Appendix 4: IR ABSORPTION FREQUENCIES
advertisement

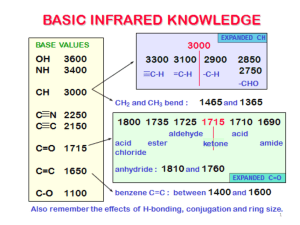
Appendix 4: IR ABSORPTION FREQUENCIES TABLE 1: GENERAL ABSORPTION RANGES Abbreviations: (s) = strong, (m) = medium, (v) = variable, (w) = weak BOND TYPE C−H COMPOUND TYPE Alkanes (aliphatic sp3 C−H stretch) (C−H bend) Alkenes (olefinic sp C−H stretch) 2 (C−H bend) Alkynes (acetylenic sp C−H stretch) (C−H bend) Aromatic rings (aryl C−H stretch) (C−H bend) C−C C=C C≡C … C−−C C−O C=O O−H N−H C−N C≡N N−O Alkanes (C−C stretch) Alkenes Alkynes Aromatic rings (in-plane vibrations) Alcohols, ethers, carboxylic acids, esters Aldehydes, ketones, carboxylic acids, esters, amides Monomeric alcohols, phenols H-bonded alcohols, phenols carboxylic acids, water contaminant Amines, Amides Amines Nitriles Nitro compounds (N−O stretch) as in C−NO2 FREQUENCY RANGE (cm−1) 2960-2850 (m-s) 1470-1350 (v) 3080-3020 (m) 1000-675 (v) (See details in Table 2) ~ 3300 (s) ~ 630 (s) 3000-3100 (v) (often weak) 675-870 (See details in Table 3) 1300-1100 (v) (not useful) 1680-1640 (v) 2260-2100 (v) 1500, 1600 (v) (usually 4 peaks, see example p.A-12) 1300-1080 (s) 1800−1650 (s) (See details given in Table 5.) 3640-3610 (v) 3600-3200 (broad) 3000-2500 (broad) 3710 & 1630 3500-3300 (m) 1360-1180 (v) 2260-2210 (v) 1600-1500 (s) 1400-1300 (s) TABLE 2: C−H BENDING OF ALKENES (in cm-1) 920-910 (s) RCH=CH2 cis-RCH=CHR & 1000-990 (s) R2C=CH2 900-880 (s) 730-675 (v) trans-RCH=CHR 975-965 (s) Characteristic Absorptions of Aromatic Compounds There are four regions of absorptions for aromatic compounds. You will be mainly concerned with only Region I this semester. Region I: C−H stretch slightly above 3000 cm-1 (Indicative of presence of benzene ring) Region II: Overtones & combination bands at 2000-1670 cm-1 (See Table 4) Region III: C=C in-plane vibrations at 1667-1429 cm-1: four bands at ~1600 (s), 1580 (w, as shoulder), 1500 (s) and 1460 (usually obscured by others). Absence of these bands is fair assurance that there is no aromatic ring in the compound. Region IV: C−H bend at 1000-670 cm-1 (See Table 3) A-11 A-12 APPENDIX 4: IR ABSORPTION FREQUENCIES TABLE 3: For aromatic rings, out-of-plane C−H bending (in cm-1) monosubstituted m-disubstituted ~750 (s) (range 770-730) R R & ~700 (s) (range 710-690) ~780 (v) (range 810-750) R p-disubstituted o-disubstituted R ~750 (s) (range 770-735) R R ~830 (v) (range 840-810) R IR Spectrum of Propylbenzene (Typical Absorptions of Aromatic Compounds) Region IV 698 cm-1 743 cm-1 monosubstituted C−H bend Region III 1496 & 1453 cm-1 ←1538 cm-1 ←1584 cm-1 Region II aromatic overtones C=C in-plane vibration Table 4: IR Absorption Overtones of Substituted Benzenes These are weak absorptions that may not be observed if the sample is not concentrated enough. They are helpful in determining the type of substitution if no interfering absorption is present, such as that of C=O. 1941, 1870, 1801, 1604 cm-1 (monosubstituted shown here) Region I aryl C−H stretch 3100-3050 cm-1 APPENDIX 4: IR ABSORPTION FREQUENCIES A-13 Characteristic IR Absorptions of Carboxylic Acid Derivatives TABLE 5: IR Absorptions of Carboxylic Acid Derivatives Functional Group ketone Frequency C=O 1710 cm−1 acid C=O 1710 cm−1 O−H 2500−3500 cm−1 C=O 1735 cm−1 ester amide acid chloride acid anhydride nitrile C=O 1640−1680 cm−1 N−H 3200−3500 cm−1 C=O 1800 cm−1 C=O 1800 and 1750 cm−1 C≡N 2200 cm−1 Comments lower if conjugated, higher if strained lower if conjugated broad, on top of C−H stretch lower if conjugated, higher if strained two peaks for R−CO−NH2 one peak for R−CO−NHR' very high frequency two peaks just above 2200 cm−1 (continued on the next page)