Lab 3: Atomic Spectroscopy
advertisement

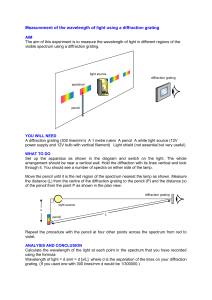
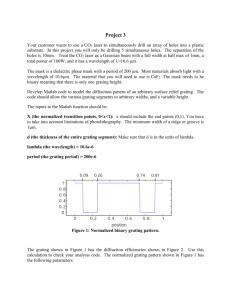
Physics 128 Fall 2004 Lab 3: Atomic Spectroscopy Week of October 4. Note the pre-lab assignment. Introduction: The Hydrogen spectrum played a rather important role in the development of our understanding of atomic structure. Unlike most elements, the H spectrum was known to follow a relatively simple empirical expression (the Balmer-Rydberg formula) as early as 1889. However, it was not until Niels Bohr (1885 1962) proposed his theory of the hydrogen atom in 1913 that the H spectrum was “explained”. Bohr received the 1922 Nobel Prize in physics for his work on understanding the structure of atoms. Although we now have a more complete and accurate theory of atomic structure, Bohr’s model was an important turning point in the understanding of the physics of atoms. His work focused on the (observable) transitions between energy states, rather than the more classical concept of orbital motions (which aren’t observable). The importance of the energy states, rather than the motion of particles, is a hallmark of quantum mechanics. In today’s lab you will make a fairly accurate determination of the Rydberg constant R and compare it to the Bohr’s theoretical value. Apparatus: 1. The lab apparatus consists of a computer driven spectrometer and a light source as shown below. Light from the discharge tube shines through a narrow slit onto a series of mirrors that direct the light onto a reflection grating. The grating is a mirror-like surface upon which a large number of evenly spaced lines have been inscribed. As you will discover in the pre-lab homework, the spectrometer uses this diffraction grating to separate out the light according to wavelength. from computer to computer motor photodiode and amplifier plane mirror diffraction grat plane mirror ing exit slit entrance slit hemispherical mirror 2. After the light diffracts off the grating, two mirrors direct the light through a narrow exit slit where onto a photosensitive semiconductor diode. NO FOOD OR DRINK IS PERMITTED IN THE LAB. Page 1 of 3 discharge tube Physics 128 Fall 2004 3. The photodiode produces a signal that is logarithmically proportional to the intensity of the light falling on the detector. This signal is amplified and can be measured by the computer. 4. The computer can send a series of voltage pulses to a motor attached to the diffraction grating to control the orientation of the grating (and hence the wavelength of the light passed to the detector.) The computer monitors the signal from the photodiode as it changes the orientation of the grating and can produce a plot of intensity vs. wavelength (a spectrum). Experiment: 1. The relationship between the energy and wavelength of a photon is E = hc / λ . Since the energy is inversely proportional to the wavelength and since it is the wavelength that is most directly measured (being dependent upon the orientation of the grating), spectrometers generally refer to a photon’s energy in inverse wavelength. This quantity 1/λ is what will be plotted along the horizontal axis of the spectra. 2. Since the apparatus doesn’t have anyway to determine the orientation of the diffraction grating directly, the instrument must be calibrated before beginning the experiment. (Your instructor may have already done this.) Measuring a known spectrum and defining the position of a prominent peak to be of a particular wavelength does this. After this has been done, the computer can keep track of how much the grating has moved and thus “knows” what wavelength corresponds to what orientation of the grating. 3. To run the spectrum program, type "\SPECTRUM" at the DOS prompt. The program is menu driven, and already set up to give you the full spectrum (1.0-2.5x104 cm-1) in a minimum amount of time. If you want further detail in any of your measurements, it is easy to change the parameters 4. Measure the H spectrum and make sure you can distinguish as many peaks as possible above the noise. You may want to narrow in on some of the peaks to get a more accurate measurement. 5. You should also take a look at the spectra of other elements before finishing. In particular, measure and save to your notebook a copy of the Rb spectrum. Analysis: 1. From your printout, determine the wavelength (and uncertainty) associated with each peak. 2. See if the position of these peaks follow the Balmer-Rydberg. Make your best determination of the Rydberg constant R (and it’s uncertainty) from your data and compare it to the accepted value of R= (10 973 731.534 ±0.013) m-1 as measured by more accurate spectrometers. Compare it also to the value expected from Bohr’s theory. Group Oral Report: At the beginning of the next lab you will present your results as a group. This should last about 10-15 minutes. Be prepared to answer questions like the following: • How is light spread out to measure the spectrum? • What is the function of the entrance/exit slits? • What are the important contributors to the uncertainty in this measurement? • Why don’t you observe more of the peaks predicted by the Balmer-Rydberg forumula? NO FOOD OR DRINK IS PERMITTED IN THE LAB. Page 2 of 3 Physics 128 Fall 2004 Exercise to understand how a diffraction grating works. A diffraction grating is usually made of a glass, metal or plastic film in which a series of closely spaced lines have been scribed. These lines, that may number 7500 per cm or so, act like scattering centers for the incident wave. Drawn below is a cross-sectional view of a diffraction grating and indicated the propagation direction of the incident and scattered waves as well as a close-up of two scattering centers. B A' A B' α β d (a) (b) (c) (d) (e) If the grating has N lines per cm, what is the spacing between the lines? Calculate the length of the heavy lines shown in the close-up. What is the difference between the path lengths for lines A-A' and B-B' in the close-up? Determine the condition for constructive interference from a grating. A light beam composed of two wavelengths 650 nm (red) and 450 nm (blue) is shined on the grating such that α=π/2. The grating has 7500 lines per cm. Calculate the angle at which the first intensity maximum will appear for each wavelength. NO FOOD OR DRINK IS PERMITTED IN THE LAB. Page 3 of 3