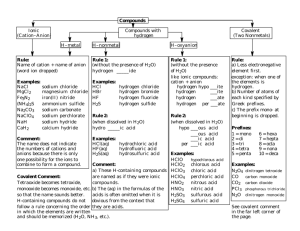
cheat sheet 2007
advertisement
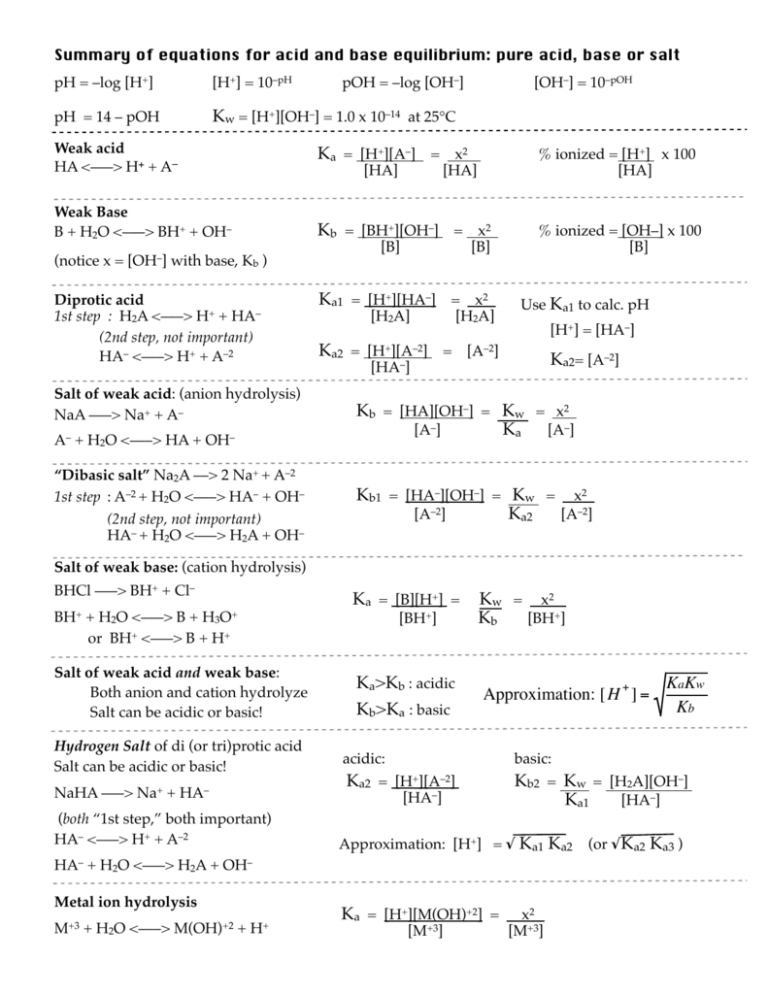
Summary of equations for acid and base equilibrium: pure acid, base or salt pH = –log [H+] [H+] = 10–pH pH = 14 – pOH Kw = [H+][OH–] = 1.0 x 10–14 at 25°C Weak acid HA <–––> H+ + A– pOH = –log [OH–] Ka = [H+][A–] = x2 Weak Base B + H2O <–––> BH+ + OH– [HA] [OH–] = 10–pOH [HA] Kb = [BH+][OH–] = x2 (notice x = [OH–] with base, Kb ) Diprotic acid 1st step : H2A <–––> H+ + HA– (2nd step, not important) HA– <–––> H+ + A–2 [B] [B] Ka1 = [H+][HA–] = x2 [H2A] [H2A] Ka2 = [H+][A–2] = [A–2] [HA–] Salt of weak acid: (anion hydrolysis) NaA –––> Na+ + A– % ionized = [H+] x 100 [HA] % ionized = [OH–] x 100 [B] Use Ka1 to calc. pH [H+] = [HA–] Ka2= [A–2] Kb = [HA][OH–] = Kw = x2 [A–] Ka [A–] A– + H2O <–––> HA + OH– “Dibasic salt” Na2A ––> 2 Na+ + A–2 1st step : A–2 + H2O <–––> HA– + OH– (2nd step, not important) HA– + H2O <–––> H2A + OH– Kb1 = [HA–][OH–] = Kw = x2 [A–2] Ka2 [A–2] Salt of weak base: (cation hydrolysis) BHCl –––> BH+ + Cl– Ka = [B][H+] = BH+ + H2O <–––> B + H3O+ or BH+ <–––> B + H+ Salt of weak acid and weak base: Both anion and cation hydrolyze Salt can be acidic or basic! Hydrogen Salt of di (or tri)protic acid Salt can be acidic or basic! [BH+] Ka>Kb : acidic Kb>Ka : basic Kw = x 2 Kb [BH+] Approximation: [ H + ] = NaHA –––> Na+ + HA– acidic: +][A–2] Ka2 = [H€ [HA–] (both “1st step,” both important) HA– <–––> H+ + A–2 Approximation: [H+] = √ Ka1 Ka2 HA– + H2O <–––> H2A + M+3 + H2O <–––> basic: Kb2 = Kw = [H2A][OH–] Ka1 [HA–] OH– Metal ion hydrolysis M(OH)+2 + H+ Ka = [H+][M(OH)+2] = [M+3] KaKw Kb x2 [M+3] (or √Ka2 Ka3 ) € For Buffer and titration pH problems: For mixtures of weak acid + its salt; or Weak base + its salt, Or Weak acid+ strong base; or Weak base + strong acid (In other words there is a source of both acid and conj base, or base and conj acid.) So [H+] ≠ [A–] and [OH–]≠ [BH+], so equations on back don’t work! Use this Equation: The Henderson-Hasselbalch equation [H + ]= K a [HA] [Acidic form] _ = K a [Basic form ] [A ] pH = pK a + log [A _ ] [Basic form] = pK a + log [HA] [Acidic form ] Weak acid: Acid= Acidic form, Salt = Basic form ; pKa= – log Ka Weak base: first get Ka = Kw : Base = Basic form, Salt = Acidic form: Then use same equation! Kb € Adding acids, bases to weak acid, base solutions, salts and Buffers Use the Henderson-Hasselbalch equation: Adding strong base : adds to the Basic form (A–) and takes away from the Acidic form (HA) Adding strong acid : adds to the Acidic form (HA) and takes away from the Basic form (A–) Titration problems : at equivalence point : VaMa(#H) = VbMb(#OH) Titration curves : Strong acid and strong base (Assume Ma=Mb) Initial pH : [H+] = Ma (Molarity of strong acid) Before equivalence point: volume of acid remaining = start volume – vol. base added total volume = start volume + vol. base added (vol acid remaining) (Ma) = (total vol.) (M2) : [H+] = M2 At the equivalence point: [H+] = [OH–] After equivalence point : volume of excess base = vol. base added – start volume total volume = start volume + vol. base added (vol excess base) (Mb) = (total vol.) (M2) : [OH–] = M2 Titration curves: Weak acid and strong base (Assume Ma=Mb) Initial pH : [H+] from original weak acid solution (other side!) Before equivalence point: Use the Henderson Hasselbach equation (Add strong base to weak acid as described above) Special case = “Half neutralized”: [H+] = Ka of the weak acid or pH = pKa At the equivalence point: [OH–] from the diluted salt solution (other side!) After equivalence point : same as strong acid/strong base titration above: Calculate [OH–] from diluted excess base.