Chemistry 103 Lab 2: Reactions and stereochemistry of
advertisement
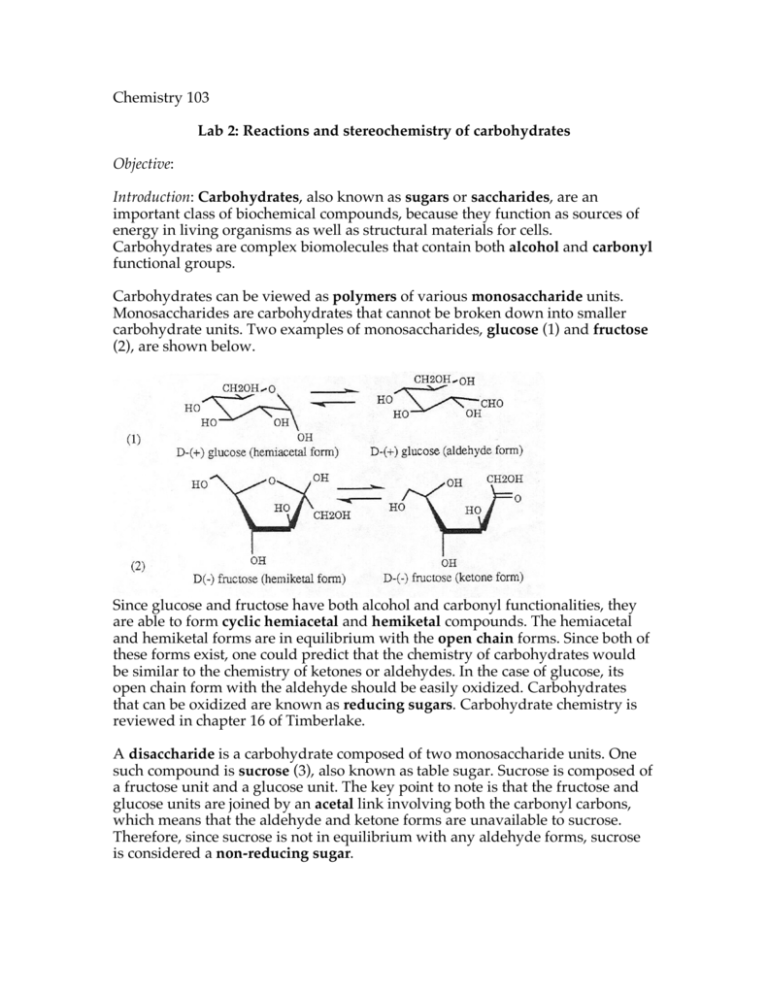
Chemistry 103 Lab 2: Reactions and stereochemistry of carbohydrates Objective: Introduction: Carbohydrates, also known as sugars or saccharides, are an important class of biochemical compounds, because they function as sources of energy in living organisms as well as structural materials for cells. Carbohydrates are complex biomolecules that contain both alcohol and carbonyl functional groups. Carbohydrates can be viewed as polymers of various monosaccharide units. Monosaccharides are carbohydrates that cannot be broken down into smaller carbohydrate units. Two examples of monosaccharides, glucose (1) and fructose (2), are shown below. Since glucose and fructose have both alcohol and carbonyl functionalities, they are able to form cyclic hemiacetal and hemiketal compounds. The hemiacetal and hemiketal forms are in equilibrium with the open chain forms. Since both of these forms exist, one could predict that the chemistry of carbohydrates would be similar to the chemistry of ketones or aldehydes. In the case of glucose, its open chain form with the aldehyde should be easily oxidized. Carbohydrates that can be oxidized are known as reducing sugars. Carbohydrate chemistry is reviewed in chapter 16 of Timberlake. A disaccharide is a carbohydrate composed of two monosaccharide units. One such compound is sucrose (3), also known as table sugar. Sucrose is composed of a fructose unit and a glucose unit. The key point to note is that the fructose and glucose units are joined by an acetal link involving both the carbonyl carbons, which means that the aldehyde and ketone forms are unavailable to sucrose. Therefore, since sucrose is not in equilibrium with any aldehyde forms, sucrose is considered a non-reducing sugar. In contrast, lactose (4) is a disaccharide composed of a galactose unit and a glucose unit. In lactose, the hemiacetal bond is formed with an alcohol oxygen of glucose and therefore the aldehyde functionality of the glucose unit is available. Thus, lactose is a reducing sugar. Another important aspect of sugars is that the body uses the monosaccharide glucose as its source of energy. In order for energy to be obtained from sucrose, the organism must first break it down into glucose. This reaction entails the hydrolysis of the acetal linkage and, in an organic chemistry lab, this type of hydrolysis is normally done by heating sucrose in a strong acid solution such as 3M HCl. However, most organisms need to maintain a roughly neutral pH and cannot tolerate such acidic conditions. Fortunately, living organisms can hydrolyze carbohydrates to gain glucose by the use of enzymes. Enzymes are proteins that accelerate biochemical reactions, such as the hydrolysis of the acetal linkage, without the need for acidic conditions. Invertase is one of the enzymes that can hydrolyze sucrose to glucose and fructose at pH 7. In this experiment, you will test sucrose and lactose for reducing action by reacting them with Tollen’s and Benedict’s reagents. In the Tollen’s test, silver ion is reduced to metallic silver. In the Benedict’s test, copper (II) is reduced to copper (I); there will be a definite color change. You will also hydrolyze sucrose to its fructose and glucose components by the two different methods mentioned above. One solution of sucrose will be heated in 3M HCl and another solution will be reacted with the enzyme invertase. In order to see if your hydrolyses were successful, you will test for the presence of reducing sugars (inferring the presence of glucose and fructose) after the reactions have been completed. Also, after the hydrolyses, you will make use of polarimetry to detect the presence of glucose. Sucrose, fructose and glucose are chiral molecules (they are not superimposable on their mirror images) and therefore they have the ability to rotate plane-polarized light, a phenomenon that can be measured by a polarimeter. Sucrose rotates plane-polarized light in a quite positive (+) direction (as viewed from detector to source) while fructose does so in a quite negative (–) direction. By taking a polarimeter reading after your hydrolysis, you can infer the presence of fructose by getting a strong negative polarimeter reading. Safety issues: Hot hydrochloric acid is used in this lab, so goggles must be worn. Materials • nine test tubes • sucrose •distilled water • 3M hydrochloric acid • invertase • lactose •37°C water bath •5% silver nitrate solution •5% sodium hydroxide solution • 2% ammonium nitrate solution • Benedict’s reagent Procedure 1. Add 0.50 g of sucrose to three different test tubes, and label them “1”, “2” and “3”. 2. To test tube 1, add 10 mL of distilled water (this will be a “blank” solution). To test tube 2, add 10 mL of 3M HCl. To test tube 3, add 10 mL of distilled water and 2 mg of invertase. Mix each thoroughly. 3. Place the three test tubes in a 37°C water bath for 50 minutes. Shake test tube 3 periodically. 4. While you are waiting for the three solutions to incubate, you will test sucrose and lactose for reducing action. Label a test tube “4”, and add 0.4 g sucrose and 7 mL of distilled water. Label a test tube “5”, and add 0.4 g lactose and 7 mL of distilled water. Perform the Tollen’s and Benedict’s tests to the contents of test tubes 4 and 5. Record all of your observations in your lab notebook by setting up a data table: solution Tollen’s result Benedict’s result sucrose + water lactose + water Tollen’s test: To an empty test tube, add 10 drops of 5% silver nitrate solution and 2 drops of 5% sodium hydroxide. Add enough 2% ammonium nitrate to just dissolve the black precipitate (shake the tube to mix). Be sure not to add an excess of ammonium hydroxide. Once the solution is clear, add 4 drops of your sugar solution. Formation of a silver mirror on the interior of the test tube is a positive test for a reducing sugar. Gentle heating in the 37°C water bath will facilitate the reaction. Benedict’s test: In an empty test tube, add 5 mL of the Benedict’s reagent to 2 mL of your sugar solution. Heat these mixtures in a 37°C water bath for 10 minutes. A reducing sugar will produce a red, green or yellow precipitate. 5. Take polarimeter readings for solution 1, 2 and 3 after the incubation period is done. The instructor will show you how to use the polarimeter. A negative (–) value indicates the presence of fructose and glucose, while a positive (+) value indicates the presence of sucrose. Note: you may need to filter the solution in test tube 3 before you take a polarimeter reading; a cloudy solution may affect the reading. Specific rotations of various sugars sucrose a = + 66.5° fructose a = – 93° glucose a = + 52° Questions 1. Write an objective for this experiment in the appropriate section of your lab notebook. The objective should not be long, but contain enough words to account for the major goal(s) of this lab. 2. a. Draw the products of the acid hydrolysis of lactose. b. Draw the aldehyde form of lactose. 3. What can you conclude from the results of the Benedict’s and Tollen’s tests? In other words, which sugars are reducing, and which are non-reducing, according to the results of your tests? This answer should be longer than a couple words. 4. Describe, using the polarimetry data, what sugars were generated in test tubes 2 and 3. Please support you claims with evidence!








