27 Apr 2004
15:6
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
10.1146/annurev.arplant.55.031903.141633
Annu. Rev. Plant Biol. 2004. 55:289–313
doi: 10.1146/annurev.arplant.55.031903.141633
c 2004 by Annual Reviews. All rights reserved
Copyright First published online as a Review in Advance on January 7, 2004
PLASTID TRANSFORMATION IN HIGHER PLANTS
Pal Maliga
Waksman Institute, Rutgers University, Piscataway, New Jersey 08854-8020;
Department of Plant Biology, Rutgers University, New Brunswick, New Jersey 08901;
email: maliga@waksman.rutgers.edu
Key Words plastid genetics, plastid markers, protein expression, gene knockouts,
gene containment
■ Abstract Plastids of higher plants are semi-autonomous organelles with a small,
highly polyploid genome and their own transcription-translation machinery. This review provides an overview of the technology for the genetic modification of the plastid
genome including: vectors, marker genes and gene design, the use of gene knockouts and over-expression to probe plastid function and the application of site-specific
recombinases for excision of target DNA. Examples for applications in basic science
include the study of plastid gene transcription, mRNA editing, photosynthesis and evolution. Examples for biotechnological applications are incorporation of transgenes in
the plastid genome for containment and high-level expression of recombinant proteins
for pharmaceutical and industrial applications. Plastid transformation is routine only
in tobacco. Progress in implementing the technology in other crops is discussed.
CONTENTS
INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
SOMATIC CELL GENETICS OF THE PLASTID . . . . . . . . . . . . . . . . . . . . . . . . . . .
APPROACHES TO PLASTID TRANSFORMATION . . . . . . . . . . . . . . . . . . . . . . . .
Stable Transformation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Episomal Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Transient Expression . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
DNA DELIVERY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
GENETIC MARKERS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Primary Positive Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Secondary Positive Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Negative Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Reporter Genes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Combination of Visual and Selective Markers . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
GENE DESIGN . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
PROBLEMS WITH DATA INTERPRETATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
IMPLEMENTATION IN DIFFERENT SPECIES . . . . . . . . . . . . . . . . . . . . . . . . . . . .
APPLICATIONS IN BASIC SCIENCE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Gene Knockouts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
1543-5008/04/0602-0289$14.00
290
290
292
292
293
294
294
295
295
296
296
296
297
297
297
298
298
298
289
24 Apr 2004
19:37
290
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
Overexpression . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Transcription . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
RNA Editing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Photosynthesis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Evolution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
APPLICATIONS IN BIOTECHNOLOGY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Agronomic Traits . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Expression of Recombinant Proteins . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Marker Gene Elimination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Containment by Plastid Localization . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
PERSPECTIVES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
299
299
300
301
301
302
302
303
303
304
304
INTRODUCTION
Plastids are plant cellular organelles with their own genome and transcriptiontranslation machinery. The plastid genome (plastome or ptDNA) is a highly polyploid, circular double-stranded DNA 120 kb to 180 kb in size, encoding ∼120
genes. A salient feature of the plastid genome in most higher plant species is
duplication of a large (∼25 kb) region in an inverted orientation (112, 148, 160).
Plastid is the general organelle category encompassing proplastids, the progenitors
of all plastid types and chloroplasts (green plastids), chromoplasts (yellow or red,
in some fruits and flowers), and different types of white plastids such as the amyloplasts (starch containing) and elaioplasts (oil containing) (48). Plastid functions
include photosynthesis and starch, amino acid, lipid, and pigment biosynthesis.
Most plastid proteins are encoded in 2100–3600 nuclear genes, the products of
which are translated on cytoplasmic ribosomes and imported into plastids (89).
Boynton, Gillham, and colleagues (18) first achieved plastid transformation
in 1988 in a unicellular alga, Chlamydomonas reindhartii, followed in 1990 by
transformation of the plastid genome in tobacco, which occurred in my laboratory
(151). Plants with transformed plastid genomes are termed transplastomic (96).
This review focuses on the methodology of plastid transformation in higher plants
and selected applications in basic science and biotechnology. General and specialized reviews on this topic have been published elsewhere (3, 12, 14, 59, 98, 100,
138).
SOMATIC CELL GENETICS OF THE PLASTID
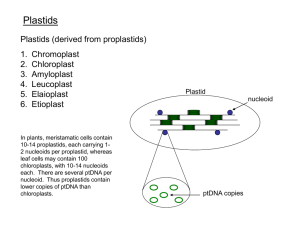
Each particular type of plastid carries identical ptDNA copies, which are attached
to membranes (77, 124, 125) in clusters called plastid nucleoids (86). The number
of plastids and ptDNA copies per cell is highly variable depending on the cell type
(9). In tobacco, the meristematic cells contain 10–14 proplastids, each containing
1–2 nucleoids per organelle, whereas leaf cells may contain 100 chloroplasts, with
10–14 nucleoids each, ∼10,000 ptDNA per cell (9, 157). Plastid transformation
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
P1: GDL
291
involves transforming one or a few ptDNA copies, followed by gradually diluting
plastids carrying nontransformed copies on a selective medium. Although there
may be as many as 10,000 ptDNA copies in a cell, physical contact and recombination are probably feasible only within a nucleoid. This suggests that the genetic
unit of transformation and sorting is probably the nucleoid (Figure 1A).
A study of plastid sorting in heteroplastomic tobacco cells shows preferential
propagation of plastids with antibiotic resistance markers on a selective medium
(106). The plastid markers were streptomycin and lincomycin resistance; the heteroplastomic cells were obtained by protoplast fusion. Cell lines established in the
presence of streptomycin or lincomycin had only one parental plastid type with the
appropriate antibiotic resistance marker; in the absence of antibiotic selection both
plastid types were maintained. Reaching the homoplastomic state was estimated
to take ∼20 cell divisions.
Current protocols for plastid transformation employ strategies to obtain homoplastomic plants by segregating genome copies and organelles in somatic cells.
The most common approach to plastid transformation in tobacco is transformation
of chloroplasts in leaves and regeneration of shoots from the transformed cells
on a selective medium (151, 153). Formation of homoplastomic cells is accelerated by chloroplast to proplastid dedifferentiation, with a concomitant reduction
in nucleoid (ptDNA) number in tissue culture cells (157), then a rebuilding of the
organelle and nucleoid (ptDNA) numbers in regenerated plants (Figure 1B).
Transplastomic shoots regenerated from leaves after bombardment are always
chimeras. Spectinomycin and kanamycin resistance, conferred by the expression
of chimeric genes, is not cell autonomous in regenerating shoots or in seedling
cotyledons. [An exception is rrn16-based spectinomycin resistance in seedling
cotyledons (151).] Lack of cell autonomous expression means that, in chimeric
shoots, nontransformed sectors also have a resistant (green) phenotype (Figure 2A,
see color insert), although they become bleached when cut out and placed in direct contact with the selective medium (see Genetic Markers, below). Resistant
phenotype of nontransformed cells in a chimeric plant is due to cross-protection
by transformed cells. However, transformed and nontransformed sectors can be
readily identified by color (green or white) in knockout plants lacking a photosynthetic gene (2) or by green fluorescent protein (GFP) accumulation (Figure 2B)
(72), which are cell autonomous traits.
The preferred method to obtain homoplastomic tobacco plants is regenerating
new shoots from the transplastomic sectors, which are then rooted (151, 153).
Homoplastomic plants from the chimeric shoots can also be obtained in the seed
progeny, as long as the transplastomes are present in the cell layer that contributes
to the maternal germline (2). In dicots this is the L2 layer, the phenotype of
which is visible at the leaf margins (113). Homoplastomic plants can be obtained
directly from tissue culture cells if cells (protoplasts) are first cultured to form
undifferentiated callus, and plant regeneration is delayed until plastid segregation
is complete. However, extended propagation of cells in tissue culture is undesirable
24 Apr 2004
19:37
292
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
because it causes chromosome rearrangements and polyploidization that affect
plant fertility.
APPROACHES TO PLASTID TRANSFORMATION
Two approaches have been applied to stable genetic modification of plastids: integration of transforming DNA by homologous targeting and introduction of independently replicating shuttle vectors. There have also been attempts to study
gene expression in plastids from transiently introduced DNA. Thus far only stable
integration of the transforming DNA yielded satisfactory results.
Stable Transformation
Plastid transformation vectors are E. coli plasmid derivatives with cloned ptDNA
sequences (1–2 kb) that flank both sides of a selectable marker gene and cloning
sites. The ptDNA sequences serve as targeting regions to direct integration into the
plastid genome (Figure 3A). The plastid vector is propagated in E. coli, and then
introduced into plastids where the marker gene and the gene of interest integrate in
the targeted region by two homologous recombination events. The E. coli vector
part does not carry a plastid replication origin and is subsequently lost.
Plastid targeting sequences do not have special properties and may derive from
any part of the plastid genome. Inserting a marker gene did not interfere with
expression of flanking plastid genes at 14 intergenic regions listed in Table 1.
(An exception is the petB-petD intergenic region, where insertion of aadA made
the plants dependent on sucrose) (107). Plastid DNA fragments containing the
14 neutral intergenic insertion sites could potentially be developed into plastid
Figure 3 Plastid transformation to obtain marker-free tansplastomic plants. (A) Plastid
vectors target insertion of marker gene (m) and gene of interest (goi) via the left (L) and right
(R) targeting sequences (153). Two loxP sites (open triangles) flank the marker gene (m is
floxed) in T1-ptDNA to facilitate its removal by CRE, the P1 phage site-specific recombinase
(26, 56). (B) Plastid-targeted CRE is expressed from a nuclear gene and excises floxed marker
gene to yield T2 marker-free transplastome.
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
PLASTID TRANSFORMATION
293
TABLE 1 Intergenic regions where transgenes were inserted in the plastid genome
Insertion site
Species
References
trnH/pbA
N. tabacum
(21)
trnG/trnfM
N. tabacum
L. esculentum
(17)
(121)
ycf3/trnS
N. tabacum
(64, 74)
rbcL/accD
N. tabacum
(153)
petA/psbJ
N. tabacum
(15, 64, 74)
50 rps12/clpP
N. tabacum
(85, 132)
petD/rpoA
N. tabacum
(74, 149)
ndhB/rps7
B. napus
(62)
3 rps12/trnV
N. tabacum
A. thaliana
O. sativa
L. fendleri
(140, 173)
(134)
(72)
(135)
trnV/rrn16
N. tabacum
(140, 141)
rrn16/trnI
N. tabacum
(140, 151)
trnI/trnA
N. tabacum
(33, 109, 140)
trnN/trnR
N. tabacum
(64, 172)
rpl32/trnL
N. tabacum
(43, 80, 158)
0
vectors. So far only two of these, the trnV/30 rps12 (173) and trnG/trnfM (121)
targeting regions, were made convenient for cloning by removing unnecessary
restriction sites and providing a cluster of unique cloning sites.
Episomal Maintenance
Plastid replication origins can be incorporated in transformation vectors for episomal maintenance. Such plasmids are termed shuttle vectors because they are
maintained as plasmids in E. coli by replication using the ColE1 replication origin
and as extrachromosomal elements in plastids using a plastid ori sequence. An
opportunity to develop such vectors was provided by the serendipitous discovery
of a small (868 bp; NICE1), breakaway circular ptDNA fragment derived from a
vector, which was transmitted through seed (142). A shuttle vector was constructed
by incorporating NICE1 sequences in an E. coli plasmid and a plastid selectable
marker. The shuttle vectors were present in both extrachromosomal and integrated
form, and were used to demonstrate gene conversion between vector and ptDNA
sequences (144). The shuttle vectors were not practical because they were rapidly
lost in the absence of selection.
A conceptual design for plastid shuttle vectors utilizing chloramphenicol resistance as a selective marker gene has been described (32). No published data confirm
24 Apr 2004
19:37
294
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
plastid transformation with the vectors, or recovery of transplastomic clones by
selection for chloramphenicol resistance.
Transient Expression
Expression of transiently introduced DNA was also studied in plastids. β-glucuronidase (GUS) expression was shown in isolated chloroplasts after polyethylene
glycol (PEG) treatment introduced the transforming DNA into protoplasts (136).
Evidence for transient green fluorescent protein (GFP) expression in plastids was
reported after biolistic DNA delivery (60) or microinjection of plastid reporter
genes (76).
During the early 1990s several papers were published on transient expression
of chloramphenicol acetyltransferase and GUS from plastid gene constructs after
biolistic delivery of chimeric genes to cells (for example, 37, 168). Because activity
derived from plastid and nuclear expression was not distinguished, the conclusions
of these papers are now considered obsolete.
DNA DELIVERY
A critical development for the progress of organelle biology was the biolistic DNA
delivery enabling transformation of Chlamydomonas chloroplasts (18), yeast mitochondria (67), and higher plant chloroplasts (151) pioneered by John Sanford
and colleagues during the late 1980s. The original gun with particles accelerated
by a gunpowder charge (75) was quickly replaced by a cleaner version, using highpressure He gas as propellant (66). The particle gun remains unchanged since the
early 1990s, except that an adaptor was introduced to simultaneously accommodate seven macrocarriers (Hepta adaptor; Biorad, Hercules, CA). Tungsten or gold
particles work equally well. Biolistic delivery is the system of choice for most
laboratories, as manipulation of leaves, cotyledons, or cultured cells in tissue culture requires less experience than the alternative PEG treatment of protoplasts.
Protocols for DNA coating of particles (97), selection in tobacco leaf cultures
(153), and transformation of chloroplasts in leaves (10) have been published.
PEG-mediated transformation of plastids requires enzymatically removing the
cell wall to obtain protoplasts, then exposing the protoplasts to purified DNA in
the presence of PEG. The protoplasts first shrink in the presence of PEG, then lyse
due to disintegration of the cell membrane. Removing PEG before the membrane
is irreversibly damaged reverses the process. PEG treatment was first used to test
transient expression of GUS in chloroplasts (136), then for stable transformation
of the plastid genome (49, 111). A review of the method (80) and a step-by-step
protocol of plastid transformation by PEG treatment are available (79).
Additional approaches for DNA delivery to plastids have also been tried. The
first attempt at plastid transformation used an Agrobacterium binary transformation vector (38). We now understand that sophisticated nuclear targeting of the
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
P1: GDL
295
transferred DNA makes use of Agrobacterium for plastid transformation a challenge. Microinjection, which looks promising for transient gene expression (76),
has not yet yielded stable transplastomic clones.
GENETIC MARKERS
Primary Positive Selection
Primary markers are suitable for selectively amplifying a small number of transformed ptDNA copies. Currently known primary markers are resistance to spectinomycin, streptomycin, and kanamycin, which inhibit protein synthesis on
prokaryotic-type plastid ribosomes. These antibiotics inhibit greening, cell division, and shoot formation in tobacco culture. Therefore, greening, faster proliferation, and shoot formation were used to identify transplastomic clones on a selective
medium. The first transplastomic clones were obtained by spectinomycin selection.
Because spectinomycin allows slow proliferation of nontransformed tobacco cells
it was assumed that the choice of a drug that enables such “nonlethal” selection
is important to recover transplastomic clones (96, 151). However, transplastomic
clones were soon identified by kanamycin selection using an antibiotic concentration that is considered “lethal” (50 mg/L) (20). Thus, slow proliferation of nontransformed cells on a selective medium is not an essential feature of the selection
scheme.
Initial transformation vectors carried a plastid 16S rRNA (rrn16) gene with
point mutations that prevent binding of spectinomycin or streptomycin to the 16S
rRNA (140, 141, 151). The rrn16 target site mutations are recessive, and were
∼100-fold less efficient than the currently used dominant aadA gene (153). Streptomycin resistance encoded in the rps12 ribosomal protein gene was also included
in an early vector (140). Unexplored plastid mutations that could be used in vectors are lincomycin resistance, encoded in the 23S rRNA (29, 30), and triazine
resistance, encoded in psbA (31). Plastid mutations conferring resistance to spectinomycin, streptomycin, and lincomycin were also described in Solanum nigrum
(70). Solanum nigrum vectors with spectinomycin resistance (rrn16) and streptomycin resistance (rps12) markers were used to transform tobacco plastids (71).
More efficient primary plastid markers are chimeric genes in which the coding
segment of a bacterial antibiotic detoxifying enzyme is expressed from plastid signals. The aadA gene encodes aminoglycoside 300 -adenylyltransferase (AAD) also
called aminoglycoside nucleotidyltransferase [ANT(300 )-I] (128). AAD inactivates
spectinomycin and streptomycin (GenBank X02340, M10241) and was used to
select transplastomic clones in Chlamydomonas (50) and tobacco (153). The neo
(aph(30 )IIa) gene encodes neomycin phosphotransferase II [NPTII; APH(30 )-II]
(GenBank V00618) (128), and was used to select transplastomic clones in tobacco
(20). The aphA-6 gene encodes aminoglycoside phosphotransferase or APH(30 )VI (GenBank X07753) (128), and was used to select transplastomic clones by
kanamycin and amikamycin resistance in Chlamydomonas (8) and by kanamycin
24 Apr 2004
19:37
296
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
resistance in tobacco (64). Direct selection for spectinomycin resistance (153,
173) and for highly expressed kanamycin resistance genes (pHK30, pHK34) (83)
(A.K. Azhagiri, unpublished results), on average, yield one transplastomic line in
a bombarded leaf sample, although the values in this laboratory varied between 0.5
and 5.0.
Another potentially useful marker is a plant nuclear gene encoding betaine aldehyde dehydrogenase (BADH), which confers resistance to the toxic compound betaine aldehyde (BA) (36). BA selection is supposedly 25-fold more efficient than
spectinomycin selection (34). This claim has triggered research in other laboratories to confirm the advantage of selecting transplastomic clones by BA resistance.
So far direct selection of transplastomic tobacco clones by resistance to BA alone
is not confirmed (S.M. Whitney, J.T. Andrews, T. Golds & H.U. Koop, unpublished
results). It may be relevant that no transplastomic plants that carry the BADH gene
as the only selective marker have been reported.
Secondary Positive Selection
Use of secondary selective markers is dose dependent; they are not suitable to
select transplastomic clones when only a few ptDNA copies are transformed,
but will confer a selective advantage when most genome copies are transformed.
Examples for secondary markers are genes that confer resistance to the herbicides phosphinothricin (PPT) (92, 167) or glyphosate (167) or to the antibiotic
hygromycin [based on expression of the bacterial hygromycin phosphotransferase
gene (Z. Svab & S. Corneille, unpublished results)].
Negative Selection
The ability to identify loss-of-function of a conditionally toxic gene forms the
basis of negative selection. A negative selection scheme in plastids utilizes the
bacterial cytosine deaminase (CD) enzyme encoded in the codA gene (126). CD
catalyzes deamination of cytosine to uracil, enabling use of cytosine as the sole
nitrogen and pyrimidine source. CD is present in prokaryotes and in many eukaryotic microorganisms, but is absent in higher plants. 5-fluorocytosine is converted to
5-fluorouracil, which is toxic to cells. This negative selection scheme was utilized
to identify seedlings on 5-fluorocytosine-medium from which codA was removed
by the CRE-loxP site-specific recombinase (26).
Reporter Genes
The E. coli GUS and the Aequorea victoria GFP are reporter enzymes that allow
tracking gene expression, but do not confer a selective advantage or disadvantage
to plastids. GUS enzymatic activity expressed in chloroplasts has been measured
using fluorogenic assays (43, 141, 143, 172) and visualized by histochemical staining (141). GFP is a visual marker, allowing direct imaging of the fluorescent gene
product in living cells. Its chromophore forms autocatalytically in the presence of
oxygen and fluoresces green when absorbing blue or UV light. GFP has been used
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
P1: GDL
297
to detect transient gene expression (60) and stable transformation events (117, 130,
133, 135) in chloroplasts. GFP was fused with the aadA gene product (AAD) to
be used as a bifunctional visual and selective (spectinomycin resistance) marker
gene (72).
Combination of Visual and Selective Markers
Koop and colleagues (74) developed an ingenious scheme for rapidly identifying
transplastomic sectors using pigment-deficient tobacco knockout plants as recipients. In the knockout plants, an antibiotic resistance gene (aadA) replaces a plastid
gene that causes chlorophyll deficiency. The transformation vector carries the photosynthetic gene to restore green pigmentation and a second antibiotic resistance
gene to favor maintenance of transformed plastids. Homoplastomic sectors and
plants can be readily identified by the green color, a strategy that significantly
reduces the time required to obtain homoplastomic plants.
GENE DESIGN
A modular approach for transgene assembly was developed to conveniently shuffle
50 -regulatory regions, coding segments, and 30 -regulatory regions based on the
pUC18/19 polycloning site (for reviews, see 98, 100).
The 50 regulatory regions are provided in a PL cassette, which includes a promoter (P) and translation control sequences (L, leader). The translation control
sequences may be the mRNA 50 -UTR, or the 50 -TCR that includes the 50 -UTR and
the coding region N terminus (82, 83). The plastid genome contains many promoters (90). Biotechnological applications have focused on the strong, sigma70-type
rRNA operon (Prrn) promoter recognized by the plastid-encoded plastid RNA
polymerase. Prrn was fused with translation control sequences of plastid and phage
origin to facilitate translation of the encoded recombinant proteins. The 50 -UTR is
typically a truncated and mutant form of native sequences. Protein accumulation
from the same (Prrn) promoter may vary as much as 10,000-fold depending on the
choice of translation control signals (43, 82, 83, 169, 172; reviewed in 100).
The 30 -regulatory region or T cassette encodes the mRNA 30 -UTR, typically
including a stem-loop structure. The 30 -regulatory region is important for mRNA
stability (108). Most commonly used T cassettes derive from the plastid psbA,
rbcL, and rps16 genes (100).
PROBLEMS WITH DATA INTERPRETATION
Although the plastid marker genes are designed for expression in plastids, some
copies may fortuitously integrate in the nucleus and express from an upstream promoter to yield spectinomycin/streptomycin or kanamycin resistant clones (20, 28).
Transplastomic clones can be readily distinguished from the products of nuclear
insertion by the altered ptDNA on DNA gel blots.
24 Apr 2004
19:37
298
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
Spontaneous mutation to spectinomycin resistance is very common in all species
tested so far, including tobacco (151, 152), potato (133), Arabidopsis (134), and
Lesquerella (135). [So far, no spontaneous mutation for kanamycin resistance
has been found in tobacco, but it is common in Chlamydomonas chloroplasts
(57).] Transplastomic clones differ from spontaneous mutants by resistance to
both spectinomycin and the unselected streptomycin (Figure 2C) (153).
Plastid genome fragments, at least in some species, are present in the nuclear (5,
118) and/or in the mitochondrial genomes (110). For example, rice chromosome 10
alone carries two large ptDNA insertions (33 kb and 131 kb) encompassing almost
the entire ptDNA (118). Such nuclear or mitochondrial copies of the plastid rbcL
(5), ndh (78), and ycf9 (7, 95) genes are indicated by a persistent, weak wild-type
signal on DNA gel blots of total cellular DNA. However, the weak signal should
be absent on blots prepared with ptDNA from purified chloroplasts (101, 120),
and nontransformed, wild-type seedlings should be absent in the seed progeny
(101). A technically more demanding approach to verifying the plastid location
of transgenes utilizes PCR analysis of pulsed-field gel electrophoresis-purified
ptDNA (154).
IMPLEMENTATION IN DIFFERENT SPECIES
Although plastid transformation in higher plants was achieved in 1990 (151), it
is routine only in tobacco (153, 173). Plastid transformation has also been successful in two other solanaceous species, potato (133) and tomato (121). Plastid transformation in Arabidopsis thaliana (134) and the related Brassica napus
(62) and Lesquerella fendleri (135) was feasible but inefficient. Plastid transformation of embryogenic cultures in rice could be readily obtained. Rice plants
regenerated from the transformed culture were heteroplastomic (72), suggesting
that only refining the tissue culture system is required to obtain homoplastomic
plants. Spectinomycin resistance (aadA) was the marker of choice in each species,
except rice, in which streptomycin resistance was used to select transplastomic
clones. Spectinomycin selection in cereals is not an option because rice and maize
plastid rRNAs naturally have the mutations that prevent spectinomycin binding
(46, 72).
APPLICATIONS IN BASIC SCIENCE
Gene Knockouts
Targeted knockout of plastid genes involves construction of a transformation vector in which a selectable marker replaces the target gene in a larger ptDNA fragment (Figure 4). Selection for antibiotic resistance results in replacement of the
target gene with the selective marker in the ptDNA. Table 2 contains the list of
28 genes that have been targeted for deletion. Twenty-five genes have been deleted.
Positively identifying essential plastid genes has been problematic since attempts to
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
P1: GDL
299
Figure 4 Targeted deletion of a plastid gene (G2) by replacement with marker
gene (M1) in vector.
obtain homoplastomic ycf1, ycf2 (42), and clpP1 (132) knockout lines by targeted
insertion of a selective marker have failed. Evidence for an essential role for the
ClpP1 protease subunit was obtained by the CRE-loxP site-specific recombination
system (85). The clpP1 gene in the plastid genome was first flanked with directly
oriented loxP sites (floxed). A CRE gene was then introduced into the nucleus
by pollination. The nuclear-encoded, plastid-targeted CRE entered the plastids
and excised the floxed clpP1 copies. Loss of the clpP1 gene product, the ClpP1
protease subunit, led to ablation of the shoot system of tobacco plants, suggesting
that ClpP1-mediated protein degradation is essential for shoot development.
Overexpression
The advantage of overexpression as a research tool was shown by the unexpected
discovery of site-specific RNA editing trans-factors based on over-expression of an
edited RNA segment (23) and a clP1-specific mRNA maturation factor based on
overexpression of the clpP1 50 -UTR in a chimeric transcript (84). Overexpression
was used to probe the plastid accD function by replacing the weak accD promoter
with the strong rrn promoter (94). Another example for overexpression involved
relocating the nuclear anthranilate synthase gene to plastids to boost tryptophan
production (170).
Transcription
The field of plastid gene transcription also benefits from plastid transformation.
The plastid rpoA (127) and rpoB (1) tobacco knockout plants played a critical role
in recognizing that the plastid-encoded plastid RNA polymerase (PEP) and the
nuclear-encoded phage-type RNA polymerase (NEP) transcribe distinct groups
of genes (55, 127). Reporter genes expressing GUS and GFP have been utilized
to identify PEP and NEP promoter elements. Of the PEP promoters, dissection
in vivo was reported for the blue-light-regulated psbD promoter (1, 158), the rRNA
operon PEP promoter (150), the rbcL promoter (129), and the psbA promoter (58).
24 Apr 2004
19:37
300
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
TABLE 2 Plastid genes targeted for deletion
Gene
Function
References
rpoA
PEP
(40, 127)
rpoB
PEP
(2, 40)
rpoC1
PEP
(40, 127)
rpoC2
PEP
(127)
trnV
tRNA-Val(GAC)
(26, 56)
sprA
RNA
(147)
oriA
DNA replication
(109)
DNA replication
(109)a
ndhA
Ndh
(78)
ndhB
Ndh
(61, 131)
ndhC
Ndh
(19, 78)
ndhH
Ndh
(78)
ndhI
Ndh
(78)
ndhJ
Ndh
(19)
ndhK
Ndh
(19, 78)
rbcL
Rubisco
(68)
psbA
PSII
(6)
psbE
PSII
(156)
psbF
PSII
(156)
psbL
PSII
(156)
psbJ
PSII
(54, 156)
clpP1
oriB
∗
Protease
(132)∗ (85)
a
ycf1
?
(42)a
ycf2a
?
(42)a
ycf3
PSI
(122)
ycf6/petN
cyt. B6/f
(53)
ycf9/lhbA/psbZ
PSII
(7, 95, 120, 155)
ycf10
?
(154)
a
No homoplastomic knockout plant was reported.
Of the promoters recognized by the NEP, the clpP1 (137) and atpB (166) promoters
have been subjected to in vivo dissection.
RNA Editing
RNA editing in plastids involves posttranscriptional C to U nucleotide conversions
(11, 160). Plastid transformation has been an important tool in studying RNA
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
P1: GDL
301
editing in chimeric transcripts consisting of a series of small mRNA segments
transcriptionally fused with a reporter gene (23). Sequences required for RNA
editing are contained in a small segment (13, 24); RNA editing depends on sitespecific depletable trans-factors (22, 23, 116); if a site is not edited in species it is
an indication that the capacity to edit the site is absent (15) unless the species has
sites that share specificity factor(s) with the heterologous site (22).
Photosynthesis
Because many photosynthetic genes are encoded in the plastid genome, plastid
transformation is a necessary tool to probe and improve photosynthesis. Studies
in higher plants focused on photosynthetic gene knockouts (Table 2) and Rubisco
engineering (for a review, see 3). Rubisco in higher plants is composed of plastidencoded large and nucleus-encoded small subunits (LS and SS, encoded in the
plastid rbcL and nuclear rbcS genes, respectively). The plastid-encoded tobacco
rbcL gene was replaced with a heterologous sunflower gene (69), genes from
nongreen algae (165), cyanobacteria (Synechococcus PCC6301) (69), and the αproteobacterium Rhodospirillum rubrum (163, 164). Replacing the tobacco rbcL
gene with the Rhodospirillum rubrum Form II Rubisco yielded a fully photoautotrophic, fertile plant (163). Allotopic expression of the Rubisco subunits has been
explored by relocating the plastid rbcL gene to the nucleus (68), and the nuclear
small subunit gene to the plastid genome (162, 171).
Evolution
During evolution most of the ∼3000 genes encoded in the genome of the photosynthetic endosymbiont migrated to the nucleus (89, 102). Plastid transformation
enabled addressing experimentally the mechanistic details of gene transfer. The
frequency of DNA transfer from plastids to the nucleus was tested by incorporating a nuclear kanamycin resistance gene in the plastid genome, then selecting
for a transfer event to the nucleus by selecting for expression of a kanamycin resistance gene. The frequencies of transfer were 1 in 16,000 in the seed progeny
(63) and significantly lower (1 in 5,000,000) in somatic cells (145). Of course,
acquiring a nuclear promoter by a transferred plastid gene is several orders of magnitude less likely than expressing a plastid gene that brings along its own nuclear
promoter. The feasibility of relocating a plastid gene to the nucleus was also tested
(68). First, the plastid-encoded rbcL copy was removed by targeted deletion yielding a Rubisco-deficient plant. Absence of a functional Rubisco in the knockout
plants established that none of the ∼15 nuclear rbcL copies contained in chunks
of incorporated ptDNA fragments are functional. The LS coding region was then
extended at the N terminus to provide a plastid-targeting transit sequence and was
incorporated in the nucleus of knockout plants. Rubisco levels were restored up
to ∼10% of the wild-type levels. Successfully relocating the plastid rbcL gene to
the nucleus supports the view that gene migration from plastids to the nucleus is
an ongoing process.
24 Apr 2004
19:37
302
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
APPLICATIONS IN BIOTECHNOLOGY
Agronomic Traits
In field crops, the most common transgenic traits are resistance to insects and herbicides expressed from nuclear genes. Both types of genes have been successfully
engineered for plastid expression (Table 3). Expression of the B.t. insecticidal
protein from nuclear genes required construction of synthetic, codon-modified
genes to improve translation, protect the mRNA from degradation, and prevent
early translation termination. In contrast, the B.t. insecticidal protein genes were
expressed in plastids from bacterial coding segments (39, 81, 103, 115; for a review,
see 100). Commercially useful versions of commonly used herbicide resistance
genes are also available. Tobacco plants with some degree of glyphosate tolerance
were obtained by overexpression of the sensitive form of 5-enolpyruvylshikimate3-phosphate synthase (EPSPS) target enzyme in plastids (33). Field-level tolerance
to glyphosate was obtained by expression in plastids of prokaryotic EPSPS genes;
the required protein levels were higher (∼5% of TSP) than when EPSPS was
expressed from nuclear genes (169). An interestingly split EPSPS gene was developed with gene containment in mind, with one half of the protein encoded in
a nuclear gene and the second half in a plastid gene. Intein trans-splicing resulted
TABLE 3 Plastid transgenes for biotechnological applications
Gene
Function
References
bar
Herbicide res./PPT
(65, 92, 167)
CP4
Herbicide res./glyphosate
(167, 169)
Ic-EPSPSc
Herbicide res./glyphosate
(25)
cry1Ac
B.t. Insecticidal prot.
(103)
cry2Aa2
B.t. insecticidal prot
(39, 81)
cry1Ia5
B.t. insecticidal prot.
(115)
Somatotropin
Human growth hormone
(139)
CTB
Cholera toxin B subunit
(35)
TetC
Tetanus vaccine
(159)
HSA
Human serum albumin
(45)
PBP
Protein-based polymer
(51)
ASA2
Tryptophan biosynthesis
(170)
MS1-99
Antimicrobial peptide
(41)
phb operon
Polyhydroxybutyrate
(91)
TPS1
Trehalose phosph. synthase
(88)
merA
Mercuric ion reductase
(123)
merB
Organomercurial lyase
(123)
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
P1: GDL
303
in reconstituting the herbicide-resistant EPSPS enzyme in plastids (25). Pollen
transmission from this crop can lead to the transfer of only part of the herbicideresistance gene, which is insufficient to confer glyphosate tolerance. Field-level
tolerance to herbicides containing PPT as an active ingredient was obtained by
expressing bar in the plastid genome (92, 167).
Expression of Recombinant Proteins
To meet the demands for production capacity of recombinant proteins, there is
significant interest in plant-based production of vaccines, antibodies, and industrial enzymes (47, 87, 93, 146). Transgene expression in tobacco plastids reproducibly yields protein levels in the 5% to 20% range (for a review, see 100).
A salient feature of plastid expression is the importance of post-transcriptional
regulation; from the same promoter proteins may accumulate in a 10,000-fold
range (for references, see Gene Design, above). Since all codons are relatively
frequently used, codon optimization yields only a modest 2- to 3-fold increase
in protein accumulation levels (92, 159, 169). Transcripts derived from genes of
diverse sources were stable in plastids, including bacterial genes with relatively
high levels of adenine and thymine (high-AT) (103, 115) (159) and guanine and
cytosine (high-GC) (92), synthetic mRNAs (159), and plant (170) and human (45)
cDNAs. This suggests the compatibility of the plastid’s RNA degradation machinery with mRNAs from diverse sources, avoiding the need to construct synthetic
genes for plastid expression. Furthermore, there is no protein glycosylation in
plastids (159).
Marker Gene Elimination
The interest in developing marker elimination systems for plastids was driven by
regulatory concerns to avoid releasing antibiotic resistance genes in transplastomic
crops, the desire to reuse the relatively few available plastid marker genes, and the
metabolic burden imposed by expressing marker genes. Three systems are available for marker gene elimination. The first system relies on the loop-out of the
marker gene through directly repeated sequences (65). This system is practical
only in exceptional cases, when introducing secondary markers, such as herbicide resistance genes (65), is the desired objective. A second approach involves
cotransforming two independently targeted plastid transgenes and segregating out
the ptDNA with the marker gene at the heteroplastomic stage (167). The third
and most efficient approach uses vectors with floxed marker genes, which can be
removed with the CRE site-specific recombinase. Although convenient vectors
with floxed marker genes have not yet been reported for the introduction of passenger genes, the feasibility of the approach was shown by excising aadA (56),
codA (26) and clpP1 (85) genes (Figure 3B). Important for the application of
CRE in plastids is that no detrimental ptDNA rearrangements persist once CRE is
removed (27).
24 Apr 2004
19:37
304
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
Containment by Plastid Localization
Transgene flow is a problem within a species, when pollen from one stand of the
crop finds its way into another stand (119), or when transgenes are incorporated in
weedy relatives (44). Effectively controlling intraspecific transgene flow in species
with a strict maternal inheritance of plastids can be achieved by incorporating the
transgenes in the plastid genome. Examples for species with strict maternal inheritance of plastids are Zea mays, Glycine max, Oryza sativa, and Arabidopsis
thaliana, a group to which two thirds of the angiosperm species belong (52, 105,
114). Since more sensitive, selectable plastid markers are available, strict maternal inheritance of plastids has been questioned in Nicotiana tabacum and Nicotiana plumbaginifolia. Two publications reported low-level pollen transmission of
plastome-encoded streptomycin resistance (104) and tentoxin resistance (4) at a
frequency of 0.07% to 2.5% of the progeny, respectively. Low frequency (0.03%)
pollen transmission of plastids was also reported in a Setaria italica (foxtail or
birdseed millet) cross (161). In each case, at least one of the parental lines was a
cytoplasmic substitution line, in which the nucleus of one species was combined
with the plastids and/or mitochondria of another species. Strict maternal inheritance is known to break down in species hybrids (73), and that may explain low
frequency of paternal pollen transmission in these examples.
PERSPECTIVES
During the past decade plastid transformation has become a principal tool of plastid biology. The most important task for the coming years will be implementing
plastid transformation in the major crops. The key to progress will be identifying bottlenecks in the recalcitrant species and combining suitable tissue culture
systems with efficient molecular tools. In agronomic applications, incorporating
transgenes in the plastid genome instead of the nucleus will be an excellent tool
to control gene flow in crops with maternal plastid inheritance. Plastid localization will be a significant improvement as compared to the present practice of
incorporating transgenes in the nuclear genome, when 100% of pollen leaving
the field carries the transgenic information. Part of breeding transplastomic crops
will require testing paternal plastid transmission and, if necessary, screening for
lines in which paternal transmission of plastids does not occur. From here on,
the feasibility of functional transfer of plastid genes to the nucleus will also be a
consideration. A predictable outcome is a glut of new smart gene designs, such as
editing-dependent genes (23), genes with plastid introns (16), and split genes (25),
which minimize the opportunity for expression of plastid genes after transfer to
the nucleus. Although basic science applications, such as probing photosynthetic
functions and plastid gene expression will remain important, plastid transformation has now reached a more mature phase when it is expected to make a broader
impact through agricultural and industrial applications.
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
P1: GDL
305
ACKNOWLEDGMENTS
I thank members of my laboratory for their comments during the preparation of
the manuscript; Arun Azhagiri for assistance with the figures; and John Andrews,
Ralph Bock, Timothy Golds, Jonathan Gressel, Hans-Ulrich Koop, Jeffrey Staub,
and Spencer Whitney for communicating unpublished results and supplying copies
of their manuscripts prior to publication. The National Science Foundation Biochemistry and Eukaryotic Genetics Program, The Rockefeller Foundation, Monsanto Co., and Rutgers F&A Special Research Grant supported research in this
laboratory.
The Annual Review of Plant Biology is online at http://plant.annualreviews.org
LITERATURE CITED
1. Allison LA, Maliga P. 1995. Lightresponsive and transcription-enhancing
elements regulate the plastid psbD core
promoter. EMBO J. 14:3721–30
2. Allison LA, Simon LD, Maliga P. 1996.
Deletion of rpoB reveals a second distinct
transcription system in plastids of higher
plants. EMBO J. 15:2802–9
3. Andrews JT, Whitney SM. 2003. Manipulating ribulose bisphosphate carboxylase/oxygenase in the chloroplasts of
higher plants. Arch. Biochem. Biophys.
414:159–69
4. Avni A, Edelman M. 1991. Direct selection for paternal inheritance of chloroplasts in sexual progeny of Nicotiana.
Mol. Gen. Genet. 225:273–77
5. Ayliffe AM, Timmis JN. 1992. Tobacco
nuclear DNA contains long tracts of homology to chloroplast DNA. Theor. Appl.
Genet. 85:229–38
6. Baena-Gonzales E, Allahverdieva Y, Svab
Z, Maliga P, Josse EM, et al. 2003.
Deletion of the tobacco plastid psbA
gene triggers post-transcriptional upregulation of thylakoid-associated terminal oxidase (PTOX) and the NAD(P)H
complex. Plant J. 35:704–16
7. Baena-Gonzales E, Gray JC, Tyystjärvi
E, Aro EM, Mäenpää P. 2001. Abnormal regulation of photosynthetic electron
transport in a chloroplast ycf9 inactiva-
8.
9.
10.
11.
12.
13.
14.
15.
16.
tion mutant. J. Biol. Chem. 276:20795–
802
Bateman JM, Purton S. 2000. Tools for
chloroplast transformation in Chlamydomonas: expression vectors and a new
dominant selectable marker. Mol. Gen.
Genet. 263:404–10
Bendich AJ. 1987. Why do chloroplasts
and mitochondria contain so many copies
of their genome? Bioessays 6:279–82
Bock R. 1998. Analysis of RNA editing
in plastids. Methods 15:75–83
Bock R. 2000. Sense from nonsense: How
the genetic information of chloroplasts
is altered by RNA editing. Biochimie
82:549–57
Bock R. 2001. Transgenic plastids in basic
research and plant biotechnology. J. Mol.
Biol. 312:425–38
Bock R, Hermann M, Kössel H. 1996.
In vivo dissection of cis-acting determinants for plastid RNA editing. EMBO J.
15:5052–59
Bock R, Hippler M. 2002. Extranuclear inheritance: functional genomics in
chloroplasts. Prog. Bot. 63:106–31
Bock R, Kössel H, Maliga P. 1994. Introduction of a heterologous editing site into
the tobacco plastid genome: The lack of
RNA editing leads to a mutant phenotype.
EMBO J. 13:4623–28
Bock R, Maliga P. 1995. Correct splicing
24 Apr 2004
19:37
306
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
of a group II intron from a chimeric reporter gene transcript in tobacco plastids.
Nucleic Acids Res. 23:2544–47
Bock R, Maliga P. 1995. In vivo testing of
a tobacco plastid DNA segment for guide
RNA function in psbL editing. Mol. Gen.
Genet. 247:439–43
Boynton JE, Gillham NW, Harris EH,
Hosler JP, Johnson AM, et al. 1988.
Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 240:1534–38
Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ. 1998. Identification of a
functional respiratory complex in chloroplasts through analysis of tobacco mutants
containing disrupted plastid ndh genes.
EMBO J. 17:868–76
Carrer H, Hockenberry TN, Svab Z, Maliga P. 1993. Kanamycin resistance as a selectable marker for plastid transformation
in tobacco. Mol. Gen. Genet. 241:49–56
Carrer H, Maliga P. 1995. Targeted insertion of foreign genes into the tobacco
plastid genome without physical linkage
to the selectable marker gene. Biotechnology 13:791–94
Chateigner-Boutin AL, Hanson MR.
2002. Cross-competition in transgenic
chloroplasts expressing single editing
sites reveals shared cis elements. Mol.
Cell. Biol. 2002:8448–56
Chaudhuri S, Carrer H, Maliga P. 1995.
Site-specific factor involved in the editing
of the psbL mRNA in tobacco plastids.
EMBO J. 14:2951–57
Chaudhuri S, Maliga P. 1996. Sequences
directing C to U editing of the plastid psbL
mRNA are located within a 22 nucleotide
segment spanning the editing site. EMBO
J. 15:5958–64
Chin HH, Kim GD, Marin I, Mersha
F, Evans TC, et al. 2003. Protein transsplicing in transgenic plant chloroplast:
Reconstruction of herbicide resistance
from split genes. Proc. Natl. Acad. Sci.
USA 100:4510–15
Corneille S, Lutz K, Svab Z, Maliga P.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
2001. Efficient elimination of selectable
marker genes from the plastid genome by
the CRE-lox site-specific recombination
system. Plant J. 72:171–78
Corneille S, Lutz KA, Azhagiri AK, Maliga P. 2003. Identification of functional
lox sites in the plastid genome. Plant J.
35:753–62
Cornelissen M, Vandewiele M. 1989. Nuclear transcriptional activity of the tobacco plastid psbA promoter. Nucleic
Acids Res. 17:19–29
Cséplö A, Eigel L, Horváth GV,
Medgyesy P, Herrmann RG, Koop HU.
1993. Subcellular location of lincomycin
resistance in Nicotiana mutants. Mol.
Gen. Genet. 236:163–70
Cséplö A, Maliga P. 1984. Large scale isolation of maternally inherited lincomycin
resistance mutations, in diploid Nicotiana
plumbaginifolia protoplast cultures. Mol.
Gen. Genet. 196:407–12
Cséplö A, Medgyesy P, Hideg E, Demeter
S, Marton L, Maliga P. 1985. Triazineresistant Nicotiana mutants from photomixotrophic cell cultures. Mol. Gen.
Genet. 200:508–10
Daniell H. 1993. Foreign gene expression
in chloroplasts of higher plants mediated
by tungsten particle bombardment. Meth.
Enzymol. 217:536–56
Daniell H, Datta R, Varma S, Gray S, Lee
SB. 1998. Containment of herbicide resistance through genetic engineering of
the chloroplast genome. Nat. Biotechnol.
16:345–48
Daniell H, Khan MS, Allison L. 2002.
Milestones in chloroplast genetic engineering: an environmentally friendly era
in biotechnology. Trends Plant Sci. 7:84–
91
Daniell H, Lee SB, Panchal T, Wiebe
PO. 2001. Expression of the native
cholera toxin B subunit gene and assembly of functional oligomers in transgenic tobacco chloroplasts. J. Mol. Biol.
311:1001–9
Daniell H, Muthukumar B, Lee SB. 2001.
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
37.
38.
39.
40.
41.
42.
43.
44.
45.
Marker free transgenic plants: Engineering the chloroplast genome without the
use of antibiotic selection. Curr. Genet.
39:109–16
Daniell H, Vivekananda J, Nielsen BL, Ye
GN, Tewari KK, Sanford JC. 1990. Transient foreign gene expression in chloroplasts of cultured tobacco cells after biolistic delivery of chloroplast vectors.
Proc. Natl. Acad. Sci. USA 87:88–92
De Block M, Schell J, Van Montagu
M. 1985. Chloroplast transformation by
Agrobacterium tumefaciens. EMBO J.
4:1367–72
De Cosa B, Moar W, Lee SB, Miller M,
Daniell H. 2001. Overexpression of the
Bt cry2Aa2 operon in chloroplasts leads
to formation of insecticidal crystals. Nat.
Biotechnol. 19:71–4
De Santis-Maciossek G, Kofer W, Bock
A, Schoch S, Maier RM, et al. 1999. Targeted disruption of the plastid RNA polymerase genes rpoA, B and C1: molecular
biology, biochemistry and ultrastructure.
Plant J. 18:477–89
DeGray G, Rajasekaran K, Smith F, Sanford JC, Daniell H. 2001. Expression of an
antimicrobial peptide via the chloroplast
genome to control phytopathogenic bacteria and fungi. Plant Physiol. 127:852–62
Drescher A, Ruf S, Calsa T, Carrer H,
Bock R. 2000. The two largest chloroplast
genome-encoded open reading frames of
higher plants are essential genes. Plant J.
22:97–104
Eibl C, Zou Z, Beck A, Kim M, Mullet J,
Koop HU. 1999. In vivo analysis of plastid psbA, rbcL and rpl32 UTR elements by
chloroplast transformation: tobacco plastid gene expression is controlled by modulation of transcript levels and translation
efficiency. Plant J. 19:333–45
Ellstrand NC, Prentice HC, Hancock JF.
1999. Gene flow and introgression from
domesticated plants into their wild relatives. Annu. Rev. Ecol. Syst. 30:539–63
Fernandez-San Milan A, Mingo-Castel A,
Miller M, Daniell H. 2003. A chloroplast
46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
P1: GDL
307
transgenic approach to hyper-express and
purify Human Serum Albumin, a protein
highly susceptible to proteolytic degradation. Plant Biotechnol. J. 1:71–19
Fromm H, Edelman M, Aviv D, Galun
E. 1987. The molecular basis for rDNAdependent spectinomycin resistance in
Nicotiana chloroplasts. EMBO J. 6:3233–
37
Giddings G, Allison G, Brooks D, Carter
A. 2000. Transgenic plants as factories
for biopharmaceuticals. Nat. Biotechnol.
18:1151–55
Gillham NW. 1994. Organelle Genes and
Genomes. New York: Oxford Univ. Press
Golds T, Maliga P, Koop HU. 1993.
Stable plastid transformation in PEGtreated protoplasts of Nicotiana tabacum.
Biotechnology 11:95–97
Goldschmidt-Clermont M. 1991. Transgenic expression of aminoglycoside adenine transferase in the chloroplast: A
selectable marker of site-directed transformation of chlamydomonas. Nucleic
Acids Res. 19:4083–89
Guda C, Lee SB, Daniell H. 2000. Stable expression of a biodegradable proteinbased polymer in tobacco chloroplasts.
Plant Cell Rep. 19:257–62
Hagemann R. 1992. Plastid genetics in
higher plants. In Cell Organelles, ed. RG
Herrmann, pp. 66–96. Wien, New York:
Springer-Verlag
Hager M, Biehler K, Illerhaus J, Ruf
S, Bock R. 1999. Targeted inactivation
of the smallest plastid genome-encoded
open reading frame reveals a novel and
essential subunit of the cytochrome b6 f
complex. EMBO J. 18:5834–42
Hager M, Mermann M, Biehler K,
Krieger-Liszkay A, Bock R. 2002. Lack of
the small plastid-encoded PsbJ polypeptide results in a defective water-splitting
apparatus of photosysem II, reduced photosystem I levels, and hypersensitivity to
light. J. Biol. Chem. 277:14031–39
Hajdukiewicz PTJ, Allison LA, Maliga P.
1997. The two RNA polymerases encoded
24 Apr 2004
19:37
308
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
by the nuclear and the plastid compartments transcribe distinct groups of genes
in tobacco plastids. EMBO J. 16:4041–48
Hajdukiewicz PTJ, Gilbertson L, Staub
JM. 2001. Multiple pathways for Cre/loxmediated recombination in plastids. Plant
J. 27:161–70
Harris EH, Boynton JE, Gillham NW.
1994. Chloroplast ribosomes and protein
synthesis. Microbiol. Rev. 58:700–54
Hayashi K, Shiina T, Ishii N, Iwai K,
Ishizaki Y, et al. 2003. A role of −35 element in the initiation of transcription at
psbA promoter in tobacco plastids. Plant
Cell Physiol. 44:334–41
Heifetz PB. 2000. Genetic engineering of
the chloroplast. Biochimie 82:655–66
Hibberd JM, Linley PJ, Khan MS, Gray
JC. 1998. Transient expression of green
fluorescent protein in various plastid types
following microprojectile bombardment.
Plant J. 16:627–32
Horvath EM, Peter SO, Joët T, Rumeau
D, Cournac L, et al. 2000. Target inactivation of the plastid ndhB gene in tobacco
results in an enhanced sensitivity of photosynthesis to moderate stomatal closure.
Plant Physiol. 123:1337–50
Hou BK, Zhou YH, Wan LH, Zhang ZL,
Shen GF, et al. 2003. Chloroplast transformation in oilseed rape. Transgenic Res.
12:111–14
Huang CY, Ayliffe MA, Timmis JN. 2003.
Direct measurement of the transfer rate of
chloroplast DNA into the nucleus. Nature
422:72–76
Huang FC, Klaus SMJ, Herz S, Zuo Z,
Koop HU, Golds TJ. 2002. Efficient plastid transformation in tobacco using the
aphA-6 gene and kanamycin selection.
Mol. Gen. Genom. 268:19–27
Iamtham S, Day A. 2000. Removal of
antibiotic resistance genes from transgenic tobacco plastids. Nat. Biotechnol.
18:1172–76
Johnston SA. 1990. Biolistic transformation: microbes to mice. Nature 346:776–
77
67. Johnston SA, Anziano PQ, Shark K, Sanford JC, Butow RA. 1988. Mitochondrial
transformation in yeast by bombardment
with microprojectiles. Science 240:1538–
41
68. Kanevski I, Maliga P. 1994. Relocation of
the plastid rbcL gene to the nucleus yields
functional ribulose-1,5-bisphosphate carboxylase in tobacco chloroplasts. Proc.
Natl. Acad. Sci. USA 91:1969–73
69. Kanevski I, Maliga P, Rhoades DF,
Gutteridge S. 1999. Plastome engineering of ribulose-1,5-bisphosphate carboxylase/oxygenase in tobacco to form a sunflower large subunit and a tobacco small
subunit hybrid. Plant Physiol. 119:133–
41
70. Kavanagh TA, O’Driscoll KM, McCabe
PF, Dix PJ. 1994. Mutations conferring
lincomycin, spectinomycin, and streptomycin resistance in Solanum nigrum
are located in three different chloroplast
genes. Mol. Gen. Genet. 242:675–80
71. Kavanagh TA, Thanh ND, Lao NT, McGrath N, Peter SO, et al. 1999. Homeologous plastid DNA transformation in
tobacco is mediated by multiple recombination events. Genetics 152:1111–22
72. Khan MS, Maliga P. 1999. Fluorescent
antibiotic resistance marker to track plastid transformation in higher plants. Nat.
Biotechnol. 17:910–15
73. Kiang AS, Connolly V, McConnell DJ,
Kavanagh TA. 1994. Paternal inheritance of mitochondria and chloroplasts
in Festuca pratensis-Lolium perenne intergeneric hybrids. Theor. Appl. Genet.
87:681–88
74. Klaus SMJ, Huang FC, Eibl C, Koop HU,
Golds TJ. 2003. Rapid and proven production of transplastomic tobacco plants
by restoration of pigmentation and photosynthesis. Plant J. 35:811–21
75. Klein TM, Wolf ED, Wu R, Sanford JC.
1987. High-velocity microprojectiles for
delivering nucleic acids in living cells. Nature 327:70–73
76. Knoblauch M, Hibberd JM, Gray JC, Van
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
77.
78.
79.
80.
81.
82.
83.
84.
Bel AJE. 1999. A galistan expansion femtosyringe for microinjection of eukaryotic
organelles and prokaryotes. Nat. Biotechnol. 17:906–9
Kobayashi T, Takahara M, Miyagishima
S, Kuroiwa H, Sasaki N, et al. 2002. Detection and localization of chloroplastencoded HU-like protein that organizes chloroplast nucleoids. Plant Cell
14:1579–89
Kofer W, Koop HU, Wanner G, Steinmuller K. 1998. Mutagenesis of the genes
encoding subunits A, C, H, I, J and K
of the plastid NAD(P)H-plastoquinoneoxidoreductase in tobacco by polyethylene glycol-mediated plastome transformation. Mol. Gen. Genet. 258:166–73
Koop HU, Kofer W. 1995. Plastid transformation by polyethylene glycol treatment of protoplasts and regeneration of
transplastomic tobacco plants. In Gene
Transfer to Plants., ed. I Potrykus,
G Spangenberg, pp. 75–82. BerlinHeidelberg-New York: Springer-Verlag
Koop HU, Steinmüller K, Wagner H,
Rössler C, Eibl C, Sacher L. 1996. Integration of foreign sequences into the tobacco plastome via PEG-mediated protoplast tranformation. Planta 199:193–201
Kota M, Daniell H, Varma S, Garczynski SF, Gould F, Moar WJ. 1999. Overexpression of the Bacillus thuringiensis
(Bt) Cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt-resistant insects. Proc. Natl.
Acad. Sci. USA 96:1840–45
Kuroda H, Maliga P. 2001. Complementarity of the 16S rRNA penultimate stem
with sequences downstream of the AUG
destabilizes the plastid mRNAs. Nucleic
Acids Res. 29:970–75
Kuroda H, Maliga P. 2001. Sequences
downstream of the translation initiation codon are important determinants
of translation efficiency in chloroplasts.
Plant Physiol. 125:430–36
Kuroda H, Maliga P. 2002. Overexpression of the clpP 50 -UTR in a
85.
86.
87.
88.
89.
90.
91.
92.
93.
94.
P1: GDL
309
chimeric context causes a mutant phenotype suggesting competition for a clpPspecific RNA maturation factor in tobacco
chloroplasts. Plant Physiol. 129:1600–
606
Kuroda H, Maliga P. 2003. The plastid
clpP1 gene is essential for plant development. Nature 425:86–89
Kuroiwa T. 1991. The replication, differentiation, and inheritance of plastids with
emphasis on the concept of organelle nuclei. Int. Rev. Cytol. 128:1–62
Kusnadi A, Nikolov Z, Howard J. 1997.
Production of recombinant proteins in
transgenic plants: practical considerations. Biotechnol. Bioeng. 56:473–84
Lee SB, Kwon HB, Kwon SJ, Park SC,
Jeong MJ, et al. 2003. Accumulation of
trehalose within transgenic chloroplasts
confers draught tolerance. Mol. Breed.
11:1–13
Leister D. 2003. Chloroplast research in
the genomic age. Trends Genet. 19:47–56
Liere K, Maliga P. 2001. Plastid RNA
polymerases in higher plants. In Regulation of Photosynthesis, ed. B Anderson,
EM Aro, pp. 29–49. Dordrecht: Kluwer
Acad.
Lössl A, Eibl C, Harloff HJ, Jung
C, Koop HU. 2003. Polyester synthesis in transplastomic tobacco (Nicotiana tabacum L.): significant contents of
polyhydroxybutyrate are associated with
growth reduction. Plant Cell Rep. 21:891–
99
Lutz KA, Knapp JE, Maliga P. 2001. Expression of bar in the plastid genome confers herbicide resistance. Plant Physiol.
125:1585–90
Ma JK. 2000. Genes, greens, and vaccines. Nat. Biotechnol. 18:1141–42
Madoka Y, Tomizawa KI, Mizoi J,
Nishida I, Nagano Y, Sasaki Y. 2002.
Chloroplast transformation with modified
accD operon increases acetyl-Co-A carboxylaase and causes extension of leaf
longevity and increase in seed yield in tobacco. Plant Cell Physiol. 43:1518–25
24 Apr 2004
19:37
310
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
95. Mäenpää P, Gonzalez EB, Khan MS, Gray
JC, Aro E-M. 2000. The ycf9 (orf62) gene
in the plant chloroplast genome encodes a
hydrophobic protein of stromal thylakoid
membranes. J. Exp. Bot. 51:375–82
96. Maliga P. 1993. Towards plastid transformation in higher plants. Trends Biotech.
11:101–7
97. Maliga P. 1995. Biolistic transformation
of tobacco cells with nuclear drug resistance genes. In Methods in Plant Molecular Biology—A Laboratory Course
Manual, ed. P Maliga, DF Klessig, AR
Cashmore, W Gruissem, J Varner, pp. 37–
54. Plainview: Cold Spring Harbor Lab.
98. Maliga P. 2002. Engineering the plastid genome of higher plants. Curr. Opin.
Plant Biol. 5:164–72
99. Maliga P. 2003. Transformation in plastids. In Encyclopedia of Applied Plant
Sciences, ed. B Thomas, D Murphy, D
Murray, pp. 392–402. London: Academic.
100. Maliga P. 2003. Progress towards commercialization of plastid transformation
technology. Trends Biotech. 21:20–28
101. Maliga P, Nixon P. 1998. Judging the
homoplastomic state of plastid transformants. Trends Plant Sci. 3:4–6
102. Martin W, Rujan T, Richly E, Hansen A,
Cornelsen S, et al. 2002. Evolutionary
analysis of Arabidopsis, cyanobacterial,
and chloroplast genomes reveals plastid
phylogeny and thousands of cyanobacterial genes in the nucleus. Proc. Natl. Acad.
Sci. USA 99:12246–51
103. McBride KE, Svab Z, Schaaf DJ, Hogan
PS, Stalker DM, Maliga P. 1995. Amplification of a chimeric Bacillus gene
in chloroplasts leads to an extraordinary
level of an insecticidal protein in tobacco.
Biotechnology 13:362–65
104. Medgyesy P, Pay A, Marton L. 1986.
Transmission of paternal chloroplasts in
Nicotiana. Mol. Gen. Genet. 204:195–98
105. Mogensen HL. 1996. The hows and whys
of cytoplasmic inheritance in seed plants.
Am. J. Bot. 83:383–404
106. Moll B, Polsby L, Maliga P. 1990. Strepto-
107.
108.
109.
110.
111.
112.
113.
114.
115.
116.
mycin and lincomycin resistances are selective plastid markers in cultured Nicotiana cells. Mol. Gen. Genet. 221:245–50
Monde RA, Greene JC, Stern DB. 2000.
Disruption of the petB-petD intergenic region in tobacco chloroplasts affects petD
RNA accumulation and translation. Mol.
Gen. Genet. 263:610–18
Monde RA, Greene JC, Stern DB. 2000.
The sequence and secondary structure of
the 30 -UTR affect 30 -end maturation, RNA
accumulation, and translation in tobacco
chloroplasts. Plant Mol. Biol. 44:529–42
Mühlbauer SK, Lössl A, Tzekova L, Zou
Z, Koop HU. 2003. Functional analysis of
plastid DNA replication origins in tobacco
by targeted inactivation. Plant J. 32:175–
84
Nakazano M, Hirai A. 1993. Identification of the entire set of transferred chloroplast DNA sequences in the mitochondrial
genome of rice. Mol. Gen. Genet. 236:
341–46
O’Neill C, Horvath GV, Horvath E, Dix
PJ, Medgyesy P. 1993. Chloroplast transformation in plants: polyethylene glycol
(PEG) treatment of protoplasts is an alternative to biolistic delivery systems. Plant
J. 3:729–38
Palmer JD. 1985. Comparative organization of chloroplast genomes. Annu. Rev.
Genet. 19:325–54
Poethig S. 1989. Genetic mosaics and cell
lineage analysis in plants. Trends Genet.
5:273–77
Reboud X, Zeyl C. 1994. Organelle inheritance in plants. Heredity 72:132–40
Reddy VS, Leelavathi S, Selvapandiyan
A, Raman R, Giovanni F, et al. 2002.
Analysis of chloroplast transformed tobacco plants with cry1Ia5 under rice psbA
transcriptional elements reveal high level
expression of Bt toxin without imposing
yield penalty and stable inheritance of
transplastome. Mol. Breed. 9:259–69
Reed ML, Lyi SM, Hanson MR. 2001.
Edited transcripts compete with unedited
mRNAs for trans-acting editing factors in
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
117.
118.
119.
120.
121.
122.
123.
124.
125.
126.
higher plant chloroplasts. Gene 272:165–
71
Reed ML, Wilson SK, Sutton CA, Hanson MR. 2001. High-level expression of
a synthetic red-shifted GFP coding region
incorporated into the chloroplasts. Plant
J. 27:257–65
Rice Chromosome 10 Sequencing Consortium. 2003. In-depth view of structure,
activity, and evolution of rice chromosome 10. Science 300:1566–69
Riegel MA, Lamond M, Preston C,
Powles SB, Roush RT. 2002. Pollenmediated movement of herbicide resistance between commercial canola fields.
Science 296:2386–88
Ruf S, Biehler K, Bock R. 2000. A small
chloroplast-encoded protein as a novel architectural component of the light-harvesting antenna. J. Cell Biol. 149:369–77
Ruf S, Hermann M, Berger IJ, Carrer H,
Bock R. 2001. Stable genetic transformation of tomato plastids: foreign protein expression in fruit. Nat. Biotechnol. 19:870–
75
Ruf S, Kössel H, Bock R. 1997. Targeted inactivation of a tobacco introncontaining open reading frame reveals a
novel chloroplast-encoded photosystem Irelated gene. J. Cell Biol. 139:95–102
Ruiz ON, Hussein HS, Terry N, Daniell
H. 2003. Phytoremediation of organomercurial compounds via chloroplast genetic
engineering. Plant Physiol. 132:1344–52
Sato N, Albrieux C, Joyard J, Douce R,
Kuroiwa T. 1993. Detection and characterization of a plastid DNA-binding protein which may anchor plastid nuceloids.
EMBO J. 12:555–61
Sato N, Ohta N. 2001. DNA binding specificity and dimerization of the DNA binding domain of the PEND protein in the
chloroplast envelope membraine. Nucleic
Acids Res. 29:2244–50
Serino G, Maliga P. 1997. A negative selection scheme based on the expression
of cytosine deaminase in plastids. Plant J.
12:697–701
P1: GDL
311
127. Serino G, Maliga P. 1998. RNA polymerase subunits encoded by the plastid
rpo genes are not shared with the nucleusencoded plastid enzyme. Plant Physiol.
117:1165–70
128. Shaw KJ, Rather PN, Hare RS, Miller GH.
1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside modifying enzymes. Microbiol. Rev. 57:138–
63
129. Shiina T, Allison L, Maliga P. 1998. rbcL
transcript levels in tobacco plastids are
independent of light: reduced dark transcription rate is compensated by increased
mRNA stability. Plant Cell 10:1713–22
130. Shiina T, Hayashi K, Ishii N, Morikawa K,
Toyoshima Y. 2000. Chloroplast tubules
visualized in transplastomic plants expressing green fluorescent protein. Plant
Cell Physiol. 41:367–71
131. Shikanai T, Endo T, Hashimoto T, Yamada
Y, Asada K, Yokota A. 1998. Directed disruption of the tobacco ndhB gene impairs
cyclic electron flow around photosystem
I. Proc. Natl. Acad. Sci. USA 95:9705–9
132. Shikanai T, Shimizu K, Ueda K,
Nishimura Y, Kuroiwa T, Hashimoto T.
2001. The chloroplast clpP gene, encoding a proteolytic subunit of ATPdependent protease, is indispensable for
chloroplast development in tobacco. Plant
Cell Physiol. 42:264–73
133. Sidorov VA, Kasten D, Pang SZ, Hajdukiewicz PTJ, Staub JM, Nehra NS.
1999. Stable chloroplast transformation in
potato: use of green fluorescent protein as
a plastid marker. Plant J. 19:209–16
134. Sikdar SR, Serino G, Chaudhuri S, Maliga
P. 1998. Plastid transformation in Arabidopsis thaliana. Plant Cell Rep. 18:20–
24
135. Skarjinskaia M, Svab Z, Maliga P. 2003.
Plastid transformation in Lesquerella
fendleri, an oilseed Brassicacea. Transgenic Res. 12:115–22
136. Sporlein B, Streubel M, Dahlfeld G, Westhoff P, Koop HU. 1991. PEG-mediated
24 Apr 2004
19:37
312
137.
138.
139.
140.
141.
142.
143.
144.
145.
146.
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
P1: GDL
MALIGA
plastid transformation: a new system for
transient gene expression assays in chloroplasts. Theor. Appl. Genet. 82:717–
22
Sriraman P, Silhavy D, Maliga P. 1998.
The phage-type PclpP-53 plastid promoter comprises sequences downstream
of the transcription initiation site. Nucleic
Acids Res. 26:4874–79
Staub JM. 2002. Expression of recombinant proteins via the plastid genome.
In Handbook of Industrial Cell Culture:
Mammalian, Microbial and Plant Cells,
ed. SR Parekh, VA Vinci, pp. 261–80.
Totowa, NJ: Humana
Staub JM, Garcia B, Graves J, Hajdukiewicz PTJ, Hunter P, et al. 2000.
High-yield production of a human therapeutic protein in tobacco chloroplasts.
Nat. Biotechnol. 18:333–38
Staub JM, Maliga P. 1992. Long regions
of homologous DNA are incorporated into
the tobacco plastid genome by transformation. Plant Cell 4:39–45
Staub JM, Maliga P. 1993. Accumulation
of D1 polypeptide in tobacco plastids is
regulated via the untranslated region of
the psbA mRNA. EMBO J. 12:601–6
Staub JM, Maliga P. 1994. Extrachromosomal elements in tobacco plastids. Proc.
Natl. Acad. Sci. USA 91:7468–72
Staub JM, Maliga P. 1994. Translation of
psbA mRNA is regulated by light via the
50 -untranslated region in tobacco plastids.
Plant J. 6:547–53
Staub JM, Maliga P. 1995. Marker rescue from the Nicotiana tabacum plastid
genome using a plastid Escherichia coli
shuttle vector. Mol. Gen. Genet. 249:37–
42
Stegemann S, Hartmann S, Ruf S, Bock R.
2003. High-frequency gene transfer from
the chloroplast genome to the nucleus.
Proc. Natl. Acad. Sci. USA 100:8828–33
Stoger E, Sack M, Fischer R, Christou P. 2002. Plantibodies: applications,
advantages and bottlenecks. Curr. Opin.
Biotechnol. 13:161–66
147. Sugita M, Svab Z, Maliga P, Sugiura
M. 1997. Targeted deletion of sprA from
the tobacco plastid genome indicates that
the encoded small RNA is not essential
for pre-16S rRNA maturation in plastids.
Mol. Gen. Genet. 257:23–27
148. Sugiura M. 1992. The chloroplast
genome. Plant Mol. Biol. 19:149–68
149. Suzuki JY, Maliga P. 2000. Engineering of
the rpl23 gene cluster to replace the plastid
RNA polymerase alpha subunit with the
Escherichia coli homologue. Curr. Genet.
38:218–25
150. Suzuki JY, Sriraman P, Svab Z, Maliga
P. 2003. Unique architecture of the plastid ribosomal RNA operon promoter recognized by the multisubunit RNA polymerase (PEP) in tobacco and other higher
plants. Plant Cell 15:195–205
151. Svab Z, Hajdukiewicz P, Maliga P.
1990. Stable transformation of plastids in
higher plants. Proc. Natl. Acad. Sci. USA
87:8526–30
152. Svab Z, Harper EC, Jones JD, Maliga
P. 1990. Aminoglycoside-300 -adenyltransferase confers resistance to spectinomycin
and streptomycin in Nicotiana tabacum.
Plant Mol. Biol. 14:197–205
153. Svab Z, Maliga P. 1993. High-frequency
plastid transformation in tobacco by selection for a chimeric aadA gene. Proc.
Natl. Acad. Sci. USA 90:913–17
154. Swiatek M, Greiner S, Kepm S, Drescher
A, Koop HU, et al. 2003. PCR analysis
of pulsed-field gel electrophoresis-purifed
plastid DNA, a sensitive tool to judge the
hetero-/homoplastomic status of plastid
transformants. Curr. Genet. 43:45–53
155. Swiatek M, Kuras R, Sokolenko A, Higgs
D, Olive J, et al. 2001. The chloroplast
gene ycf9 encodes a photosystem II (PSII)
core subunit, PsbZ, that participates in
PSII supramolecular architecture. Plant
Cell 13:1347–67
156. Swiatek M, Regel RE, Meurer J, Wanner G, Pakrasi H, et al. 2003. Effects of
selective inactivation of individual genes
for low-molecular-mass subunits on the
24 Apr 2004
19:37
AR
AR213-PP55-12.tex
AR213-PP55-12.sgm
LaTeX2e(2002/01/18)
PLASTID TRANSFORMATION
157.
158.
159.
160.
161.
162.
163.
164.
assembly of photosystem II, as revealed
by chloroplast transformation: the psbEFLJ operon in Nicotiana tabacum. Mol.
Gen. Genom. 268:699–710
Thomas MR, Rose RJ. 1983. Plastid number and plastid ultrastructural changes
associated with tobacco mesophyll protoplast culture and plant regeneration.
Planta 158:329–38
Thum KE, Kim M, Morishige DT, Eibl
C, Koop HU, Mullet JE. 2001. Analysis of the barley chloroplast psbD
light-responsive promoter elements in
transplastomic tobacco. Plant Mol. Biol.
47:353–66
Tregoning J, Nixon P, Kuroda H, Svab Z,
Clare S, et al. 2003. Expression of tetanus
toxin fragment C in tobacco chloroplasts.
Nucleic Acids Res. 31:1174–79
Wakasugi T, Tsudzuki T, Sugiura M.
2001. The genomics of land plant chloroplasts: gene content and alteration of genomic information by RNA editing. Photosynth. Res. 70:107–18
Wang T, Li Y, Shi Y, Reboud X, Darmency H, Gressel J. 2003. September 25.
Low frequency transmission of a plastidencoded trait in Setaria italica. Theor.
Appl. Genet. doi: 10.1007/s00122-0031424-8
Whitney SM, Andrews TJ. 2001. The
gene for the ribulose-1,5-bisphosphate
carboxylase/oxygenase (Rubisco) small
subunit relocated to the plastid genome
of tobacco directs the synthesis of small
subunits that assemble into Rubisco. Plant
Cell 13:193–205
Whitney SM, Andrews TJ. 2001.
Plastome-encoded bacterial ribulose1,5-bisphosphate carboxylase/oxygenase
(RubisCO) supports photosynthesis and
growth of tobacco. Proc. Natl. Acad. Sci.
USA 98:14738–43
Whitney SM, Andrews TJ. 2003. Photosynthesis and growth of tobacco with
substitutited bacterial rubisco mirror the
properties of the introduced enzyme.
Plant Physiol. 133:287–94
P1: GDL
313
165. Whitney SM, Baldet P, Hudson GS, Andrews TJ. 2001. Form I Rubiscos from
non-green algae are expressed abundantly
but not assembled in tobacco chloroplasts.
Plant J. 26:535–47
166. Xie G, Allison LA. 2002. Sequences upstream of the YRTA core region are essential for transcription of the tobacco atpB
NEP promoter in chloroplasts in vivo.
Curr. Genet. 41:176–82
167. Ye GN, Colburn S, Xu CW, Hajdukiewicz
PTJ, Staub JM. 2003. Persistance of unselected transgenic DNA during a plastid
transformation and segregation approach
to herbicide resistance. Plant Physiol.
133:402–10
168. Ye GN, Daniell H, Sanford JC. 1990. Optimization of delivery of foreign DNA into
higher-plant chloroplasts. Plant Mol. Biol.
15:809–19
169. Ye GN, Hajdukiewicz PTJ, Broyles D,
Rodriquez D, Xu CW, et al. 2001. Plastidexpressed
5-enolpyruvylshikimate-3phosphate synthase genes provide high
level glyphosate tolerance in tobacco.
Plant J. 25:261–70
170. Zhang XH, Brotherton JE, Widholm JM,
Portis AR. 2001. Targeting a nuclear anthranilate synthase alpha-subunit gene to
the tobacco plastid genome results in enhanced tryptophan biosynthesis. Return
of a gene to its pre-endosymbiotic origin.
Plant Physiol. 127:131–41
171. Zhang XH, Ewy RG, Widholm JM, Portis AR. 2002. Complementation of the
nuclear antisense rbcS-induced photosynthesis deficiency by introducing an rbcS
gene into the tobacco plastid genome.
Plant Cell Physiol 43:1302–13
172. Zou Z, Eibl C, Koop HU. 2003. The stemloop structure of the tobacco psbA 50 UTR
is an important determinant of mRNA stability and translation efficiency. Mol. Gen.
Genom. 269:340–49
173. Zoubenko OV, Allison LA, Svab Z, Maliga P. 1994. Efficient targeting of foreign
genes into the tobacco plastid genome.
Nucleic Acids Res. 22:3819–24
Maliga.qxd
4/24/2004
8:32 PM
Page 1
PLASTID TRANSFORMATION
See legend on next page
C-1
Maliga.qxd
C-2
4/24/2004
8:32 PM
Page 2
MALIGA
Figure 1 Sorting ptDNA at the organelle and cellular levels yields homoplastomic
plants. (A) Chloroplast (CHL) to proplastid (PP) dedifferentiation accelerates ptDNA
sorting. Transformed and nontransformed nucleoids (N) in plastids are symbolized
with red and blue circles, respectively. Transformed and nontransformed ptDNA in
enlarged nucleoids are red and blue circles, respectively. ptDNA are anchored to
membranes by proteins (black dots). Nucleoid 1 is heteroplastomic and is the progenitor of homoplastomic transgenic (1a) and wild-type (1b) proplastids. Homoplastomic proplastid differentiates into chloroplast (bottom). Wild-type proplastids (2, 1b)
are antibiotic sensitive and divide more slowly. (B) Reduction in plastid number during chloroplasts (∼100 per leaf cell; green, elongated ) to proplastid (∼10–14 per
meristematic cell; greenish, oval) transition and lack of exact duplication of the cytoplasm accelerates formation of homoplastomic cells. On top is a leaf mesophyll cell
with one transformed chloroplast (red) and nucleus (Nu). Meristematic cell 1 is heteroplastomic. Cleavage of the cytoplasm yields one meristematic cell with transformed chloroplasts only (1a) and one with wild-type plastids (1b). Cell 1a is the
progenitor of homoplastomic mesophyll cells (1c). Reproduced with permission
(99).
Maliga.qxd
4/24/2004
8:32 PM
Page 3
PLASTID TRANSFORMATION
C-3
Figure 2 Selection of transplastomic clones by spectinomycin resistance. (A) Spectinomycin inhibits callus formation, greening, and shoot regeneration from tobacco
leaf segments on shoot regeneration medium. Transplastomic clones are resistant to
spectinomycin and are identified as green shoots or calli (153). (B) The shoots are
chimeric, visualized by accumulation of green fluorescent protein in transplastomic
sectors. Spectinomycin resistance is not cell autonomous as sensitive sectors are also
green. (72) (C) Spontaneous spectinomycin resistant mutants are sensitive (top),
transplastomic clones are resistant to streptomycin (bottom) when cultured on a
selective streptomycin (500 mg/L) medium (153).