Addressing Operational Challenges in Conducting Clinical Trials
advertisement
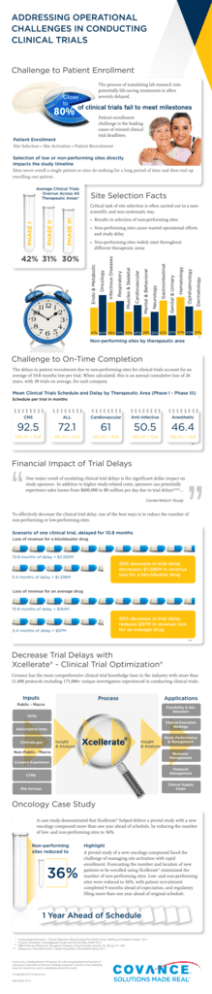
ADDRESSING OPERATIONAL CHALLENGES IN CONDUCTING CLINICAL TRIALS Challenge to Patient Enrollment The process of translating lab research into potentially life-saving treatments is often severely delayed. Close to 80% of clinical trials fail to meet milestones Patient enrollment challenge is the leading cause of missed clinical trial deadlines. Patient Enrollment Site Selection • Site Activation • Patient Recruitment Selection of low or non-performing sites directly impacts the study timeline Sites never enroll a single patient or sites do nothing for a long period of time and then end up enrolling one patient. Average Clinical Trials Overrun Across All Therapeutic Areas* Site Selection Facts • Results in selection of non-performing sites PHASE III • Non-performing sites cause wasted operational efforts and study delay Dermatology Ophthalmology Hematology Genital & Urinary Gastrointestinal Neurology Mental & Behavioral Cardiovascular Respiratory Oncology Endo & Metabolic 42% 31% 30% Muscles & Skeletal • Non-performing sites widely exist throughout different therapeutic areas Infectious Diseases PHASE I PHASE II Critical task of site selection is often carried out in a nonscientific and non-systematic way. 41% 60% 49% 51% 35% 45% 28% 35% 52% 30% 57% 43% 37% ** Non-performing sites by therapeutic area Challenge to On-Time Completion The delays in patient recruitment due to non-performing sites for clinical trials account for an average of 10.8 months lost per trial. When calculated, this is an annual cumulative loss of 26 years, with 30 trials on average, for each company. Mean Clinical Trials Schedule and Delay by Therapeutic Area (Phase I - Phase III) Schedule per trial in months CNS ALL Cardiovascular Anti-infective Anesthetic 92.5 72.1 61 50.5 46.4 DELAY = 10.8 DELAY = 10.8 DELAY = 10.8 DELAY = 10.8 DELAY = 10.8 *** Financial Impact of Trial Delays One major result of escalating clinical trial delays is the significant dollar impact on study sponsors. In addition to higher study-related costs, sponsors can potentially experience sales losses from $600,000 to $8 million per day due to trial delays***... CenterWatch Study To effectively decrease the clinical trial delay, one of the best ways is to reduce the number of non-performing or low-performing sites. Scenario of one clinical trial, delayed for 10.8 months Loss of revenue for a blockbuster drug 10.8 months of delay = $2.592M 50% decrease in trial delay decreases $1.296M in revenue loss for a blockbuster drug 5.4 months of delay = $1.296M Loss of revenue for an average drug 10.8 months of delay = $194M 50% decrease in trial delay reduces $97M in revenue loss for an average drug 5.4 months of delay = $97M **** Decrease Trial Delays with Xcellerate® - Clinical Trial Optimization® Covance has the most comprehensive clinical trial knowledge base in the industry with more than 11,000 protocols including 175,000+ unique investigators experienced in conducting clinical trials. Inputs Process Applications Public - Macro Feasibility & Site Selection 1572s Clinical Execution Strategy Subscription Data Insight & Analysis Clintrials.gov ® Xcellerate Insight & Analysis Non-Public - Macro Study Performance & Management Resource Management Covance Experience Financial Management CTMS Clinical Supply Chain Site Surveys Oncology Case Study A case study demonstrated that Xcellerate® helped deliver a pivotal study with a new oncology compound more than one year ahead of schedule, by reducing the number of low- and non-performing sites to 36%. Non-performing sites reduced to 36% Highlight A pivotal study of a new oncology compound faced the challenge of managing site activation with rapid enrollment. Forecasting the number and location of new patients to be enrolled using Xcellerate® minimized the number of non-performing sites. Low- and non-performing sites were reduced to 36%, with patient recruitment completed 9 months ahead of expectation, and regulatory filing more than one year ahead of original schedule. 1 Year Ahead of Schedule * ** *** **** Cutting Edge Information, “Clinical Operations Benchmarking Per-Patient Costs, Staffing and Adaptive Design”, 2011 Covance Xcellerate® knowledgebase CLabs and Clinical Data, 2006-2010 R&D Costs and Returns by Therapeutic Category. Drug Information Journal, Vol. 38, pp 211-223 Datasource: PharmaCircle 2011 Global Drug Sales, CenterWatch Study 2011 Covance Inc., headquartered in Princeton, NJ, is the drug development business of Laboratory Corporation of America® Holdings (LabCorp®). Covance is the marketing name for Covance Inc. and its subsidiaries around the world. © Copyright 2015 Covance Inc. INFCDS001-0714