cases, deep stromal striae or binding; in fact, they may report opacification of the cornea can be better comfort (except late in the seen, which is indicative of further day) because of the decreased lid potential issues. In most cases, however, the cornea will eventually interaction and movement. If an extended wear patient exremold to a stable shape if the lens is removed (Figure 3).6 Regardless, hibits lens adhesion, changing their wearing schedule to daily wear education on spectacle blur and will likely resolve the issue. During sleep, the pressure from the eyelids squeezes out the tear layer under the GP lens, which results in lens binding.7 If lens binding occurs with a daily wear patient, however, the fit of the lens will need to be altered. When the lens decenters, the secondary and peripheral curve junctions contact the flatter areas of the cornea.7,8 This, combined with pressure from the eyelids, leads to lens adherence. Try altering the centration and curvature of the lens to achieve a more centered fit and check to make sure the curves are well blended. If normal modifications do not work, consider switching to an aspheric back surface design. Note that another potential cause of lens adherence is dry eye, specifically aqueous deficient dry eye, so it is important to check your patient for any type of dry eye during their exam. RCCL 1. Nichols J, King-Smith PE. Thickness of the pre- and post-contact lens tear film measured in vivo by interferometry. Invest Ophthalmol Vis Sci. 2003 Jan;44(1):68-77. 2. Van der worp E, de Brabander J, Swarbrick HA, Hendrikse F. Evaluation of signs and symptoms in 3 and 9 o’clock staining. Optom Vis Sci. 2009 Mar;86(3):260-5. 3. Holden T, Bahr K, Koers D, et al. The effect of secondary curve liftoff on peripheral corneal desiccation. Am J Optom Physiol Opt 1987;64:113. 4. Henry VA, Bennett ES, Forrest JM. Clinical investigation of the Paraperm EW rigid gas permeable contact lens. Am J Optom & Physiol Opt 1987;64:313-320. 5. Bennett, E. et al. Clinical Contact Lens Practice. Lippincott, Williams, and Wilkins. Philadelphia, PA. 2005. Pp 351. 6. Bailey SC. Contact lens complications. Optometry Today. June 4 1999. 7. Bennett, E. et al. Clinical Contact Lens Practice. Lippincott, Williams, and Wilkins. Philadelphia, PA. 2005. Pp 346-8. 8. Swarbrick HA, Holden BA. Rigid gas permeable lens binding: significance and lens factors. Am J Optom Physiol Opt. 1987;64(11)815-823. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 030_RCCL0415_gpe.indd 31 31 3/27/15 3:45 PM Corneal Consult By James Thimons, OD Managing Acute Corneal Hydrops in Keratoconus Using a structured approach to determine which factors impede healing will lead to success with these often challenging scenarios. C 32 inflammation and IOP-lowering drugs. Some patients, however, may require surgery if corneal edema persists or resultant corneal scarring affects visual clarity. Conservative medical intervention is usually sufficient to stave off the more concerning consequences of perforation, but overall has little impact on visual outcome or duration of disease. Thus, doctors are turning to other, more advanced methods of disease management. Recently, there has been renewed interest in the use of intracameral SF6 and C3F8 gases, which have been shown to lead to Photo: Edward Boshnick, OD orneal hydrops, an uncommon complication seen in patients with corneal ectatic disorders, is characterized by the leakage of aqueous through a tear in Descemet’s membrane, which leads to a rolling of the edge and subsequent gaping of the posterior surface of the cornea. This allows the aqueous fluid to intrude into the cornea, producing an acute edematous response, relative changes in corneal architecture and clinical symptomatology. Patients typically present with a rapid decrease in visual acuity, photophobia and pain. There is also notable localized edema and, in some instances, visible ectasia in the areas most compromised. Corneal hydrops is estimated to occur in 2% to 3% of patients with keratoconus, with the majority presenting between ages 20 and 40. While some literature supports an increased rate of occurrence in males, there is no notable prevalence by race. In most cases, the location of the hydrops is inferior to the apex of the cone. When questioned, patients frequently admit to significant eye rubbing. In some instances, severe coughing and/or heavy lifting have also been associated with onset of disease. Down’s syndrome may be a risk as well. Most cases heal naturally over a period of several months when treated using conservative methods, which include topical cycloplegic agents, corticosteroids to reduce Fig. 1. Placement of intracameral C3F8 (non-expansile concentration) in a different patient than the one described here. Note: the patient was instructed to lie flat with their eyes looking up towards the ceiling, so that the gas can occlude the break in the posterior cornea. faster improvement compared to more conservative treatments.1,2 Intrastromal venting incisions, used to delimit the large vacuoles that occur in some patients, have also demonstrated some success but are still relatively experimental. Overall, more research is needed on the efficacy of these and other emerging treatments. CASE STUDY A recent case of corneal hydrops that presented to my office is emblematic of the conventional characteristics of the disease, and serves as a good example of conservative vs. more aggressive surgical treatment. TW, a 42-year-old black female, was initially seen for a complaint of progressive change in visual function relative to longstanding keratoconus, and a recent history of possible progression noted by her current clinician. The consultation identified significant apical scarring in her right eye, and notably less scarring in the left. The best-corrected visual acuity with spectacles was 20/300 OD, which did not improve on pinhole, and approximately 20/80 OS with minimal pinhole improvement. Her previous history was positive for contact lens wear, although discomfort as well as poor acuity had decreased the patient’s wearing activity to a minimal level over the last six to 12 months. The remainder of her examination elements (i.e., intraocular pressure, lenticular assessment and posterior pole) were relatively normal. The patient was advised that several options were available: she could forgo intervention at this time, be fit with a complex hybridtype or scleral contact lens with the hope of achieving better results than REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 010_RCCL0415_CC.indd 32 3/27/15 3:42 PM Photo: Edward Boshnick, OD Fig. 2. Hydrops resolved. her current standard hard lens, or undergo a corneal transplant. The patient elected to consider the options and was scheduled for another appointment in three months, with plans to return sooner if a decision was made. Approximately six weeks after her initial evaluation, the patient contacted the on-call service on a weekend and indicated that she had developed a notable decrease in vision, along with severe pain and photophobia. I saw her on the same day, and it was evident she had a significant corneal hydrops and a visible Descemet’s membrane tear. This was accompanied by notable apical ectasia in the zone of the hydrops. The presentation of the cornea was 3+/4 edema, focally located over the zone of the hydrops, which consisted of epithelial microcystic edema and intrastromal cysts and vacuoles. The diameter of the area was approximately 4.5mm to 5.0mm. Visual acuity was hand motion at two feet with no pinhole improvement. I discussed treatment options with the patient, who elected to proceed with conservative therapy, which included a topical antibiotic (ciprofloxacin) QID to prevent infection, one drop of a cycloplegic (atropine) BID, the use of a hypertonic saline ointment (Muro 128) QID and an ocular antihypertensive agent to lower pressure and decrease the posterior forces on the cornea contributing to architectural change. The patient was also placed on topical Durezol QID, and a bandage lens was inserted—primarily for comfort, but also to prevent perforation. The patient was observed over several weeks and, as is typical with hydrops, the intrastromal edema that was present at the initial episode began to resolve, as did the photophobia. At the initial threeday follow-up, the pain had lessened significantly with the atropine and Durezol, and the inflammatory response present in the cornea showed a mild decrease. Additionally, the pressure had been reduced from 16mm Hg to 10mm Hg. The patient’s bandage lens was removed in order to view the corneal surface; upon removal, a decrease in the microcystic edema was observed and the stromal vacuoles and clefts appeared stable. I ordered current medication levels be maintained, but decreased the atropine to QD. At the two-week follow-up, I reduced the steroids to BID and stopped the atropine. I continued the antibiotics but reduced the dose to BID. The bandage lens was also removed at two weeks and the cornea was competent with a negative Seidel. The patient called two days after the bandage lens was discontinued and indicated that she had developed a foreign body sensation, so I reinstituted lens wear. I maintained her on the antihypertensive agents for approximately four weeks. Additionally, she used the hypertonic saline ointment four times daily throughout the treatment period. By the fourth week, the patient’s vision had returned to 20/200 best-corrected in the affected eye. Additionally, the microcystic edema had reduced dramatically, and the stromal edema had begun to demonstrate a coalescence of the vacuoles, which were notably present at the initial clinical assessment. It should be noted in cases like this one that therapeutic options like hybrid or scleral lenses are less viable because of the scar formation, which is almost universal in patients of this type following a severe hydrops. I discussed the potential for a therapeutic penetrating keratoplasty as the option of choice, and TW is actively considering the possibility once the eye stabilizes. I anticipate that, given her current, relatively poor visual rehabilitative potential and the fact that the other eye also shows notable keratoconus, a penetrating keratoplasty would be a reasonable option to rehabilitate visual function and give her overall better visual potential in the years to come. RCCL 1. Panda A, Aggarwal A, Madhavi P, et al. Management of acute corneal hydrops secondary to keratoconus with intracameral injection of sulfur hexachloride (SF6). Cornea. 2007;26(9):1067-9. 2. Basu S, Vaddavalli PK, Ramappa M, et al. Intracameral perfluoropropane gas in the treatment of acute corneal hydrops. Am J Ophthalmol 2011:118(5):934-9. REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 010_RCCL0415_CC.indd 33 33 3/27/15 3:42 PM Out of the Box By Gary Gerber, OD Are You a Mentalist? Probably not. And neither are your staff members. Clear communication with your team is key to the success of your practice. I f you’ve attended one of my lectures at a meeting or trade show, chances are you’ve probably also seen me do magic—specifically, a type known as mentalism. Instead of pulling a rabbit out of a hat, mentalists use whatever is in the imagination of their audiences to complete their illusions. Mentalism is essentially magic of the mind and frequently deals with predictions about the future. So, here’s a prediction I’ll make for everyone reading this article and, since you’re reading it for the first time and I’m not standing in front of you, there is no possible way I could have set this up in advance! My prediction is that the practice-building challenge you encounter most frequently is—wait for it—staff management. How’d I do? (Email me, I’d love to know!) And, the number one reason why so many doctors struggle with staff management is because they aren’t mentalists. In other words, they haven’t figured out how to predict the future! But what’s the connection between understanding the future and staff management? While it’s likely most of us do not know what our personal futures hold, chances are many of us have a good idea regarding the future of our practice. And, we have an obligation as practice leaders and CEOs to communicate this future to our staff. Great staff management and leadership involves understanding the long view and communicating that view to your staff—something that isn’t always easy. 34 AVOID MYOPIC LEADERSHIP Due to the stresses and day-to-day pressures of running a practice, many of us succumb to “leadership myopia” and are only able to focus on what is directly in front of us at a given moment. As with all behaviors, staff members see your acute laser focus on the “here and now” and assume that if it’s important to you, it should be important to them too. The problem with this thought process is that bigpicture goals, practice values and your very reason for being a practice can get lost in this short-term view. People are people, and patients are patients. You’ll always have acute patient management issues to deal with. Unless you routinely set aside uninterrupted time to discuss with your staff why you are doing what you do with each patient on a day-to-day basis, they will never adopt your long-term view (i.e., your mentalist’s prediction, if you will) of the future. Here’s an example: Mr. Late runs into the office out of breath and says, “I ran out of contact lenses and I’m on my way to work. Can I have one more pair to hold me over?” As instructed at your last staff meeting, your staff correctly checks his record and sees that his last examination was 16 months ago. Your clinical recommendation is once per year. So, the staff member says, “I’m sorry, you’re overdue for your exami- nation, so we can’t give you any more lenses.” Volumes have already been written about what may or may not happen next, and putting 10 of us in a room to discuss the best course of action would make for a nice fireside chat—or perhaps a barroom brawl. However, what is rarely discussed is how to put your own view of how this situation should be handled into the minds of your staff. “BIG PICTURE GOALS AND PRACTICE VALUES CAN GET LOST.” What is your long-term, futuristic macro goal for your practice and the micro short-term goal for this particular patient? The policy to deal with this individual patient is already known to every practice (i.e., either give the patient lenses or don’t, with any necessary explanations or caveats) but the reason for doing so, in the context of your big picture practice vision, is rarely articulated to staff. In other words, “Give or don’t give the lenses because our practice philosophy and long-term goals are…” is not usually discussed. This way, your staff members are able to better adhere to these long-term goals in the face of such situations. So, hone your mentalist skills and start communicating your goals and aspirations to your staff. Your day-to-day operations will run much smoother. RCCL REVIEW OF CORNEA & CONTACT LENSES | APRIL 2015 034_RCCL0415_otb.indd 34 3/27/15 4:48 PM All eyes deserve clariti. clariti 1 day—now available for practices everywhere. The world’s first and only family of silicone hydrogel daily disposable contact lenses designed for every patient type—sphere, toric and multifocal. High Oxygen Transmissibility High Water Content Low Modulus UV Protection Affordable Upgrade Now you can prescribe all of your patients with healthy, comfortable, affordable silicone hydrogel 1 day lenses— which will make all eyes very happy indeed. To learn more, contact your CooperVision representative today or visit CooperVision.com/practitioner. RO0115_Cooper Clariti.indd 1 12/22/14 11:42 AM Hello Miru. Bye, bye blister pack. Introducing Miru 1day, the world’s thinnest package for daily disposable contact lenses. Miru’s ultra lightweight 1mm thin package is about 1/8th the thickness of a traditional blister pack >`Ü>ÃëiVwV>Þ`iÛi«i`ÌÀi`ÕViÌ iÀÃvVÀL>VÌ>>Ì°7 i«ii`]Ì i lens is presented on a special disk, oriented correctly for proper insertion. To learn more and request trials, please visit: miru.meniconamerica.com ©2014 Menicon America, Inc. Miru is a registered trademark of Menicon Company Ltd. RCCL0315_Menicon.indd 1 2/23/15 11:15 AM