Development and Evaluation of a Logical Delta Check for Identifying
advertisement

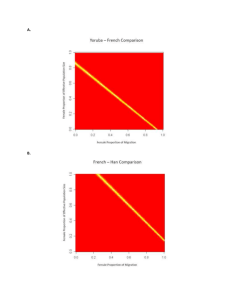
Development and Evaluation of a Logical Delta Check for Identifying Erroneous Blood Count Results in a Tertiary Care Hospital Ira Miller, MD, PhD Context.—Delta checks have been suggested to increase patient safety by identifying preanalytic and analytic errors, including wrong name mislabeling on the sample tube. Objective.—To implement an effective and practical complete blood cell count (CBC) delta check by optimizing specificity and sensitivity using weighted deltas of multiple parameters. Design.—The mean red blood cell volume (MCV) delta (.3.0 fL) check was retrospectively assessed. The composite CBC delta (CCD) test was formulated using serial same-patient CBC data and random interpatient CBCs. The logical delta check (LDC) ignores CCD failures due to platelet change only. The effect of LDC implementation was evaluated. Results.—The MCV delta check test recognized only 3 of 6 confessed mislabeled specimens in the initial review period, whereas all were identified using the CCD. The LDC flagged 2% (205 of 13 234) of eligible results, one- third as many as the MCV delta check. The CCD and LDC checks revealed 20 presumed or confirmed mislabeling events, only half of which were caught by the MCV delta check. Thirty-four percent of LDC failures not due to transfusion reflected problematic results, including presumed or confirmed wrongly labeled patient samples (36% of flags for real problems). Implementation of the LDC, requiring immediate verbal feedback to the caregivers, was associated with more retracted erroneous results in patients’ medical records. Conclusions.—The MCV delta check test was found not to have led to correction of errors in our laboratory due to impractically low specificity and sensitivity. The LDC is a useful tool for identifying preanalytic and analytic specimen problems, including wrong name mislabeling on the sample tube. (Arch Pathol Lab Med. 2015;139:1042–1047; doi: 10.5858/arpa.2014-0494-OA) M quality management plan that covers all areas of the laboratory and includes benchmarking key measures of laboratory performance.’’2(GEN.13806, .20316) Two programs are offered in the Q-Tracks Continuous Quality Monitoring Program, namely, Patient Identification Accuracy and Corrected Results. In the former instance, phlebotomists record problems with patient wristbands; in the latter, the fraction of corrected laboratory results (a portion of which may have been due to mislabeled specimens) is recorded. The Clinical Laboratory Improvement Act also requires laboratories to monitor, assess, and correct problems identified in preanalytic, analytic, and postanalytic systems.2 The greatest emphasis on preventing labeling errors has been in the area of blood banking, where transfusion of the wrong blood product may result in a rapid and fatal reaction. In a multi-institutional study, Grimm et al3 estimated a frequency of at least 1 per 2500 samples for misidentified specimens sent for blood typing, and incidence was positively correlated with phlebotomy by nonlaboratory personnel. Their estimate included errors unveiled through a mismatch of the sample blood type with the historical patient blood type. The safety standard of The Joint Commission is for 2 qualified people to verify blood samples sent to the blood bank. However, any mislabeled blood sample has the potential for adverse repercussions. islabeled laboratory specimens have the potential to lead to adverse patient outcomes through misdiagnosis, delayed treatment, and inappropriate treatment. For this reason, The Joint Commission, formerly The Joint Commission on Accreditation of Healthcare Organizations, has listed ‘‘Improve the Accuracy of Patient Identification’’ as the first goal of the 2014 National Patient Safety Goals.1 The standards instituted are to use at least 2 patient identifiers for any specimen and to label the specimens in the presence of the patient. The Joint Commission requires accredited hospitals to regularly collect and analyze performance data. Proof of compliance with these standards will undoubtedly be incorporated into upcoming accountability measures used in accreditation and determining top performers. According to the general checklist of the College of American Pathologists, accredited laboratories must have ‘‘a Accepted for publication December 2, 2014. From the Department of Pathology, Rush University Medical Center, Chicago, Illinois. The author has no relevant financial interest in the products or companies described in this article. Reprints: Ira Miller, MD, PhD, Department of Pathology, Rush University Medical Center, 1653 W Congress Pkwy, Chicago, IL 60612-3833 (e-mail: Ira_Miller@rush.edu). 1042 Arch Pathol Lab Med—Vol 139, August 2015 CBC Logical Delta Check—Miller Wagar and colleagues tracked mislabeling events at their institution before and after implementation of performance improvement strategies, which included an electronic event reporting system, and concluded that ‘‘strategies that focus longitudinally on specimen labeling errors can significantly reduce errors, therefore improving patient safety.’’4(p1662) They lowered the rate of recognized mislabeled specimens from an average of more than 20 per month in the first 7 months to less than 1 per month in the last 10 months in their study period. However, they did not state the details of how mislabeled specimens were recognized, and they suggested that ‘‘the number of mislabeled specimens observed in this study underestimates the actual frequency of mislabeled specimens.’’4(p1667) A misidentified specimen test result may be obvious to a caregiver if a result is reported on a test that was not ordered or if the result differs substantially from the expected result. It may be identified by laboratory personnel to reconcile an entry on the preceding day’s log of rejected specimens, which includes those for which no test has been ordered. Also, it might be suspected by the caregiver or laboratory when there is significant variation from a recent prior result from that test on the same patient, the so-called delta checks. The practice of delta checking in hematology was first reported by Nosanchuk and Gottman5 in 1974 as a broad quality control for preanalytic and analytic errors. In an analysis of simulated delta check rule performance, Strathmann and colleagues6 found that the mean red blood cell volume (MCV) had the highest positive predictive value and the fewest false positives. In 2005, the International Consensus Group for Hematology Review7 published suggested criteria for action following automated complete blood cell count (CBC) and white blood cell differential analysis. This list of actionable items included delta rules for changes in white blood cells, MCV, and platelets. The action suggested for a failure of the MCV delta was to ‘‘verify sample integrity/identity.’’ The authors did not suggest a specific delta value to trigger action. Our laboratory had long used a delta check of the MCV value in patients’ CBCs to suggest the possibility of a specimen error, including misidentification. We followed a protocol whereby serial MCVs within 3 days on the same patient that differ by more than 3.0 fL prevented the automatic release, or autoverification, of the entire CBC panel and prompted the instrument operator to release the results with a footnote in the medical record indicating either that the change correlates with a charted interim transfusion or that the caregiver should confirm the specimen identity or redraw the sample. This MCV delta check procedure was instituted at a time before autoverification was in place, when test volume was lower, staffing was higher, and test turnaround time was longer. In their original study, before introduction of computers into the laboratory, Nosanchuk and Gottmann5 reported that the results of 10% of tests failed 1 or more delta checks, but they believed that improvement in the ‘‘standard of reliability and confidence’’ justified the expenditure. The present study began as an evaluation of the effectiveness of the MCV delta check as currently used in our institution for unveiling labeling errors. That analysis led to the proposal and testing of a composite CBC delta (CCD) check and subsequently to the logical delta check (LDC), with improved specificities and sensitivities. This streamlined tool is suitable for implementation in the institutional patient safety insurance program. Arch Pathol Lab Med—Vol 139, August 2015 MATERIALS AND METHODS Initial Survey of the MCV Delta Check Failures On 110 days between November 2010 and March 2011, CBCs were performed on 1 or more of 3 automated blood analyzers (2100XE; Sysmex Corporation, Kobe, Japan). Instrument printouts were collected for patient samples failing the MCV delta check, defined as a change of 3.0 fL or more compared with the most recent test within the preceding 3 days. The electronic medical record of the patient was interrogated to determine the following: (1) whether the MCV delta flag was triggered accurately, (2) whether comparison with subsequent and prior counts suggested that a mislabeling error had occurred, (3) whether the patient had been transfused in the interim, and (4) whether other conditions or therapies might account for the change in MCV. Test results were presumed to have come from mislabeled specimens if multiple CBC parameters changed, with cell counts moving in opposite directions, and subsequent results, which were almost always available, returning to the previous values. Cases were not considered mislabeled if changes could be attributed to fluid dilution, poor mixing (no change in red blood cell distribution width [RDW] or the mean cell hemoglobin [MCH]), or clinical situations such as postoperative samples without transfusion or hydration of patients. Optimization of the CCD Forty-nine random patients with MCV delta check failures were chosen from the initial survey group, each with more than 30 sequential CBC tests (up to 280 tests per patient, with an average of approximately 50) performed on Sysmex 2100XE instruments. For some patients, data from more than 1 hospitalization were analyzed. The CBC results were transferred into a spreadsheet (Excel; Microsoft Corporation, Redmond, Washington). For each of the independent parameters of the CBC, comparison was made between all serial CBCs performed within 5 days of each other for each patient’s data set (intraindividual deltas), and a separate comparison was made between the most recent CBCs of each patient (interindividual deltas, up to 5 million comparisons). For intraindividual deltas, serial cases with increases in hemoglobin of more than 1.7 g/dL were excluded as indicative of interim transfusion. Using the spreadsheet frequency function with bins scaled to each CBC parameter, the frequency distribution of intraindividual and interindividual deltas for each variable was plotted. These plots were assessed visually to determine the usefulness of each parameter in discriminating interindividual comparisons from serial intraindividual comparisons. An optimized CCD was then derived by serially repeating all comparisons using the square root of the sum of the squares of the discriminating CBC parameters, each with a scaling or weighting factor derived by trial and error. The following computation for CCD was chosen: CCD ¼ SQRT ([50 3 DHb]2 þ [100 3 DMCH]2 þ [100 3 DRDW]2 þ [1.5 3 DPLT]2), where SQRT is square root, the hemoglobin concentration (Hb) is in grams per deciliter, MCH is in picograms, RDW is a percentage, and PLT (platelet) is in thousands per microliter. An initial cutoff value of 250 for flagging was suggested based on the initial training data set. (To simplify the computer programming, the same result is achieved by comparing the term within the square root sign with the cutoff value of 62 500.) Testing the CCD and LDC The CCD was programmed into the Workspace Application Module software by programmers at Sysmex. For each CBC, both MCV delta checks and CCD checks were performed on Sysmex 9000XN instruments. Data from the course of a 6-month period were reviewed to determine the discriminatory power of the MCV delta and CCD checks for specimens subsequently discovered to have been mislabeled. In addition, Workspace Application Module manager reports of failures were generated. All data for a period of 2 weeks (CCD) or 5 weeks (LDC) were assessed to determine the number of specimens flagged by each delta test, which changes CBC Logical Delta Check—Miller 1043 were attributable to transfusion, and any clinical or laboratory associations with a delta check failure. RESULTS Initial Survey of the MCV Delta Check Failures In the initial evaluation period, Sysmex operators were requested to submit instrument printouts for tests failing the MCV delta check. Five hundred ninety-one CBC results were assessed. This represented an average of 5.4 per day out of an average of 440 CBC tests per day (1%) and serves as a lower limit estimate of the MCV delta failure rate because there was no monitor of operator compliance. Review of the prior CBC results for each patient revealed that 89 tests were false calls of an MCV failure due to lack of finalization of all components of the most recent test results, causing comparison with earlier results, or due to prior retracted results read as zero values. Of the 502 true MCV delta failures, review of the medical record indicated that 288 were associated with transfusion in the interim between tests. Of the 205 MCV delta failures without interim transfusion, review of the results of subsequent CBCs, which were available in almost every case, showed similar values, indicating that the CBC test with the MCV delta failure was accurate. Analytic problems due to interinstrument calibration differences or random error contributed to MCV delta failures. For 46 tests of the MCV delta failures, the sample was retested on another similar instrument, with a mean (SD) difference of 1.0 (0.8) fL. In 28% of those cases, the MCV change due to interinstrument variability was 1.5 fL or more, or half of the change required to trigger a MCV delta check failure. Possible physiological reasons suggested from review of the patient records included red blood cell volume changes due to alterations in plasma osmolarity in patients on hemodialysis or with aggressive fluid hydration associated with diarrhea or emesis, as well as patients who had undergone surgery with no blood products given. Six mislabeled specimens were identified. The MCV delta check failure flag occurred on the erroneous test and on the subsequent test in 3 cases. In 2 cases, the flag occurred only on the subsequent test. Failure to flag the truly mislabeled specimen probably occurred because other results were still pending when the next CBC test was reported. In 1 case, there was no follow-up specimen, so it is uncertain which was mislabeled. There was no evidence of any result corrections initiated from charted MCV delta check failures because erroneous results remained in the medical record. Formulating an Optimized CCD Check An index population of 49 patients with many sequential CBC tests was used to derive a CCD check based on the hypothesis that the individual CBC parameters varied independently in a population and that sensitivity and specificity of the delta test could be improved by combining the most informative parameters. In this index patient group, more than 98% of the sequential samples were acquired less than 2 days apart, and almost all of these were next-day comparisons. Most but not all sequential samples with interim transfusion were eliminated from analysis by excluding serial samples with a rise of 1.7 g/dL or more. Comparison between random interpatient deltas and intrapatient serial CBC deltas for each of the CBC parameters suggested that hemoglobin concentration (or hematocrit), MCV, MCH, RDW, and platelet count would be useful delta parameters (representative curves are shown in Figure 1). 1044 Arch Pathol Lab Med—Vol 139, August 2015 By trial and error, the CCD check was optimized by applying scaling and weighting factors to give the greatest separation between the curves (Figure 2, A). The MCV and MCH gave similar discriminatory power and were not independently useful. The MCH was incorporated rather than the MCV because of the observation that MCV changes occurred with hydration or dialysis, which should not affect the MCH. Adding the white blood cell delta did not provide additional discriminatory power. Based on the initial sample comparisons, the computation for CCD and the cutoff value stated in the ‘‘Materials and Methods’’ section were derived. The receiver operating plot for this function is shown in Figure 2, B. This test gave a specificity of 97.6% and a sensitivity of 92.5% using the training data set from the 49 index patients. That is to say, 2.4% of the more than 2000 serial intrapatient CBC tests gave CCD values of greater than 250, and 7.5% of the 5 million interpatient comparisons gave CCD values of 250 or less, flagging one-third as many as the MCV delta check. In the same data set, using the single parameter MCV delta with a cutoff of 3.0 fL had a specificity of 94.4% and a sensitivity of only 77.4%. Testing the CCD Parameter The CCD check and the MCV delta check were compared in the subsequent 6-month period for sensitivity in detecting actually mislabeled samples discovered through other means (error forms submitted by nurses). Of 6 such specimens with prior results for comparison, all failed the CCD check with a cutoff of 250, while only 3 failed the MCV delta check (Table 1). In further assessment, the number and characteristics of all 110 specimens flagged by the CCD check out of a possible 5792 specimens (with a 3-day look-back to prior window) during a randomly chosen 2-week period were also evaluated (Table 2). The CCD check with a cutoff of 250 flagged less than half of the number of specimens flagged by the MCV delta check. The difference is significant, with a 2-tailed P , .001 (v21 ¼ 66.704). Approximately half were readily explained by interim transfusion. Review of prior and subsequent values showed that in approximately 13% of cases a large change in platelets contributed disproportionately to the high CCD value, usually in patients with platelet counts that were elevated. In another 36% of cases, review of subsequent values confirmed the shifted CBC results, and review of the medical record usually provided an explanation such as hemorrhage, surgery, delivery, or sickle cell disease, with the latter associated with a large change in RDW. In 17 cases (0.3% of all samples with recent priors for comparison), the large change in CCD value reflected an error. Dilution from intravenous fluid was apparent in 3 cases by a proportional change in concentrations of all components, usually accompanied by a rise in the MCV in the diluted sample. Erroneous red blood cell parameters due to cold agglutinins were evident as increases in the MCH in 3 cases in which the operator deviated from protocol, and improper mixing of tubes run in manual mode was the likely explanation for 2 errors. Cases of CCD failure reflecting clotted samples were excluded because these were always independently recognized and flagged by other sample or result characteristics. Finally, 5 mislabeled specimen events triggering 9 CCD failures were discovered in the 2-week period. Four mislabeled specimen events each triggered 2 high CCD values, one for the mislabeled specimen compared with the results of the correctly labeled prior and another for the CBC Logical Delta Check—Miller Figure 1. Delta curves comparing frequency distributions of representative single parameters (MCV, Hb, MCH, and RDW in A, B, C, and D, respectively) from the index population, frequency plotted on the y-axis, show random interindividual comparisons (red) and intraindividual comparisons (blue). Abbreviations: MCV, mean red blood cell volume; Hb, hemoglobin concentration; MCH, mean cell hemoglobin; and RDW, red blood cell distribution width. subsequent correctly labeled specimen compared with the mislabeled specimen results. In only 1 of these instances was the MCV delta greater than 3.0 fL. In 1 additional case, the actually mislabeled specimen was reported before the beginning of the 2-week assessment period. Several clotted specimens would have been detected but were never reported because they were already flagged based on abnormal platelet parameters. Assessment of the 14 cases with high CCD values due to a disproportionately large platelet delta suggested the possibility of further refinement of the CCD test. For all 14 of these false-positive cases, eliminating platelets from the CCD calculation—using the square root of the sum of squares of deltas of hemoglobin, MCH, and RDW (termed HMR calculation)—gave values of less 116, but all of the true-positive problem cases had HMR values of greater than 116. The LDC, requiring violation of both criteria (CCD .250 and HMR .116), was enabled in the laboratory, and at this point we changed our delta check protocol to only require follow-up of LDC failures and not Arch Pathol Lab Med—Vol 139, August 2015 of MCV delta failures. Technicians were required to determine if flagged values were associated with interim transfusion, to consider the possibilities of inadequate mixing of samples run in manual mode, and to be alert to the possibility of inadequate warming of samples with cold agglutinins in cases with increased MCH. All unexplained changes required a phone call that suggested the need for a redraw if the recent change in values could not be explained clinically. Technicians were requested to save the histogram and CBC result printouts for LDC failures and to indicate the cause and the resolution of the discrepancy. For a period of 5 weeks, characteristics of samples failing the LDC test (with a 5-day look-back window) were assessed (Table 2). Retrospective review of the patient records and laboratory information indicated that the LDC test caught 15 misidentified specimens that caused 16 delta check failures. In all but 3 cases, the erroneous results had been deleted from the record. In 2 cases, the test results had not been reported because a duplicate specimen was CBC Logical Delta Check—Miller 1045 Table 1. Comparison of CCD and MCV Delta Values for Independently Established Mislabeled Blood Specimens Sent for CBC Testing During a 6-Month Period Mislabeled Specimen No. Composite CBC Delta MCV Delta, fL 1 2 3 4 5 6 Mean 414.6 655.8 782.8 554.3 300.6 398.3 518.0 2.4 18.5 0.5 15.0 2.6 7.1 7.7 Abbreviations: CBC, complete blood cell count; CCD, composite CBC delta; MCV, mean red blood cell volume. Figure 2. Shown are optimized composite complete blood cell count (CBC) delta (CCD) frequency distribution curves (A) of random interpatient comparisons (red) and serial intrapatient comparisons (blue) using the index population CBC test results and the receiver operating curve (B) for these data. The CCD calculation uses the weighted combination of deltas of hemoglobin concentration, red blood cell distribution width, mean cell hemoglobin, and platelet count as indicated in the ‘‘Materials and Methods’’ section. recognized before release of the erroneous results. In 4 cases, error reports were submitted by the nurse responsible. One error was committed by a phlebotomist, who received a reprimand. In the other 5 cases, the results were deleted from the medical record, with a note that the specimen had ‘‘questionable integrity.’’ Specimens with problematic results for reasons other than mislabeling were also detected (Table 2). Shortening the period of look-back to prior CBCs from 5 days to 3 days would have excluded 22 of 85 false-positive specimens unrelated to transfusion, without reducing sensitivity. Since that observation was made, we have shortened our look-back to 3 days. 1046 Arch Pathol Lab Med—Vol 139, August 2015 COMMENT This study was initiated in an attempt to evaluate the effectiveness of the MCV delta test for identifying mislabeled blood specimens in our hospital, which is a tertiary care center with a varied inpatient population with medical, surgical, obstetric, psychiatric, and pediatric illness. Review of the MCV delta test indicated that, as it had been used in our hospital, it was ineffective and a nuisance. The MCV changes were often associated with interim transfusion or occurred in patients with probable osmotic imbalance such as those on dialysis. Also, calibration differences between instruments could significantly contribute to the delta value if serial samples were run on different instruments. Approximately 1% of the 860 investigated MCV delta check–flagged tests were mislabeled specimens. However, these values remained in the record, indicating either that they were not recognized by the caregiver to be erroneous or that there were barriers to documenting correction of the results in the medical record. Approximately half of the MCV delta failures were due to interim transfusion. Robust implementation of the MCV delta test by laboratory computer software was hampered by consideration only of prior samples for which all reportable tests had been released, preventing consideration of many prior samples for which there were pending unreported tests. To improve upon the MCV delta check, I used random sample results from our hospital population to initially determine which of the CBC values were most informative to distinguish interpatient samples from serial intrapatient samples, followed by the best weighting coefficients for the CCD calculation. Retrospective review of the CCD performance proved that it had higher sensitivity and specificity than the MCV delta check. Review of false-positive and false-negative cases suggested further improvement, with the LDC calculation adjusting for rapid platelet changes. Review of the patients’ medical records after implementation of the LDC showed that only 3 of the 15 erroneous result sets were not removed, suggesting that action by the technician led in most cases to real-time recognition of the error. Finally, shortening the look-back period to 3 days will further improve specificity. Based on the initial 5-week period of implementation, the LDC is predicted to flag less than 1% of total CBC results. Posttransfusion results are released after consulting the record, and other results are released after notification of the caregiver or withheld (along with other concurrent test results) at his or her request, pending CBC Logical Delta Check—Miller Table 2. Characteristics of Failures for CCD and LDC Using Cutoff Values Indicated in the Text Variable CCD Length of evaluation period, d Total CBCs performed during the evaluation period, No. Total delta checks, No. (% of CBCs with recent priors) Failed delta checks Interim transfusion, No. (%) No interim transfusion, valid based on medical record review, No. (%) Failure due to platelet change only, No. (%) Presumed or confirmed mislabeled, No. (%) Failure due to other problem, No. (%) Specimen dilution from intravenous fluid in line, No. Analytic (agglutinin or unmixed specimen), No. Failed tests Both MCV delta (.3.0 fL) and CCD, No. Both MCV delta (.3.0 fL) and CCD, presumed or confirmed mislabeled, No./total No. LDC 14 11 193 5792 (52) (n ¼ 110) 54 (49) 39 (36) 14 (13) 35 26 566 13 234 (50) (n ¼ 205) 76 (37) 85 (42) 0 9 (8), comprising 5 events 8 (7) 3 5 16 (8), comprising 15 events 28 (14) 11 17 38a Not determined 2/38, comprising 1 event 8/13 assessable LDC failures Abbreviations: CBC, complete blood cell count; CCD, composite CBC delta; LDC, logical delta check; MCV, mean red blood cell volume. a There were 269 MCV delta check failures (5% of values with recent priors) in this period. a redraw. The clinical effect of this innovation is 3-fold. First, there is increased turnaround time for the approximately 2% of specimens that would have been investigated for possible error using the MCV delta check but not with the more specific LDC. Second, early identification of mislabeled or problem specimens—with retraction of erroneous results for not only the CBC but also other tests ordered on blood tubes drawn at the same time—allows for rapid redraw and may prevent unnecessary or inappropriate clinical action. At the current rate, approximately 40% of LDC flags of nontransfused patients’ results will correspond to problem specimens or to their follow-up specimens. Third, identifying more mislabeled specimens provides a tool for laboratory personnel, nurses, and others to identify patterns of error and remediate them. Monitoring these data may be useful for ongoing quality improvement efforts. In consideration of possible limitations of this study, I note that the LDC calculation was derived and tested on a set of specimens from a single medical center with a diverse patient population. As such, it is likely to be useful for most high-volume laboratories that serve large medical centers. Additional modification may be useful to laboratories that encounter false-positive LDC failures resulting from unique clinical situations. For example, if the laboratory receives specimens from a medical center where blood loss from Arch Pathol Lab Med—Vol 139, August 2015 surgery is frequently not corrected by transfusion due to practice variation or patient values, then an additional logical step to ignore LDC failures due to hemoglobin change only may be desirable. I thank Kristin Epperson, MT, and Kathryn Williams, MT, for assistance in data collection, as well as the programmers at Sysmex Corporation. References 1. Joint Commission. National Patient Safety Goals. http://www. jointcommission.org/standards_information/npsgs.aspx. Accessed October 31, 2014. 2. College of American Pathologists. Quality Management Tools. Footnote to table, page 3. http://www.cap.org/apps/docs/proficiency_testing/2014_qmt_ catalog.pdf. Accessed October 31, 2014. 3. Grimm E, Friedberg RC, Wilkinson DS, AuBuchon JP, Souers RJ, Lehman CM. Blood bank safety practices: mislabeled samples and wrong blood in tube: a Q-PROBES analysis of 122 clinical laboratories. Arch Pathol Lab Med. 2010; 134(8):1108–1115. 4. Wagar EA, Tamashiro L, Yasin B, Hilborne L, Bruckner DA. Patient safety in the clinical laboratory: a longitudinal analysis of specimen identification errors. Arch Pathol Lab Med. 2006;130(11):1662–1668. 5. Nosanchuk JS, Gottmann AW. CUMS and delta checks: a systematic approach to quality control. Am J Clin Pathol. 1974;62(5):707–712. 6. Strathmann FG, Baird GS, Hoffman NG. Simulations of delta check rule performance to detect specimen mislabeling using historical laboratory data. Clin Chim Acta. 2011;412(21–22):1973–1977. 7. Barnes PW, McFadden SL, Machin SJ, Simson E; International Consensus Group for Hematology. The International Consensus Group for Hematology Review: suggested criteria for action following automated CBC and WBC differential analysis. Lab Hematol. 2005;11(2):83–90. CBC Logical Delta Check—Miller 1047