Covalent Bonding Worksheet
advertisement

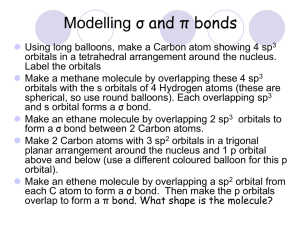
Chem 129 Dr. Baxley Covalent Bonding Worksheet Section 11.1. No Worksheet problems. Do all assigned textbook problems Section 11.2 1. a. Draw the Lewis structure of acetone, (CH3)2CO. What is the hybridization on each carbon atom in the molecule? b. On the Lewis structure, identify the type of each bond (σ or π). c. Describe the orbital location, including type of bond, of each pair of electrons in the molecule including lone pairs (for example, there are six pairs of electrons in six different σ bonds formed between an s orbital on a hydrogen and an sp3 hybrid orbital in a carbon). Section 11.3 2. a. Without looking in your book or notes (just a periodic table), which three period 2 homonuclear diatomic molecules (choose from B2, C2, N2, O2, F2, and Ne2) have 2s-2p mixing and which three do not? b. What does “mixing” mean in this case, why does it occur and what are the consequences? Chem 129 Dr. Baxley c. Draw two MO diagrams (without electrons filled in), one for the molecules with mixing and one for the molecules without mixing. Check your answer using Fig 11.20. Use these MO diagrams to answer the remaining questions. 3. The bond length in C2 is measured by neutron diffraction to be 131 pm (100 pm = 1Å). Using table 9.4 on page 343 (p 341 in 3rd ed.) in your book, does the C2 bond resemble a C−C, a C=C, or a C≡C? 4. Predict the bond order of C2 via MO theory. Does MO theory agree with the experimentally observed bond order from the previous question? Chem 129 Dr. Baxley 5. Calcium and carbon form an ionic compound, CaC2. Draw the Lewis structure of the C2 anion. What is the MO orbital configuration and the bond order of this anion. Do VBT and MO theory agree? 6. Define ionization energy. The ionization energy of O2 is lower than the ionization energy of atomic O. Explain this in terms of the where the electrons are in atomic and MO’s of O2. 7. Do you expect the ionization energy of N2 to be higher or lower than that of atomic N? Why? Chem 129 Dr. Baxley Answers 1. sp3 sp3 sp2 There are six pairs of electrons in six different σ bonds formed between an s orbital on a hydrogen and an sp3 hybrid orbital in a carbon. There are two pairs of electrons in two bonds formed between an sp3 hybrid orbitals on the outside carbons and an sp2 hybrid orbital on the central carbon. There is one pair of electrons in a σ bond between an sp2 hybrid orbital on the central carbon and an sp2 hybrid orbital on the oxygen. There is one pair of electrons in a π bond between the 2p orbital on the central carbon and the 2p orbital of the oxygen. There are two lone pairs of electrons in sp2 hybid orbitals of the oxygen. 2. a. B2, C2, and N2 have mixing. O2, F2 and Ne2 do not. b. Mixing is when two orbitals are close in energy, so the orbitals mix. Mixing occurs between the 2s orbitals 2p orbitals when the atoms are larger (in B, C and N), but do not occur with the atoms are smaller and more compact (in O, F and Ne). The consequence of mixing is that the σ2s and σ2s* MOs are lower in energy and the σ2p and σ2p* MOs are higher in energy. Specifically, the σ2p orbital becomes higher in energy than the π2p orbitals. This means that the π2p MOs will be filled before the σ2p MOs. c. See fig 11.20 in your textbook 3. Table 9.4 lists the C=C bond as 134, so the bond in C2 most resembles a double bond. 4. σ*2s π*2p σ2p π2p σ*2s σ2s Bond Order = ½ (6−2) = 2 Yes, MO theory predicts a bond order of 2, which agrees with the experimental result of bond length. Chem 129 Dr. Baxley 5. Lewis structure :C≡C: MO theory: Use the same MO diagram as above, but fill in 10 e−. The two extra electrons will go into the σ2p MO Bond order = ½ (8−2) = 3 Yes, VBT and MO theory both predict a bond order of 3. 6. Ionization energy is the amount of energy required to remove an electron. Fill in the AO diagram and the MO diagram for O and O2 and compare them: σ*2p π*2p 2p π2p σ2p σ*2s 2s AO of O σ2s MO of O2 IE is the amount of energy required to remove an electron. In O2, the highest energy electron is higher in energy than the highest energy electron in O. Therefore, less energy needs to be supplied in order to removed an electron from O2 (it already has more energy). 7. When you fill the AOs and MOs for N vs N2, you’ll find that N (with 5 v.e.) has its highest energy e− in the 2p orbital. However, N2 (with 10 v.e.) has its highest energy electron in the σ2p orbital, which is lower in energy than the atomic 2p orbital. Therefore, N2 will have a higher I.E. (more energy must be supplied to remove an electron) than N.