Homeostasis / Allostasis
advertisement
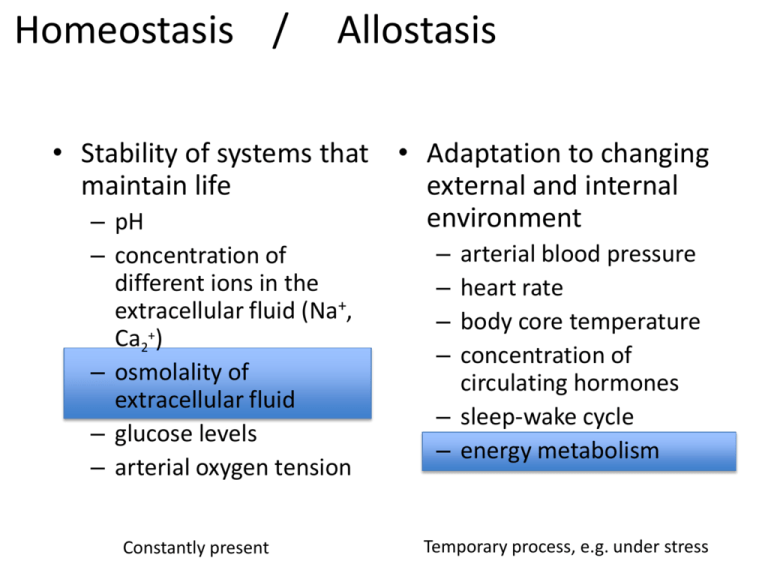
Homeostasis / Allostasis • Stability of systems that • Adaptation to changing maintain life external and internal environment – pH – concentration of different ions in the extracellular fluid (Na+, Ca2+) – osmolality of extracellular fluid – glucose levels – arterial oxygen tension Constantly present arterial blood pressure heart rate body core temperature concentration of circulating hormones – sleep-wake cycle – energy metabolism – – – – Temporary process, e.g. under stress Osmoregulation Bourque, Nature Reviews, 2008, 9: 519-531 Osmoregulation – Changes in ECF-osmolality cause H2O to flow across cell membranes to equilibrate the osmolality of the cytoplasm with that of the ECF • Altering cell volume and ionic strength – Danger for the brain, encased in the skull – Osmoregulators: maintain ECF osmolality constant around a set-point (300 mOsm / Kg) osmoconformers osmoregulators osmoconformers Osmoregulation • Values measured in an individual varies around the set point ± 3% range is normal – Changes in water evaporation or diuresis – Changes in salt excretion or intake Diuresis Natriuresis • Receptors generate a basal signal and encodes the polarity and the magnitude of a change • Receptors found in the brain and gastrointestinal tract – GI-tract: anticipatory osmoregulation • changes before plasma osmolality increase. • Information reach the brain through the vagus and Nucleus tractus solitarius Osmoregulation: hyper-, hypoosmolality • ECF hyperosmolality – – – – – stimulates the sensation of thirst activates the release of vasopressin increases natriuresis inhibits exercise-induced sweating ev. inhibits salt appetite • ECF hypoosmolality – suppress basal vasopressin secretion – Increased diuresis – Inhibiting desire to drink promotes water intake enhances water readsorption decreases Na excretion decreases water loss decreases Na absorption Osmoregulation: brain • The brain possess an intrinsic osmoreceptor – OVLT • Lesion experiments • Direct injection of hypertonic solutions • Electrophysiologic recording • Modulates thirst and vasopressin secretion • FMRI-studies show activation of the anterior region of the 3d ventricle during the onset of hypertonicity Osmoregulation: receptors • Osmoreceptors are specialized neurons with intrinsic ability to transduce osmotic perturbations into changes in the rate or pattern of action potential discharge – Detect differences between ECF-osmolality and a pre-established set-point – Encode this information in electrical signals • OVLT, SFO and supraoptic nucleus • Basal electrical activity encodes the osmotic set-point excited by hypertonic ECF inhibited by hypotonic ECF Osmoreception: cellular mechanism – Osmotic stimuli modulate nonselective stretch activated cation channels – Osmosensory transduction is a mechanical process • Increase in cation conductance caused by hypertonicity leads to decrease in cell volume • Osmosensor display reversible changes in volume that are inversely proportional to ECF-osmolality Osmoreception: cellular mechanism • Stretch inactivated cationic channels transduce osmoreception Osmoregulation: the transducer • C. elegans mutant for osm-9 lack avoidance to strongly hyperosmolar solutions • Member of the transient receptor potential (TRP) vanilloid channels – Knock-out mice for TRP1 and TRP4 Under hyperosmotic conditions: • impairment in vasopressin secretion • reduced drinking • decreased cFos expression in the OVLT • Actin network is required for osmosensory transduction • link to channels • volume dependent strain deformations of the membrane Osmoregulation: CNS integration • Integration with other visceral sensory modalities such as blood volume, blood pressure, ECF Na+ concentration and body temperature in thirst and diuresis – Extracellular thirst: decrease of volume Changes Changes in sodium appetite and natriuresis – Intracellular thirst: increase of osmolite concentration • Different types of osmotic perturbations require different combinations of physiologic responses – Hypovolemia activates the renin-angiotensin system and inhibit natriuretic peptide. Thirst elicited by AII effect on the SFO which controls the secretion of ADH from the posterior pituitary lobe. Decrease of fluid output. – Increased osmolite concentration activates OVLT and SFO and other peripheral osmoreceptors. Integration in higher brain centers. Thirst. Osmoregulation Osmoregulation: thirst • Water ingestion satiates sensation of thirst in seconds, well before ECF osmolality is corrected Anterior cingulate cortex motivate behavioural responses demanded by particular homeostatic mechanisms Insula genesis of speecific homeostatic sensations: pain, hunger and thirst Osmoregulation: salt appetite • Central pathways are not well known Parabrachial nucleus and Nucleus tractus solitarius inhibit salt appetite Osmoregulation: natriuresis • Natriuresis controlled by various hormones (aldosterone, AII, ANP) and the sympathetic nervous system Kidney innvervated polysynaptically from the OVLT, MnPO, PVN, VLM, IML and sympathetic neurons Retrograde tracing with pseurdorabies virus Osmoregulation: work in progress • Molecular structure of osmoreceptor (stretch sensitive cation channel) is unknown • Nature of cytoskeleton involvement remains to be elucidated • Acute versus chronic osmotic perturbation • Neural mechanisms whereby thirst and salt appetite become perceived at a conscious level