Matter – Properties and Changes 1 Intensive properties
advertisement
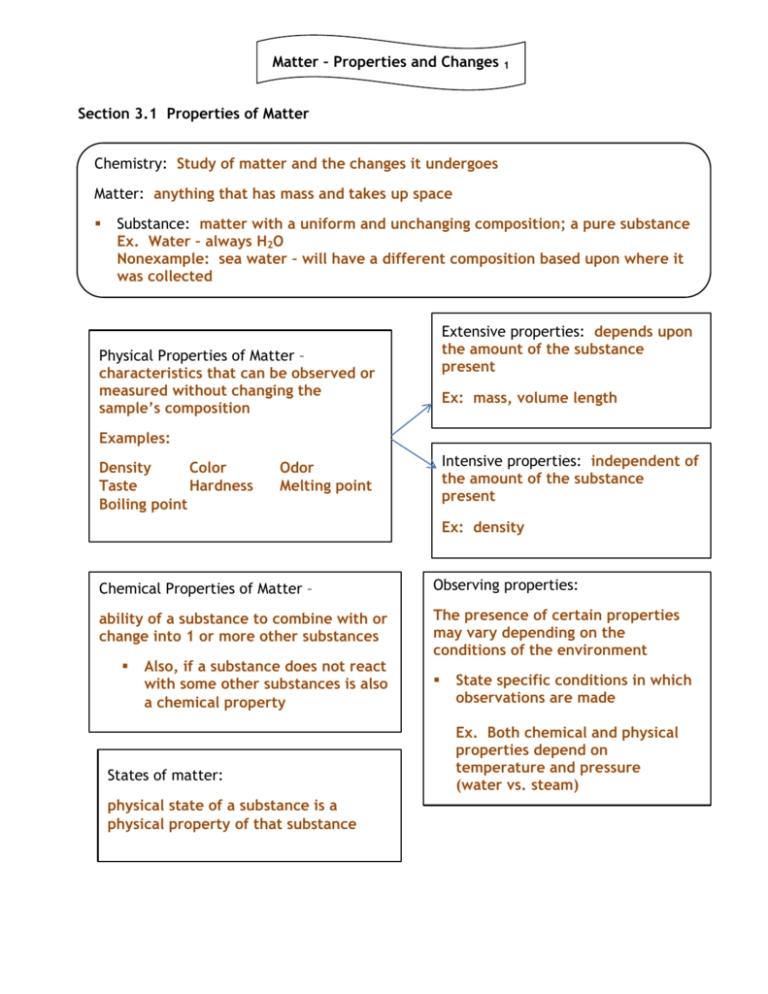
Matter – Properties and Changes 1 Section 3.1 Properties of Matter Chemistry: Study of matter and the changes it undergoes Matter: anything that has mass and takes up space Substance: matter with a uniform and unchanging composition; a pure substance Ex. Water – always H2O Nonexample: sea water – will have a different composition based upon where it was collected Extensive properties: depends upon the amount of the substance present Physical Properties of Matter – characteristics that can be observed or measured without changing the sample’s composition Ex: mass, volume length Examples: Density Color Taste Hardness Boiling point Intensive properties: independent of the amount of the substance present Odor Melting point Ex: density Chemical Properties of Matter – Observing properties: ability of a substance to combine with or change into 1 or more other substances The presence of certain properties may vary depending on the conditions of the environment Also, if a substance does not react with some other substances is also a chemical property States of matter: physical state of a substance is a physical property of that substance State specific conditions in which observations are made Ex. Both chemical and physical properties depend on temperature and pressure (water vs. steam) Matter – Properties and Changes The fact that a substance can change form is an important concept in chemistry and is included in a list of its physical properties 2 States of Matter Solid Liquid Definite shape and volume Particles are tightly packed When heated, particles expand slightly Indefinite shape, definite volume Indefinite shape, indefinite volume Takes shape of container Conforms to the shape of the container Particles are less tightly packed than solid; they are able to slide past each other (flow) May not conform to the shape of a container Incompressible Gas Expands when heated Virtually incompressible Fills the entire volume of the container Particles are far apart Easily compressed gas Gas Gas: a substance that is naturally in the gaseous state at room temperature freezing solid melting liquid Temperature goes up = energy absorbed Temperature goes down = energy released Vapor Vapor: gaseous state of a substance that is a solid or liquid at room temperature Matter – Properties and Changes 3 Section 3.2 Changes in Matter Physical changes: Chemical changes: Any change to a substance without a change in its composition o Drawing copper into a wire Crumpling up aluminum foil Cutting paper Breaking a crystal State of matter depends on: temperature, pressure of surroundings As temperature and pressure changes, most substances undergo a phase change Phase change physical change The temperature and pressure at which a substance undergoes a phase change is a physical property of that substance, just like its melting point or boiling point. Ability of a substance to combine with or change into 1 or more substances AKA: chemical reaction – o new substance formed in the reaction that has different composition and properties from the original o Crushing grapes physical change Fermenting grape juice and sugars into wine chemical change o Iron rusting (forms iron oxide) chemical change A+BC reactants (A + B) yield product (C) Verbs for chemical change: Verbs for physical change: boil, freeze, condense, vaporize, melt explode, rust, oxidize, corrode, tarnish, ferment, burn, rot Late 18th century scientists used quantitative tools to monitor chemical change. Color change generally means a Conservation of mass Measure mass carefully before and after the chemical reaction: New tools: analytical balance for measuring small changes in mass chemical change has occurred total mass remained constant o Law of Conservation of Mass mass is neither created nor destroyed during a chemical reaction; it is conserved Mass reactants = Mass products Matter – Properties and Changes 4 Indicators of a chemical reaction: Sample problem: color change and production of gas In an experiment, 10.00 g of red mercury (II) oxide powder is placed in an open flask and heated until it is converted to liquid mercury and oxygen gas. The liquid mercury has a mass of 9.26 g. What is the mass of oxygen formed in the reaction? When reaction occurs in a closed container, the gas cannot escape. Section 3.3 Mixtures of Matter Matter Mixture: combination of 2+ pure substances in which each substance retains its individual properties Most everyday matter occurs as mixtures sand and water salt and water Heterogeneous mixture (sand/water) Substances (pure) form of matter with a uniform and unchanging composition Ways to separate mixtures: Homogeneous mixture (salt/water) Constant composition throughout Does not blend smoothly throughout Mixture is not uniform 2+ substances remain distinct AKA: solution Can be solid, liquid, gas Examples: solid – steel liquid – vinegar (acetic acid & water) gas – air we breathe (nitrogen, oxygen, and others) 1. Filtration – porous barrier to separate solid from liquid 2. Distillation – separating substances based on boiling point 3. Crystallization – (rock candy) water evaporates from sugar-water, leaving sugar crystals 4. Chromatography – separate substances by their traveling across material Matter – Properties and Changes 5 Section 3.4 Elements and Compounds Matter Mixtures Pure substances Compounds Elements pure substances that cannot be separated into simpler substances by physical or chemical means. o undergo chemical changes Elements 1-92 are naturally occurring (exceptions: 43, 61 are synthetic) Unique chemical name and symbol o Symbol consists of 1, 2, or 3 letters (first letter is CAPITALIZED, rest are lowercase) a combination of 2+ different elements that are combined chemically o Ex. water, table salt, sugar Chemical formulae are written from chemical symbols Can be broken down into simpler substances by chemical means o separating a compound into its elements often requires external energy (heat, electricity) Properties of a compound are different from those of its elements Periodic Table of Elements Law of Definite Proportions 1869: Russian chemist Dmitri Mendeleev organized all of the elements known at that time into rows and columns based on similarities and their masses. Regardless of amount, a compound is always composed of the same elements in the same ratio. o 1st version of Periodic Table Rows = periods Columns = groups or families “periods” pattern of similar properties repeats from period to period Mendeleev’s table left blank spots for currently unknown elements Made predictions of elements not yet discovered by analyzing similarities among elements and patterns of repetition; predictions were accurate. H2O, NaCl, C12H22O11 Sum of the individual masses of the elements that make up the compound = mass of the compound Percent Mass Ratio of the mass of each element in a compound to the compound as a whole. Law of Multiple Proportions Different compounds may be formed from the same elements combined in different ratios. Matter – Properties and Changes 6 Sample problem: Sample problem 2: A compound is analyzed and found to be 50.0% sulfur and 50.0% oxygen. If the total amount of the sulfur oxide compound is 12.5 g, how many grams of sulfur are there? Two unknown compounds are analyzed. Compound 1 contains 5.63 g of tin and 3.37 g of chlorine, while compound II contains 2.5 g of tin and 2.98 g of chlorine. Are the compounds the same? Matter – Properties and Changes 7 Matter – Properties and Changes 8