Carbohydrates
advertisement
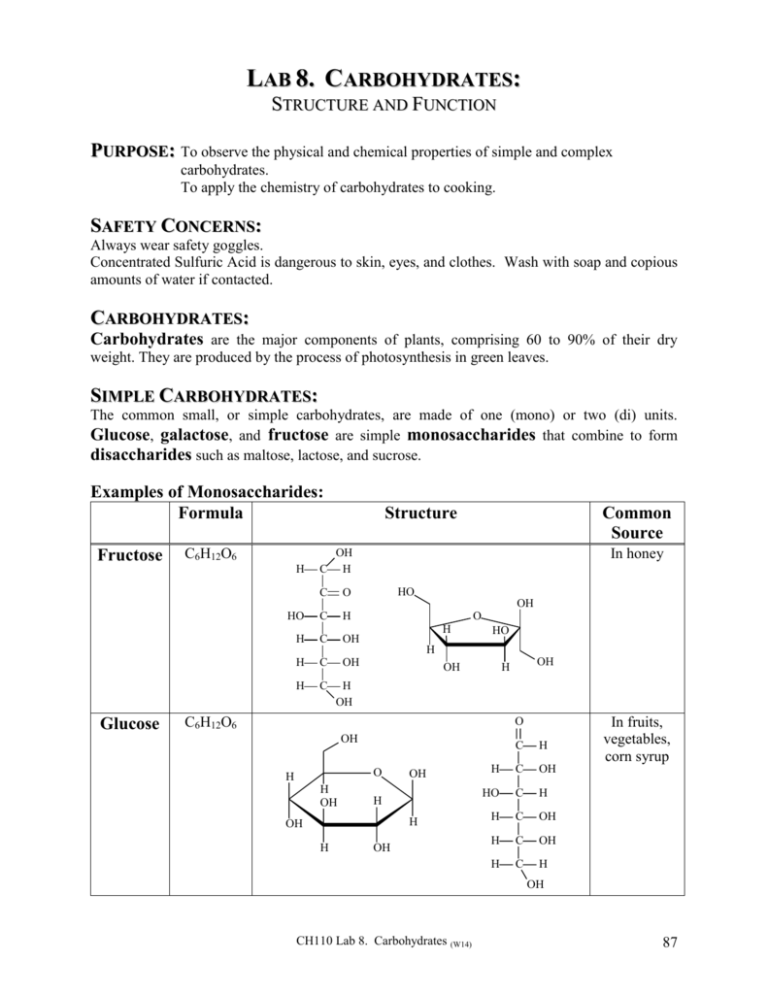
LAB 8. CARBOHYDRATES: STRUCTURE AND FUNCTION PURPOSE: To observe the physical and chemical properties of simple and complex carbohydrates. To apply the chemistry of carbohydrates to cooking. SAFETY CONCERNS: Always wear safety goggles. Concentrated Sulfuric Acid is dangerous to skin, eyes, and clothes. Wash with soap and copious amounts of water if contacted. CARBOHYDRATES: Carbohydrates are the major components of plants, comprising 60 to 90% of their dry weight. They are produced by the process of photosynthesis in green leaves. SIMPLE CARBOHYDRATES: The common small, or simple carbohydrates, are made of one (mono) or two (di) units. Glucose, galactose, and fructose are simple monosaccharides that combine to form disaccharides such as maltose, lactose, and sucrose. Examples of Monosaccharides: Formula Fructose C6H12O6 H Structure Common Source In honey OH C H C O HO C H H C OH H C OH H C HO OH O H HO H Glucose OH OH H H OH C6H12O6 O OH O H H OH OH H H OH H OH C H H C OH HO C H H C OH H C OH H C H In fruits, vegetables, corn syrup OH CH110 Lab 8. Carbohydrates (W14) 87 Examples of Disaccharides: Formula Structure OH C12H22O11 Sucrose Common Source HO H O H H OH H H OH H O H HO O OH OH H OH OH C12H22O11 Lactose In sugar cane and sugar beets In Milk OH O H H OH O OH O H OH H H H H H H OH H OH OH COMPLEX CARBOHYDRATES: The complex carbohydrates amylose, and cellulose are long chain polymers of the simple carbohydrate glucose. Examples of Complex Carbohydrates: (Polysaccharides) Common Source Amylose: Polymer of -D-glucose OH OH O H H H OH O H H H OH H OH H H OH O H H H OH H H O O O Starches: Rice, wheat, potatoes, beans OH O H OH n Cellulose: Polymer of -D-glucose Plant Fibers: Cotton, wood, stems, leaves OH OH OH H H OH O H H H OH O H H OH H H H H H O H O O H OH O H O OH OH n Amylose, and its branched relative, amylopectin, are major components of starches, the energy storage carbohydrates found in tubers and edible roots. Amylose and amylopectin are polymers of D-glucose in which the anomeric carbon of each glucose unit is in the alpha () form. Cellulose is plant structure material like that of wood, stems, and leaves. The major function of cellulose for us is not as a source of glucose for energy, but rather as fiber to keep our digestive tract clean. Cellulose is a polymer of D-glucose in which the anomeric carbon of each glucose unit is in the beta () form. 88 CH110 Lab 8. Carbohydrates (W14) SWEETNESS: Simple carbohydrates are called saccharides from the Latin term saccharum (sweet) because of their sweet taste. Many of the small carbohydrates, mono and disaccharides, are used as sweeteners. They fit into the taste receptor sites on our tongues and send the signal to our brains that we call sweet. Artificial sweeteners mimic the shape of the simple sugars and will fit into the same taste receptor sites that natural sugars do and so send to our brains a similar “sweet” signal. Artificial sweeteners are not metabolized in the same way as natural sugars. Examples of Artificial Sweeteners: Formula Sucralose C12H19O11Cl3 Structure Common Source “Splenda” OH Cl H O Cl H OH H H OH H O H HO O H OH Aspartame H C14H18O5N2 NutraSweet® “Equal” O C OH CH2 O H2N Saccharin CH Cl C CH2 O NH CH C CH3 “Sweet’N Low” O C7H5NSO3 O N H S O O SOLUBILITY: Mono and Disaccharides are soluble in water since they have many exposed OH’s along their surfaces which can easily hydrogen bond with water. Larger carbohydrates can still be soluble depending on the positioning of their OH’s. If the OH’s are abundant on the exterior of a carbohydrate (even a large one) then hydrogen bonding with water can occur and the carbohydrate can be made to dissolve in water. If, however, there is internal hydrogen bonding within a carbohydrate, or coiling that may prevent water from hydrogen bonding to the OH’s, then the carbohydrate will not dissolve in water. Starch granules, tightly coiled strands of amylose and amylopectin, are not soluble in water at ordinary temperatures and so are convenient forms in which to store the plant’s excess energy supplies. Roots and seeds are the organs in which starch is usually concentrated. Forms of amylose starch can be made to be soluble by heating in water so as to disrupt the solid packing of the strands. The coils unravel into long strands of amylose which have OH’s more exposed to hydrogen bond with water. Not only are the uncoiled strands more water soluble but they can also tangle with each other to form gels. We often use these “unraveled” starch strands as thickeners in cooking or for stiffening fabrics. CH110 Lab 8. Carbohydrates (W14) 89 Although there are some forms of soluble fiber (like in oatmeal), most plant cellulose is not water soluble even with heating. Cellulose strands have a lot of internal hydrogen bonding with themselves and each other. They form tight, twisted cables that make very strong structure material that does not unravel even in hot water. CHEMICAL REACTIVITY: Colorization of Iodine: Test for Polysaccharides: Amylose, the unbranched chain polymer of -D-glucose in starch, coils into tight spirals. Elemental iodine, I2, which is normally yellow-brown in color, will fit inside the amylose coil and complex with the OH’s inside the spiral. The resulting amylose-iodine complex is a deep blue-black color. I2 I2 I2 (yellow/brown) I2 I2 I2 Amylose/I2 Complex (blue/black) Amylose Helix Iodine, I2, is commonly used as a test for the presence of amylose starch. Legal U.S. dollar bills made from a linen fabric will not react with iodine; however, the mark of the iodine pen on bills made from starched paper will show the characteristic dark blue sign of a counterfeit. Hydrolysis: Each bond connecting monosaccharide units (the glycoside bond) can be broken by hydrolysis; the reaction with water in the presence of a catalyst. Disaccharides can thus be hydrolyzed into two monosaccharides. Polysaccharides can be hydrolyzed into shorter chains or further into simple sugars. In the laboratory we can catalyze sugar hydrolysis reactions with acid, or we can use catalytic enzymes that are specific for each carbohydrate. Sucrose is hydrolyzed into glucose and fructose by catalysis with the enzyme, sucrase. Maltose hydrolyses into glucose with maltase. Lactose hydrolyses into galactose and glucose with lactase. OH OH O H H OH H HO H H O H HO O OH OH H OH H H+, H2O or Sucrase O H HO OH H OH H H + H OH OH Glucose Sucrose OH O H OH HO H OH H OH Fructose Amylose and amylopectin are easily hydrolyzed into shorter chains of glucose called dextrins which are then further hydrolyzed into the disaccharide maltose and then to glucose (blood sugar) itself. In our bodies the hydrolysis of starches in digestion is catalyzed by the enzymes amylase and maltase. People vary in the amount of amylase in their saliva or urine. Starch (Amylose, Amylopectin) H+, H2O or Amylase Dextrins H+, H2O or Amylase Maltoses H+, H2O or Maltase D-Glucoses The hydrolysis of cellulose can be catalyzed in the laboratory with acid or by the enzyme cellulase found in certain bacteria. The human body does not contain the enzyme cellulase and so cannot convert cellulose into glucose for use as an energy source. Therefore, the long chain of cellulose stays intact for use as fiber. Oxidation: Benedict’s Test for Reducing Sugars: All of the monosaccharides and most of the disaccharides can be oxidized. When their cyclic (furanose or pyranose) hemiacetal structures open an aldehyde group formed, HC=O, is available 90 CH110 Lab 8. Carbohydrates (W14) for oxidation to a carboxylic acid. Benedict’s reagent, a basic solution of Copper (II) Sulfate, (CuSO4 in NaOH) is commonly used for this purpose. The oxidizing agent, CuSO4, is blue in color which is characteristic of many Cu2+ compounds. When the blue Cu2+ of CuSO4(aq) causes the sugar aldehyde to oxidize it becomes reduced itself to Cu1+ in the form of Copper I oxide, Cu2O(s) which is a brick red precipitate. A sugar that reduces blue Cu2+ to brick-red Cu1+ is called a reducing sugar. The color of the precipitate varies from green to gold to red depending on the concentration of the reducing sugar. O OH O H H OH OH H H OH H OH O C H H C OH HO C H H C H C H C C OH H C OH HO C H OH H C OH OH H C OH H H C Cu+2 (blue) OH Cu1+ + (brick red) H OH Glucose Gluconic Acid Tests for reducing sugars are commonly performed in medicine to identify the simple sugars present in blood or urine that may be indicators of metabolic problems or disease. Dehydration: When carbohydrates are either heated or reacted with acid, H’s and OH’s combine and leave as water, HOH. As water molecules begin to escape the carbohydrate begins to turn yellow, then brown, and then eventually black. O O C H H C OH HO C H H C OH H C OH H C H OH C H+1 or Heat O O H H C HO C H C OH H C OH H C H OH C C H H C HO C C H C C H C OH C C H C OH H C H C + H2O's complexes (black) H OH Possible Dehydration Intermediates (yellow to brown) As paper (a cellulose product processed with acid) ages it slowly dehydrates and turns yellow. Scrap-bookers prefer “acid-free” paper that will stay white longer. Caramel candy is sugar that has been heated so as to partially dehydrate. Loss of some of the OH’s from sugars results in a caramel brown color and also gives the characteristic taste of browned carbohydrates. A common method for making sauces and gravies uses the formation of a “roux”. Flour or starch is heated in oil until it browns. Water or milk is then added and the mixture heated to a thick sauce. The word “roux” is French for “red” and at some point in history came to mean flour that had been cooked long enough to change color. The fat and flour undergo browning reactions when cooked giving flavorful dehydrated molecules in much the same way as the caramelization process. If all of the OH’s on a carbohydrate are removed as water then all that remains of the compound is black carbon charcoal. This phenomenon is observed when food or wood is burned to blackness. CH110 Lab 8. Carbohydrates (W14) 91 NOTES: PROCEDURES: 1 There will be a variety of results here as personal tastes vary. ACTIONS: I. SWEETNESS: 1. Taste a small sample of each sugar or sweetener available and rank them in order of sweetness.1 (#1 being the sweetest.) 2. On the report sheet, record the predicted order of sweetness from your textbook. Compare your taste results with the predicted order and report your observations.2 II. SOLUBILITY: Do your tastes match the predicted order or are there discrepancies? 3 Use the same size sample for each item so you can compare solubilities. 4 1. Obtain 3 stoppered test tubes. 3 Into tube #1 put a corn kernel sized scoop of glucose (a monosaccharide). Into tube #2 put a corn kernel sized scoop of sucrose (a disaccharide). Into tube #3 put a corn kernel scoop of corn starch (a polysaccharide). 2. To each tube add 5 mLs water.4 Stopper and shake each tube to mix. Record the solubility of each (S = soluble, I = insoluble, and PS = only partially soluble) on the report sheet. 3. If any of the samples are not completely soluble in cold water, warm their test tubes by letting them stand in hot water or carefully5 warm them over the flame of a laboratory burner. Record the solubility of each.7 4. Save these samples for the Colorization of Iodine test (Part III). III. CHEMICAL REACTIVITY: A. Colorization of Iodine, I2: Test for Starch: 1. Take the 3 samples you used to test for solubility and number them (#1 glucose, #2 sucrose, & #3 starch6) 2. Into another tube numbered #0 put about 5 mLs water. Put it first in your line up of sugar samples to use as the control.7 3. Obtain 2 more clean test tubes. Label one tube #4. Pour ½ of the contents of starch tube #3, into tube #4. This will give 2 starch samples to compare. Into the other tube spit enough saliva to measure about 1 inch. 4. Pour the saliva into starch tube #4. 5. Set tubes (#0 through #4) aside for about 20 minutes. 6. After about 20 minutes: Into sample tubes #0-4 add 1 drop of Iodine, Potassium Iodide (I2, KI) solution.8 Stopper and shake each to mix. Observe and record the color of all samples and note the presence of amylose. 9 92 2 CH110 Lab 8. Carbohydrates (W14) This measurement does not need to be exact. Make the first measurement with a graduated cylinder and then estimate the others by comparing the levels with that of the 1st tube. 5 If heating with a flame, hold the tube with a wire test tube holder high in the flame just enough to warm it. Point the tube away from people. Avoid loss of water from heating. If water is lost add more to maintain 3 mLs of solution. 6 This experiment could be done with starch in any form. Crackers or bread crumbs also work. 7 A control sample should show no reaction. We use it as a reference to which we can compare the others to tell if a reaction has taken place. 8 Iodine, I2, turns dark blue if it complexes with the spiral form of amylose starch. 9 Not all people have the same amount of amylase in their saliva. Check the samples of other class members to see how their amylase reacted. B. Oxidation: 1. Set up a boiling water bath by filling your largest beaker 1/4th full of water and heating it on a hot plate. 2. Obtain 6 clean test tubes with stoppers labeled #1-#6. Put about 2 mLs water into each. 4 3. Leave tube #1 as the control sample. Into tube #2 put a pea sized scoop of fructose. Into tube #3 put a pea sized scoop of glucose. Into tube #4 put a pea sized scoop of sucrose. Into tube #5 put a pea sized scoop of lactose. Into tube #6 put a pea sized scoop of corn starch. Shake each tube to thoroughly mix the solutions. 10 Benedict’s reagent will react with any carbohydrate that can form a free aldehyde group in solution. These “reducing” sugars will reduce the blue Cu2+ ions in the solution to produce red Cu1+ ions instead. 4. Into each tube add 3 mL of Benedict’s reagent.10 Stopper and shake each to mix. 5. Place all 6 tubes into the boiling water bath at the same time with the stoppers on very loosely so they do not pop off during heating. Remove the tubes after 5 minutes and record any color changes. 6. On the report sheet circle the ion responsible for the color of each solution after the reaction is complete. Label each sample as reducing or nonreducing. 11 Stirring constantly keeps the sugar from burning. 12 7. Report any discrepancies in your results. Did each sample give you the results expected? Explain any anomalies. 8. Dispose of solutions in designated waste container. C. Dehydration with Heat; Caramelization: 1. Into your largest beaker put about 10 mLs of solid sucrose. Notice any steam, water vapor, which may come from the reaction. The high heat forces the O’s and H’s to leave the carbohydrate as water. Once the O’s and H’s have left as water it is just the C’s, charcoal, which are left behind. 2. Observe the color, odor, chemical changes, etc. as you heat the sucrose over a hot plate or laboratory burner while stirring constantly. 11 Stop heating when the sugar starts to turn caramel brown.12 Record all observations on your report sheet. 3. Return the beaker of caramel to the hot plate to continue the dehydration just until you observe the sugar starting to turn black. Remove from heat. 4. Clean the beaker by boiling hot tap water in it until the burnt sugar is dissolved enough to be washed out. CH110 Lab 8. Carbohydrates (W14) 93 13 D. Dehydration with Acid: 1. Into a 50 mL beaker pour solid sucrose up to about the 25 mL mark. 2. Move the beaker of sucrose to the fume hood. Pour 10 mLs concentrated Sulfuric Acid13 onto the sucrose and observe the color, odor, and chemical changes. Record all observations. 3. Place a drop14 of concentrated Sulfuric Acid on a piece of paper towel. Record your observations. IV. PRACTICAL APPLICATIONS IN COOKING: A. Preparation of Gravy: Cornstarch method 1. Mix completely 2 level Tablespoons of cornstarch15 with 1.5 teaspoons (7.5 mLs) of water in a small beaker. Play with the results of this Gak and describe its texture on the report sheet. 16 2. In a separate beaker, heat ½ cup (120 mLs) of water17 (or broth). Concentrated Sulfuric Acid is dangerous to your skin, eyes, and clothes. Do not breathe the vapors. Wash with copious amounts of water if contacted. 14 Wiping the con H2SO4 from a dripping container would get the drop of acid you need on the paper towel. 15 Other starches can be used as thickeners. Cornstarch gives a more transparent sauce. Flour gives a cloudy sauce because it contains insoluble suspended proteins. 16 18 3. With constant stirring, pour the corn starch gak into the hot water and bring to a boil for 1 minute or until smooth and bubbly. 19 4. Describe your results on the report sheet. B. Preparation of a Cheese Sauce: Roux method 1. Melt 2 level Tablespoons of shortening or margarine20 in a beaker over low heat stirring constantly. 2. Blend in 2 level Tablespoons of flour15 (and seasonings like salt, pepper, or dry mustard if making a real cheese sauce) and cook over low heat, stirring until mixture is smooth and bubbly. The flour will brown as the amylose dehydrates. 3. Remove from heat. Stir in ½ cup (120 mLs) water (milk for a real cheese sauce) then stirring constantly, bring to a boil for 1 minute or until smooth and bubbly. (If really making a cheese sauce; blend in ½ cup cheese, cut up or grated. Stir until cheese is melted.) 4. Describe your results on the report sheet. Compare this sauce made with four to that made with cornstarch. More water or starch may be added to reach the desired consistency. The gak should be cutable like a solid yet pourable like a liquid. If you pour the gak into your hand and squeeze it should crumble. 17 The liquid could be broth that would become gravy. Adding gak to a pot of boiled vegetables and meat will create a thick soup or stew. 18 If you add dry flour or starch to hot water Hbonding will occur on the surface of the starch granules and your gravy will be lumpy. 19 You can vary the proportion of starch to liquid to make your gravy thinner or thicker. 20 Any fat can be used. Gravies can be made by adding flour to the fat left behind from cooked meats like hamburger, bacon, or sausage. 94 CH110 Lab 8. Carbohydrates (W14) LAB 8. CARBOHYDRATES: PRE LAB EXERCISES: NAME_____________ DATE______________ More than one answer may be required. Give all correct answers. _____1. Which of the following are not carbohydrates? A. fructose C. aspartame E. saccharin B. glucose D. cellulose F. sucrose _____2. Amylose differs from amylopectin in that _____ A. Amylose is digestible by humans but amylopectin is not. B. Amylose is branched but amylopectin is a straight chain. C. Amylopectin is branched but amylose is a straight chain. D. Amylose is a polymer of -D-glucose but amylopectin is a polymer of -Dglucose. _____3. The major component of paper is _____ A. amylose B. cellulose C. glucose D. cellulite _____4. Cellulose is essential in human nutrition because _____ A. it is undigestible so remains as long strands that act as fiber. B. it can be hydrolysed to form glucose which will be used as blood sugar. C. it is undigestible and so acts as filler in food products so we can avoid calories. D. it can be digested to form glucose which will be used as blood sugar. _____5. Carbohydrates that are called “reducing sugars” are those that ____ A. have OH groups that are easily oxidized. B. form oxidizable aldehydes in the test solution. C. are reduced in the presence of an oxidizing agent. D. are only aldose monosaccarides. _____6. What is most important in making a carbohydrate water soluble? A. The ratio of O’s relative to C’s in the structure. B. The ratio of OH’s relative to C’s in the structure. C. The ratio of OH’s available to hydrogen bond with water relative to C’s in the structure. D. Monosaccharides are water soluble but the others are not. CH110 Lab 8. Carbohydrates (W14) 95 96 CH110 Lab 8. Carbohydrates (W14) LAB 8. CARBOHYDRATES: NAME___________________ PARTNER_________DATE___ REPORT: I. SWEETNESS: Rank in order of sweetness from 1-6 (#1 being the most sweet) Fructose Glucose Sucrose Aspartame Splenda Saccharin Order of Sweetness (Your Taste) Order of Sweetness (From Text) Compare your taste w/ text: II. SOLUBILITY: 1. Glucose 2. Sucrose 3. Starch (Amylose) Solubility (cold) Solubility (hot) 4. On the given structure of glucose, show how water molecules hydrogen bond to all possible locations: H O H O O H H O H O H H H O H H H H O H O H H O H H O H III. CHEMICAL REACTIVITY: A. Colorization of Iodine, I2: 0. Control Observation Color 1. Glucose Color 2. Sucrose Color 3. Starch (Amylose + Amylase) Color Amylose? Yes or No Yes or No (circle one) (circle one) (> 20 min w/ I2) Conclusion/Explanation/Analysis: Why did these results occur? ___ 1. 4. Starch + Saliva (Amylose) Color Amylose? Explain any Anomalies. Why does is there no blue color with Iodine when amylose reacts with saliva? A. Amylase in saliva causes the amylose chain to uncoil so Iodine is not trapped. B. Amylase in saliva reacts with iodine causing it to be unable to complex with the amylose coil. C. Amylase in saliva causes the amylose chain to break into short chains and then into glucose which does not coil and so does not trap iodine. D. Amylase in saliva will replace the I2 in the amylose coil and so remove the color. CH110 Lab 8. Carbohydrates (W14) 97 B. Oxidation: 1. Control 2. Fructose 3. Glucose 4. Sucrose 5. Lactose Cu2+ Cu2+ Cu2+ Cu2+ Cu2+ 6. Starch Color after Heating w/ Benedict’s Ion produced or (circle one) or or or or Cu1+ Cu1+ Cu1+ Cu1+ Cu1+ Cu1+ Reducing or nonreducing Reducing or nonreducing Reducing or nonreducing Reducing or nonreducing Reducing or nonreducing Reducing or nonreducing (circle one) Sugar Type or Cu2+ Conclusion/Explanation/Analysis: Why did these results occur as they did? Explain any Anomalies. 1. Show the equation for the reaction between glucose and Benedict’s reagent. ___2. Sucrose is a nonreducing sugar because A. it makes a person gain weight. C. it is a monosaccharide. B. it cannot form a free aldehyde in solution. D. it is a disaccharide. C. & D. Dehydration with Heat and Acid: Observations for Each General Explanation for All What is happening in all of these cases and why? What is responsible for the color produced? Heat on Sucrose Acid on Sucrose Acid on Cellulose IV. PRACTICAL APPLICATIONS IN COOKING: Observations, Results, Comments A1. Gak A2. Thickening B. Cheese Sauce Comparisons: 98 Compare cornstarch vs flour thickeners. Compare water vs roux methods. CH110 Lab 8. Carbohydrates (W14) LAB 8. CARBOHYDRATES: NAME_____________ DATE______________ POST LAB EXERCISES: More than one answer may be required. Give all correct answers: _____1. Why do crackers (high in amylose) have the potential to taste sweet after chewing in your mouth? A. Amylose is naturally sweet tasting. B. Amylose reacts with amylase in saliva and produces glucose which tastes sweet. C. Amylose reacts with sucrase and produces sweet tasting sucrose. _____2. Why does the blue color of Iodine disappear when amylose reacts with saliva? A. Saliva causes the amylose chain to uncoil so Iodine is no longer trapped. B. Saliva reacts with iodine causing it to be unable to complex with the amylose coil. C. Saliva causes the amylose chain to break into short chains and then into glucose which does not coil and so does not trap iodine. D. Amylase in saliva will replace the I2 in the amylose coil and so remove the color. _____3. Glucose (C6H12O6) is soluble in water but is not soluble in hexane (C6H14) because __ A. compounds that have many OH’s have polarity that attracts polar water but repels nonpolar hexane. B. compounds that contain oxygen will repel compounds that don’t. C. hexane is an organic solvent and glucose is not an organic compound. D. glucose has fewer hydrogens than hexane. _____4. Which of the following are reducing sugars? A. fructose B. glucose C. sucrose D. lactose E. amylose _____5. The gaseous substance that is driven off of sucrose when it is heated is __ A. CO2 B. SO3 C. CH4 D. H2O _____6. The gaseous substance that is driven off of sucrose when it is reacted with acid is __ A. CO2 B. SO3 C. CH4 D. H2O _____7. The black residue left over after complete dehydration of a carbohydrate is ___ A. C B. SO3 C. HSO2 D. H2O _____8. Why does a solution made of starch get thick when heated in water? A. Granules of starch uncoil to give long strands that tangle into a gel. B. Starch granules stack in an organized pattern that makes the mixture solid. C. Starch is insoluble so just forms complexes of suspended solids which thicken the mixture. CH110 Lab 8. Carbohydrates (W14) 99 100 CH110 Lab 8. Carbohydrates (W14)