Characteristics of Paramagnetic and Diamagnetic Anisotropy which
advertisement

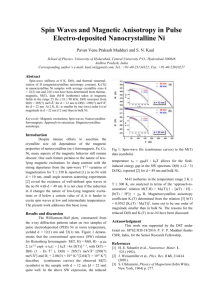
Materials Transactions, Vol. 44, No. 12 (2003) pp. 2594 to 2598 Special Issue on Structural and Functional Control of Materials through Solid-Solid Phase Transformations in High Magnetic Field #2003 The Japan Institute of Metals Characteristics of Paramagnetic and Diamagnetic Anisotropy which Induce Magnetic Alignment of Micron-Sized Non-Ferromagnetic Particles Chiaki Uyeda, Kenta Tanaka* , Ryouichi Takashima* and Makoto Sakakibara* Institute of Earth and Space Science, Graduate School of Science, Osaka University, Toyonaka 560-0043, Japan The effect of temperature is discussed on the magnetic-alignment process of micron-sized particles dispersed in a fluid medium, based on the experimental data compiled on various non-ferromagnetic materials having different concentrations of paramagnetic impurity ion. The fieldintensity required to achieve alignment decreased with temperature following the relation calculated from the Langevin theory, when the diamagnetic particles were free of paramagnetic ions. The rotational Brownian motion was considered to randomize the direction of the microcrystals in the theory. The above-mentioned temperature dependence was expected to occur for most of the diamagnetic oxides, since the oxides were expected to posses a finite amount of diamagnetic anisotropy according to a model proposed recently to explain the origin of anisotropy. The decease of temperature caused additional reduction on the field-intensity to achieve alignment, when finite amount of paramagnetic ion was contained in the particle. This was because the paramagnetic anisotropy increased which the reduction of temperature. The doping of paramagnetic ion on non-ferromagnetic materials in the course of processing a material expected to reduce the field intensity to achieve magnetic alignment at room temperature. The above findings, concerned with the reduction of field intensity to achieve magnetic alignment, may increase the possibility of practical applications of the phenomena of magnetic alignment. (Received June 20, 2003; Accepted November 14, 2003) Keywords: diamagnetic anisotropy, magnetic alignment at low magnetic field, temperature dependence of magnetic alignment, magnetic alignment of micron-sized particle, magneto-rotation, Curie temperature dependence of paramagnetic anisotropy, doping of paramagnetic ion, ceramic material, kaolinite, graphite 1. Introduction It is known that micron-sized non-ferromagnetic particles dispersed in a fluid medium generally possess an efficiency of magnetic alignment due to its diamagnetic or paramagnetic anisotropy .1,2) The magnetically stable axis of a particle may align nearly parallel to the field direction, when the fieldinduced anisotropy energy exceed the energy of rotational Brownian motion supplied from thermal motions of the fluid molecules. The process of the alignment depend on three parameters, namely the mol number of the particle N, magnetic anisotropy of the material per mol and temperature T. The effect of these parameters has not been studied systematically as yet, in spite that the controlling of the alignment process by means of these parameters is an important basis to realize alignment at low field intensity. The alignment at practical low field-intensity may produce new types of applications based on the above-mentioned alignment. Up to now, alignment has been studied mainly on biological3) and organic materials4) in a strong magnetic field above several Tesla. Magnetic alignment of inorganic materials have been performed on micro-crystals of some nonmetals, such as kaolinite, talc,5) lepidolite, fluoro-phlogopite, muscovite,6) graphite,7) Al2 O3 .8,9) and various ceramic materials.2) The number of reports on inorganic materials, nevertheless, were not large because their diamagnetic anisotropy ðÞDIA were considered to be negligibly small compared to those of the organic materials.10) The small ðÞDIA values of basic inorganic oxides were detected by a new method developed by one of the authors.11,12) The ðÞDIA values were obtained for forsterite,12) corundum, muscovite, Mg(OH)2 , Al(OH)3 , AlOOH,13) orthoclase, apophylite,14) gypsum, KDP and *Graduate Student, Osaka University ADP.15) The compiled data indicated that many of the unmeasured diamagnetic-oxides may posses a finite amount of anisotropy to cause magnetic alignment.16) Experimental evaluations of the effects of the parameters which control the magnetic alignment process, discussed above, can be examined quantitatively by adopting the inorganic materials in the alignment experiments.5–7) It was difficult, for example, to alter the value of temperature over a wide range in the experiment using a biological material. The effect of temperature is discussed in the present paper on the alignment of diamagnetic and paramagnetic particles, based on the compiled data on inorganic materials.5,7,13,19,20) It is seen that the reduction of temperature is effective in lowering the field-intensity to achieve magnetic alignment for most of the diamagnetic and paramagnetic particles. This effect is caused by the temperature dependence of the paramagnetic anisotropy ðÞPARA deriving from the impurity ions, and also by the decrease of the rotational Brownian motion.7) 2. Effects of the Parameters that Control the Magnetic Alignment The relationship between the degree of preferential alignments of the magnetically stable axes of the micron-sized particles and the field-intensity B has been analyzed consistently for various experiments,1,2) based on a method first proposed by Langevin and Curie.17) The degree of alignment was calculated from the Boltzmann distribution of the micron-sized particles at a given temperature T. The field-induced free energy U of a particle having a mol number N was used in the calculation, UðÞ ¼ ðNB2 =2Þf? þ cos2 g: ð1Þ The angle between the magnetically stable axis of the particle Order Parameter <m> Characteristics of Paramagnetic and Diamagnetic Anisotropy which Induce Magnetic Alignment of Micron-Sized Non-Ferromagnetic Particles 2595 alignment is almost completed, in order to carry out quantitative comparison between different alignment processes. It is useful to define such a value in investigating the quantitative amount of field intensity required for grain alignment in the processes of various applications as well. The field intensity where hmi reaches hmi ¼ 0:8 was defined tentatively as BS for this purpose.5–7) The value of BS is calculated from the Langevin theory as, 1 kaolinite talc graphite BS ¼ ð15kB T=NÞ1=2 : 0 0.5 1 1.5 2 2.5 Magnetic Field B/ T Fig. 1 Magnetic alignment process measured for various ceramic microcrystals dispersed in liquid ethanol. and the field direction is denoted as in the above equation. The magnetic anisotropy of the material composing the material is described as ¼ k ? ; k and ? denote the molar magnetic susceptibility in the direction parallel and perpendicular to the magnetically stable axis, respectively. The origin of has been attributed to the diamagnetic and paramagnetic anisotropy.1,2) The degree of axis-alignment is commonly described by an order-parameter hmi, which is the Boltzmann average of a function ð3 cos2 1Þ=2 calculated in terms of UðÞ as, Z hmi ¼ fð3 cos2 1Þ=2g expfUðÞ=kB Tg Z ð2Þ sin d= expfUðÞ=kB Tg sin d: Here hmi ¼ 0 and 1 correspond to completely random state and to the completely ordered state of the grains, respectively. The order parameter has been commonly used to describe the state of molecular orientation in the liquid crystals as well as the state of magnetic alignment of the micron-sized particles.1,2) The measured hmi-B relationships of various inorganic micro-crystals measured at room temperature are compiled in Fig. 1, namely for kaolinite, talc5) and graphite.7) The experimental hmi-B relationship was obtained from the light intensity IðBi Þ transmitted through the sample-suspension at an field intensity Bi .1,3,5) The field intensity was increased stepwise until IðBi Þ ¼ IðBi Þ Ið0Þ reached a constant value IS , where the alignment was almost completed. The hmi values are obtained from the relation hmi ¼ Ii =IS , which was deduced theoretically18) and confirmed experimentally by measuring the hmi-B relation for several materials which had published values determined by bulk measurements.5–7) The N value which gave the best fit to the experimental hmi-B data was adopted to describe the theoretical curves by the use of eq. (2). The adopted N value for each curve is interpreted as the average N value of the particles dispersed in the suspension.5–7,19,20,22) The state of full orientation corresponding to the state of hmi ¼ 1 cannot be obtained exactly at a finite field intensity according to eq. (2), although the alignment seem to be completed according to Fig. 1 for graphite, talc and kaolinite. It is necessary therefore to define a field intensity where the ð3Þ It is deduced from eqs. (1) and (2) that a BS value correspond to a unique hmi-B curve. The equation shows that a BS value is determined by the three parameters7) as described before, namely T, and N. The BS –N relationships were examined experimentally for inorganic materials, such as kaolin,5) talc,5) lepidorite6) and fluoro-phlogopite.6) The expected N dependence of BS described in the equation was confirmed in the range of N ¼ 1010 to 1012 mole. The BS –N relationships measured for several different materials at a constant temperature fell on different lines, which derived from the difference of values. The results revealed that the values of individual materials determined the BS values as well. The T dependence of BS expected in eq. (3) was examined experimentally between room temperature and 180 K for synthetic graphite grains, free of paramagnetic ions, by Chihara et al.7) Ethanol was chosen as the dispersing medium for the low temperature experiments. The BS -T relations observed for the two samples, namely graphite-1 and -2, followed the theoretically expected T dependence as described in Fig. 2. The differences of the BS values observed between the two graphite samples were explained by the difference of the average N values obtained for graphite-1 and -2. The measurement served as an experimental examination of the Langevin theory on magnetic alignment, which assumed that the alignment was controlled by the Brownian thermal energy. It is deduced from the results of the following section that the reduction of BS by lowering the temperature of the suspension, observed in Fig. 2, is applicable for many of the diamagnetic oxides. 0.06 0.05 log (Bs/K) 0 graphite-1 graphite-2 0.04 0.03 0.02 100 200 300 400 500 log (T/K) Fig. 2 Temperature dependence of magnetic alignment process observed for diamagnetic graphite grains. 2596 C. Uyeda, K. Tanaka, R. Takashima and M. Sakakibara Table 1 ðÞ [109 emu/g] Material 15) Diamagnetic Anisotropy Measured for Various Inorganic Oxides Material Magnetically stable axis Locality of product y 11 0:5 a-axis synthesized apophylite14) [a-c] brucite13) [c-a] 3:8 0:1 2:6 0:2 a-axis c-axis Jalgon-India, Poona-India Zimbabwe, Ural-Russia corundum16) [c-a] 0:7 0:1 c-axis synthesized 4:2 0:3 c-axis Mugla-Turkey Gerais-Brazil ADP [a-c] 16) diaspore [c-a] gibbsite13) [c-a] 1:4 0:2 c-axis graphite10) [c-a] 0:15 0:2 c-plane synthesized gypsum15) [1 -2 ] 9:6 0:2y 1 -axis Chihuahua-Mexico muscovite13) [c-a] 11 2 c-axis Minas Gerais-Brazil KDP15) [a-c] orthoclase14) [3 -2 ] 8:3 0:3y 2:1 0:1 a-axis 3 -axis synthesized Udateha-Russia, Gifu-Japan -quartz10) [a-c] 2:0 0:2 a-plane synthesized kaolinite19) [c-c? ] 12 6z c-plane Georgia-USA, SouthCarolina-USA talc20) [c-c? ] 77 38z c-axis Kouchi-Japan, Nagasaki-Japan 2 1y c-plan synthesized phlogopite6) [c-c? ] #1) Symbols [*] show that ðÞDIA were obtained from high temperature limit of -T measurement on bulk samples; see Fig. 4(a). Symbols [y] denote that the samples had high quality free of paramagnetic impurity ions, and ðÞDIA were obtained by measuring bulk samples at room temperature. ðÞDIA of phlogopile was obtained from magnetic alignment experiment on micro-crystals.6) Symbols [z] show that ðÞDIA was estimated from high temperature limit of -T relations obtained from magnetic alignment experiment on micro-crystals;.20,21) Effect of Diamagnetic Anisotropy on Magnetic Alignment The number of published ðÞDIA values on basic inorganic oxides have increased recently as mentioned before.13–15) The values are listed in Table 1. The origin of the obtained ðÞDIA values were explained consistently by assigning a constant amount of uni-axial diamagnetic anisotropy to the individual bonding orbital composing the material.12,14–16) The direction of the principle axis was assumed to be identical to the bond direction in the model. Accordingly, positive correlations were expected between measured ðÞDIA (per moll) and the differences between 2 , 2 and 2 as described in the appendix. , and denoted the direction cosines of the bond directions. As a first step, the correlations mentioned above were examined separately on three types of basic chemical bonds, namely the T-O bonds composing the tetrahedral [TO4 ] units,14) the hydrogen bonds15) and the M-O bonds composing the octahedral [MO6 ] units.16) The correlation of the hydrogen bonds were examined using the experimental ðÞDIA data of gypsum, KDP, ADP and hexagonal ice.15) The relationship for T-O bonds was examined using the data of quartz, orthoclase and apophylite.14) Finally the model was examined for the M-O bonds using the data of corundum, gibbsite, brucite13) and diaspore.16) Detailed procedures of the calculation are given in the appendix. Clear correlations were obtained for the three types of chemical bonds as described in Fig. 3. Diamagnetic anisotropy deriving from a single chemical-bond ðÞBD were estimated from the regression lines of the correlations to be 2:2, 0:63 and 0:19 [106 emu/mol] for the T-O bond, hydrogen bond and the M-O bond, respectively; equations for the regression lines are given in eqs. (A·2a)-(A·2c). It is noted that the three types of chemical bonds are the major categories of bonding orbital that compose the inorganic oxides of light elements.16) Accordingly most of the Tetrahedral unit measured ∆χ (x10-6 emu/mol) 3. 2.5 2.0 1.5 Hydrogen bond 1.0 0.5 Octahedral unit 0.5 0 -0.5 -1.0 -1.5 -2.0 calculated Σ∆ Fig. 3 Correlation between Diamagnetic Anisotropy and Bond Direction.16) diamagnetic oxide may posses a finite ðÞDIA value to cause magnetic alignment, even if their concentrations of electron spins are negligibly low. It was expected in eq. (3) that BS was proportional to the square root of T for diamagnetic material with high purity; the relation was confirmed experimentally as shown in Fig. 2. This BS -T relation is expected to occur for the abovementioned oxides as well. BS value at T ¼ 10 K for a diamagnetic particle is expected to be less than one fifth of the intensity required for alignment at room temperature, Characteristics of Paramagnetic and Diamagnetic Anisotropy which Induce Magnetic Alignment of Micron-Sized Non-Ferromagnetic Particles 2597 since BS ðT ¼ 10 KÞ=BS ðT ¼ 300 KÞ ¼ ð10=300Þ1=2 according to eq. (3). Double oxides, such as forsterite, kaolinite, muscovite and phlogopite listed in Table 1, are composed of more than one kind of chemical bonds. Hence, as a second step, the measured ðÞDIA values of these oxides should be examined to be consistent with the sum of ðÞBD values of different chemical bonds. Numerical analysis is now in progress to examine the efficiency of the above-mentioned model on double oxides. 4. Effect of Paramagnetic Anisotropy on Magnetic Alignment. 30 (a) muscovite 20 10 0 1 2 3 -10 0.0 1.0 2.0 3.0 4.0 5.0 1.0 6.0 (x10-3) T-1/103K-1 Magnetic Anistropy ∆ µ /Hm2Kg-1 Magnetic Anisotropy 10-9 emu/g The -T relations of three muscovite crystals, which have been measured to determine the ðÞDIA value, are shown in Fig. 4(a).13) The ðÞDIA values were determined from the high temperature limits of the T- relations. The high temperature limits of were obtained form hmi-B measurements as well performed for two different kaolinite samples, as shown in Fig. 4(b).19) Kaolinite is a classical ceramic-material that is difficult to synthesize; it usually contain finite amount of paramagnetic impurity ions. Liquid ethanol was adopted as the suspending medium, and the measurement was performed throughout its liquid phase temperature-region between T ¼ 200 K and 340 K. Linear -1=T relations are obtained for the two samples, confirming the Curie-temperature dependence of . The quantita- (b) Kaolinite 1 2 0.5 0.0 1.0 2.0 3.0 4.0 5.0 6.0 T-1/103K-1 Fig. 4 Temperature dependences of paramagnetic anisotropy. tive amount of ðÞDIA and ðÞPARA components are obtained from Fig. 4 for the measured materials. The ðÞPARA components, following the Curie-law, were considerably large compared to the ðÞDIA components in the low temperature region for the three muscovite samples as well as for kaolinite-1. This tendency was seen in many of the -T measurements on bulk oxide crystals, in the course of determining ðÞDIA values from the high temperature limits.13) The large ðÞPARA value with respect to the ðÞDIA value indicate that the field intensity required to achieve magnetic alignment of micron-sized particles may be reduced considerably, when some amount of paramagnetic impurity ions were contained in the crystals. It is expected that the Bs value becomes nearly proportional to temperature in the low temperature region according to eq. (3), since ðÞDIA becomes small compared to ðÞPARA . Accordingly the reduction rate of Bs for the paramagnetic grains with the decrease of temperature becomes considerably large compared to that of pure diamagnetic grains. The T dependence of Bs is expected to occur for many of the non-ferromagnetic oxides, since the ðÞPARA components were actually observed to be considerably large compared to the ðÞDIA values in many of the measured oxides as mentioned above. The efficiency of magnetic alignment at room temperature can be enhanced when the contaminated paramagnetic ions is increased in a diamagnetic insulator. It is seen in Fig. 4(b) that the value of kaolinite-1, having a larger paramagnetic susceptibility (T¼300 K ¼ 190 106 emu/mol) compared to kaolinite-2 (T¼300 K ¼ 72 106 emu/mol), is always larger compared to the value of kaolinite-2. The positive correlation between and seen for kaolinite was observed in many of the oxides in the course of the bulk T measurements mentioned above. It is concluded that doping a finite amount of paramagnetic ions during material processing can enhance the practical efficiency of magnetic alignment of non-ferromagnetic materials in many cases. Paramagnetic anisotropy of muscovite and kaolinite are considered to derive mainly from Fe2þ ions, since iron had the highest concentration (order of several mass%) among the paramagnetic impurity ions for the two materials.19,20) The amount of ðÞPARA deriving from single Fe2þ ion can be evaluated from anisotropy of g-value, gð¼ g k g ?Þ, obtained by ESR measurements.21) g can be as large as 0.1 for Fe2þ ions occupying the octahedral [MO6 ] sites of the sheet-silicates. It is noted that g-values are more or less isotropic for Fe3þ ions occupying the above-mentioned sites. Exact Fe2þ /Fe3þ ratios of the sample are therefore required to evaluate the origin of ðÞPARA values in terms of abovementioned ESR analysis. It is deduced from eq. (3) that Bs is reduced to 65 mT at T ¼ 100 K for sphere-shaped grains of 1 mm in diameter assumed for muscovite-1, according to the -T relation in Fig. 4(a). The micron-sized insulators, however, do not disperse effectively in cryogenic liquid such as N2 or Ar. A medium that can disperse micron-sized crystals at low temperature is the He gas. Magnetic alignment of micronsized graphite grains dispersed in a He gas medium at room temperature was observed using an apparatus developed for this purpose.22) Modifications of the apparatus are now 2598 C. Uyeda, K. Tanaka, R. Takashima and M. Sakakibara carried out to examine the reduction of Bs value at low temperature conditions,23) which has been proposed for diamagnetic and paramagnetic materials in the present paper. Acknowledgements This work was partially supported by the Grant in Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan (grant no. 14350008), by the Grant in Aid for Scientific Research on Priority Area ‘‘Innovative utilization of strong magnetic fields’’ (Area 767, No 15085206) and also by the Ground Research Foundation of the Japan Space Forum. REFERENCES 1) for example, G. Malet and K. Dransfeld: Topics in App. Phys. 57 (1985) 144-230. 2) for example various paper appearing in, Proc. Intern. Symp. New Magneto-Science (Jpn. Sci. Tec. Corp. 1999). 3) for example, A. Yamagishi, T. Takeuchi, T. Higashi and M. Date: Physica B 164 (1990) 222-228. 4) for example, A. Yamagishi, E. Nagao and M. Date: J. Phys. Soc. Jpn. 53 (1984) 928-931. 5) C. Uyeda, T. Takeuchi, A. Yamagishi and M. Date: J. Phys. Soc. Jpn. 60 (1991) 3234-3237. 6) C. Uyeda, T. Takeuchi, A. Yamagishi, A. Tsuchiyama, T. Yamanaka and M. Date: Phys. Chem. Minerals 20 (1993) 369-374. 7) H. Chihara, C. Uyeda, A. Tsuchiyama and T. Yamanaka: Publ. Astrom. Saoc. Jpn. 50 (1998) 149-154. 8) S. Suzuki, Y. Sakka and K. Kitazawa: Adv. Eng. Mater. 3 (2001) 490493. 9) B. Michaud, E. Beaugon, a. Sulpice, R. Tournier and J. Claverie: Mater. Trans., JIM 41 (2000) 962-966. 10) R. Guputa: Diamagnetism, ‘‘Landort Bornstein’’ New Series II (1983) 445-451. 11) C. Uyeda: Jpn. J. App. Phys. 32 (1993) L268-L270. 12) C. Uyeda: Phys. Chem. Minerals 20 (1993) 77-81. 13) C. Uyeda, K. Ohtawa and K. Okita: Jpn. J. App. Phys. 39 (2000) L514L517. 14) C. Uyeda, K. Ohtawa, K. Okita and N. Uyeda: J. Phys. Soc. Jpn. 70 (2001) 889-892. 15) C. Uyeda, K. Ohtawa, K. Okita and N. Uyeda: Jpn. J. App. Phys. 39 (2000) L890-893. 16) C. Uyeda and K. Tanaka: J. Phys. Soc. Jpn. 72 (2003) 2334-2337. 17) P. Langevin and P. Curie: C. R. Acad. Sci. Paris 15 (1910) 331-349. 18) V. Twersky: J. Opt. Soc. Am. 60 (1969) 1084-1095. 19) C. Uyeda, M. Sakakibara and K. Tanaka: Phys. Chem. Minerals 30 (2003) 425-429. 20) C. Uyeda, K. Sakakibara and K. Tanaka: Jpn. J. App. Phys. 42 (2003) L581-L584. 21) O. Ballet and J. M. D. Coey: Phys. Chem. Minerals 8 (1982) 218-219. 22) C. Uyeda, T. Komatzu, K. Sakakibara and K. Tanaka: Astron. Astrophys. 400 (2003) 805-810. 23) C. Uyeda, K. Tanaka and R. Takashima: J. Phys. Soc. Jpn., in print. Appendix Diamagnetic anisotropy are considered to originate from spatial anisotropy of the electrons localized in a substance in general; the spatial anisotropy of electrons in the inorganic oxides are considerably small compared to those of the organic molecular-crystals. It is noted that the diamagnetic susceptibility of inorganic insulator is approximately equivalent to the simple summation of the susceptibility assigned to the individual orbital consisting the material, according to the advanced Pascal’s law.10) This treatment on diamagnetic susceptibility can be expressed by a 3-demensinal -tensor of a material, assuming that each orbital posses an uni-axial anisotropy with constant value BO ¼ BO k BO ?; BO k and BO ? are the susceptibilities parallel and perpendicular to the bond direction.0,0,13,16) The field-induced free energy UðBÞ of an orbital is described as, UðBÞ ¼ ð1=2ÞB2 fBO ? þBO ða2 2 þ b2 2 þ c2 2 Þg: ðA:1Þ The direction cosines of B is defined as (a; b; c), whereas (; ; ) denote the direction cosines for the bond direction; magnetic principle axes are identical to the x-, y- and zcoordinates in the above vector components. , and are calculated directly from atomic position data for various materials. The ðÞDIA values (per moll) between x-y, y-z and z-x axes of a material are expected to be proportional to 2 2 , 2 2 and 2 2 , respectively. The relationships between experimental and calculated anisotropy were obtained separately for the three chemical bonds. The results are compiled in Fig. 3; solid circles, solid squares and open symbols show the relationships for materials composed of the T-O bonds, the M-O bonds and the hydrogen bonds, respectively. Clear correlations, as expected in the above-mentioned model, are seen for the three types of chemical bonds. Regression lines for the three types of bonds are calculated as, O-H bond : ðÞDIA ¼ 2:2ðÞ þ 0:06½106 emu/mol ðf ¼ 0:99Þ; ðA:2aÞ T-O bond : ðÞDIA ¼ 0:63ðÞ þ 0:23½106 emu/mol ðf ¼ 0:89Þ; ðA:2bÞ M-O bond : ðÞDIA ¼ 0:19ðÞ þ 0:03½106 emu/mol ðf ¼ 0:93Þ; ðA:2cÞ where the correlation factor is described as f . denote the differences between 2 , 2 and 2 described in the above equations. The gradient of the above regression line correspond to the ðÞDIA value of a single bond. The large differences seen between the obtained BO values of the chemical bond probably derive from the differences of spatial density distribution of each chemical bonds. These BO values may serve as a basic data to construct a general theory on diamagnetic anisotropy of inorganic insulators.