double replacement lab teacher answer key correct
advertisement
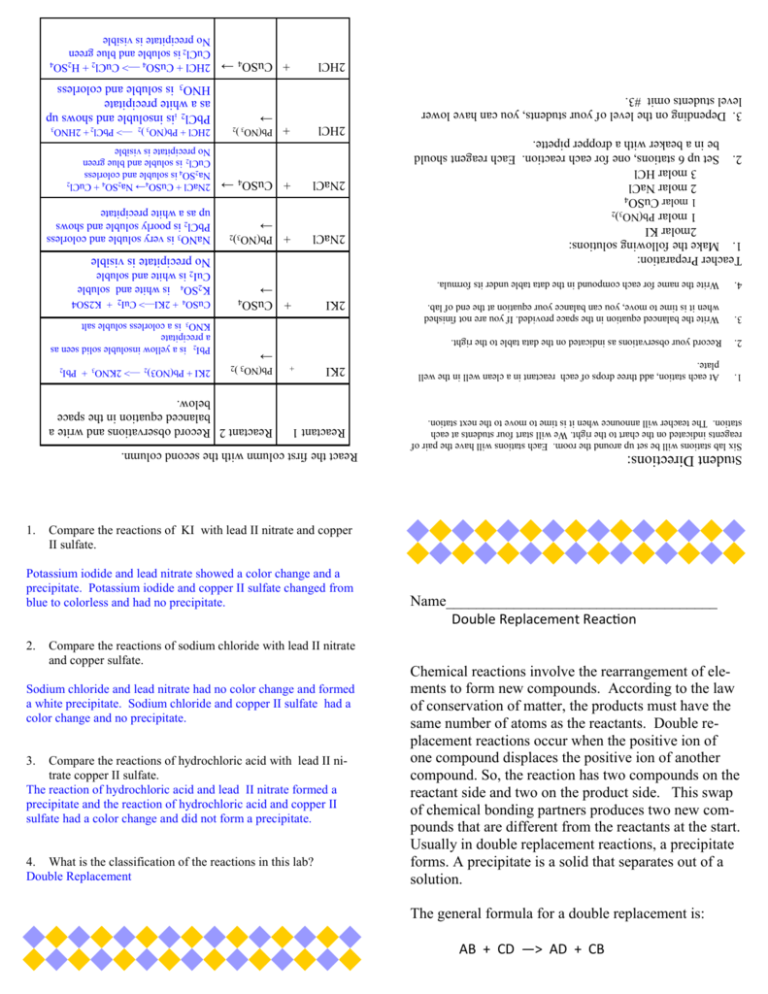
4. What is the classification of the reactions in this lab? Double Replacement Compare the reactions of hydrochloric acid with lead II nitrate copper II sulfate. The reaction of hydrochloric acid and lead II nitrate formed a precipitate and the reaction of hydrochloric acid and copper II sulfate had a color change and did not form a precipitate. 3. Sodium chloride and lead nitrate had no color change and formed a white precipitate. Sodium chloride and copper II sulfate had a color change and no precipitate. Compare the reactions of sodium chloride with lead II nitrate and copper sulfate. AB + CD —> AD + CB The general formula for a double replacement is: Chemical reactions involve the rearrangement of elements to form new compounds. According to the law of conservation of matter, the products must have the same number of atoms as the reactants. Double replacement reactions occur when the positive ion of one compound displaces the positive ion of another compound. So, the reaction has two compounds on the reactant side and two on the product side. This swap of chemical bonding partners produces two new compounds that are different from the reactants at the start. Usually in double replacement reactions, a precipitate forms. A precipitate is a solid that separates out of a solution. Name____________________________________ Double Replacement Reaction Compare the reactions of KI with lead II nitrate and copper II sulfate. Student Directions: Six lab stations will be set up around the room. Each stations will have the pair of reagents indicated on the chart to the right. We will start four students at each station. The teacher will announce when it is time to move to the next station. Write the name for each compound in the data table under its formula. 4. Write the balanced equation in the space provided. If you are not finished when it is time to move, you can balance your equation at the end of lab. 3. Record your observations as indicated on the data table to the right. 2. At each station, add three drops of each reactant in a clean well in the well plate. 1. Teacher Preparation: 1. Make the following solutions: 2molar KI 1 molar Pb(NO3)2 1 molar CuSO4 2 molar NaCl 3 molar HCl 2. Set up 6 stations, one for each reaction. Each reagent should be in a beaker with a dropper pipette. 2. Potassium iodide and lead nitrate showed a color change and a precipitate. Potassium iodide and copper II sulfate changed from blue to colorless and had no precipitate. 1. React the first column with the second column. Reactant 1 2KI + Reactant 2 Record observations and write a balanced equation in the space below. Pb(NO3 )2 → + Pb(NO3)2 2NaCl + CuSO4 → 2KI 2KI + Pb(NO3)2 —> 2KNO3 + PbI2 PbI2 is a yellow insoluble solid seen as a precipitate KNO3 is a colorless soluble salt CuSO4 + 2KI—> CuI2 + K2SO4 K2SO4 is white and soluble CuI2 is white and soluble No precipitate is visible → NaNO3 is very soluble and colorless PbCl2 is poorly soluble and shows up as a white precipitate 2HCl + Pb(NO3 )2 —> PbCl2 + 2HNO3 + Pb(NO3 )2 → 2HCl 2NaCl + CuSO4→ Na2SO4 + CuCl2 Na2SO4 is soluble and colorless CuCl2 is soluble and blue green No precipitate is visible + CuSO4 → 2NaCl 3. Depending on the level of your students, you can have lower level students omit #3. PbCl2 iis insoluble and shows up as a white precipitate HNO3 is soluble and colorless + CuSO4 → 2HCl + CuSO4 —> CuCl2 + H2SO4 CuCl2 is soluble and blue green No precipitate is visible 2HCl








