Super Cool Salol.pages
advertisement

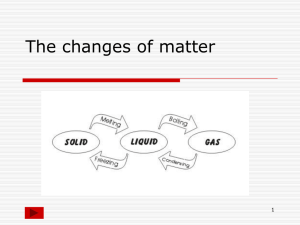
That Supercool Story of Salol ! ! One of the little experiments done in Lab 11 for Chem1160, involved the heating and melting of a compound known as Salol (Phenyl Salicylate), and then following it’s temperature and state as it cooled to room temperature. ! The main take-home point of this experiment for me was the reminder that melting and freezing (boiling too) are properties of most substances, not just the ubiquitous water molecules…and now I’ve used up all of my big words for the day… For Salol, it only took heating the compound to a temperature of about 40-45oC to get it to melt, and if we had had a thermometer in the test tube when we melted it, we would likely have seen the melting plateau of the heating curve. In fact we can represent the states of Salol on the same heating (cooling curve) that we used for water, only in this case, we have different temperatures. ! ! Notice how the temperatures for melting and boiling are different from water? That’s because the MP and BP for a substance are two of its physical properties and usually unique for each substance (pure element or compound) ! But let’s focus on the melting and freezing point, which is supposed to occur at the same temperature point for a substance; being a semantics thing…are we heating it or cooling it??? ! We didn’t have time to show the MP of the Salol with the melting point apparatus in the lab, but if we did we probably would have seen that the Salol did melt at around 41-45oC…you’ll have to take my word for that. If we had been taking temperature readings, and could follow the temperature accurately as we heated, we would likely have seen the nice step and plateau at the melting point - that we all saw during the heating of water to the boiling point. ! But when we cooled a sample of Salol that had been heated to melting and beyond (about 50oC), the nice plateau representing the freezing point wasn’t quite perfect. ! We saw the temperature dip below 41oC until about 37oC. (the dashed line) and then it started to solidify and the temperature jumped up to 41oC and stayed there until it was all solid, and then started cooling again to room temperature. ! Strange…. !There has been a lot of debate over the years about the reason this can happen, and it’s not an isolated incident - i.e exclusive to Salol. Water can also be supercooled and I suggest checking out YouTube for some 30 second videos of water bottles being supercooled. ! The questions remaining are, why and how?? ! There are some complex calculations of thermodynamics and crystalline packing coordination, that is beyond most of us…so lets avoid that. ! Let’s try and think of it in everyday terms ! Imagine a field of soldiers, all standing at attention in formation in their nice straight lines…please excuse the stickmen renderings Now, you’re the sergeant, so you yell out “Dismissed!” and they begin to scatter randomly. I don’t think it would be too surprising that they would scatter quickly and evenly, because we are creating chaos from order. They’d be moving around faster, so we know they have more kinetic energy and therefore a higher temperature…but that’s not too important to my point. The point is they were once in a nice ordered arrangement, fixed in their positions (solid) and we made them move around randomly within the parade ground - we melted them into a liquid. ! Now picture it the other way. You decide that you want them back in formation so you yell out “Fall in! You bunch of $%^ and&%$#!!”… Ahh…maybe that’s a scene from Full Metal Jacket…let’s just pretend the sergeant is nicer than that… ! Anyway, you want them to get back in formation in their straight and even lines - back to the solid arrangement. ! Would it go as smoothly? Even with the loud bad language??? ! I’m sure with practise, even the best trained soldiers would probably take more time and effort to rearrange themselves into formation…with that one arms length distance between each person. And I’m guessing there won’t be any marks on the ground for them to know where to stand. They have to rely on the formation being set by someone and then they can all build on that and eventually they’ll have their ordered straight lines back. ! This same problem confronts a substance being cooled from a liquid into a solid. Solids form crystals, like soldiers in formation, and they have to line up in a specific way, that depends on their shape and forces of attraction - or in the case of ionic compounds, so that all of the negatives are surrounded by positives, and vice-versa. Sometimes they don’t get it, even though they are moving slower then they should in order to be a liquid. It takes some seed of order to get the ball rolling. This could be an imperfection in the side of the container that makes some of them start to line up, or a seed crystal that is dropped in…or a shock wave that is sent through (tapping the side). In any event, once some of the substance particles begin to line up, the rest can follow suite and a crystalline arrangement achieved. ! I’ll admit this analogy only covers some aspects of this process. I have purposefully avoided the thermodynamic aspects (heat of fusion), and the differential cooling between the outside and the inside of the sample. These are factors that can’t be explained by soldiers in formation…at least by me. But hopefully it satisfies some of your questions about this process and maybe why we showed it in the first place…other than the point I made at the beginning. Maybe the real take-home is that the theoretical things we talk about in science may not always match up perfectly with reality…because nature is never perfect… ! ! !