Organic Chemistry II Laboratory
advertisement
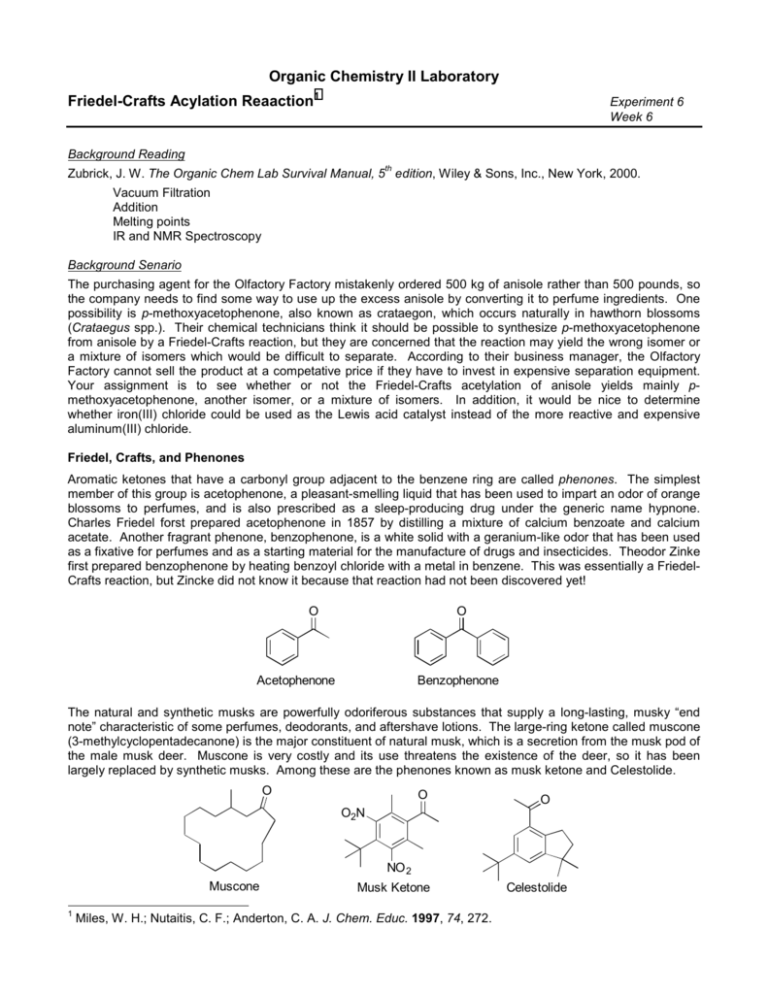
Organic Chemistry II Laboratory Friedel-Crafts Acylation Reaaction1 Experiment 6 Week 6 Background Reading th Zubrick, J. W. The Organic Chem Lab Survival Manual, 5 edition, Wiley & Sons, Inc., New York, 2000. Vacuum Filtration Addition Melting points IR and NMR Spectroscopy Background Senario The purchasing agent for the Olfactory Factory mistakenly ordered 500 kg of anisole rather than 500 pounds, so the company needs to find some way to use up the excess anisole by converting it to perfume ingredients. One possibility is p-methoxyacetophenone, also known as crataegon, which occurs naturally in hawthorn blossoms (Crataegus spp.). Their chemical technicians think it should be possible to synthesize p-methoxyacetophenone from anisole by a Friedel-Crafts reaction, but they are concerned that the reaction may yield the wrong isomer or a mixture of isomers which would be difficult to separate. According to their business manager, the Olfactory Factory cannot sell the product at a competative price if they have to invest in expensive separation equipment. Your assignment is to see whether or not the Friedel-Crafts acetylation of anisole yields mainly pmethoxyacetophenone, another isomer, or a mixture of isomers. In addition, it would be nice to determine whether iron(III) chloride could be used as the Lewis acid catalyst instead of the more reactive and expensive aluminum(III) chloride. Friedel, Crafts, and Phenones Aromatic ketones that have a carbonyl group adjacent to the benzene ring are called phenones. The simplest member of this group is acetophenone, a pleasant-smelling liquid that has been used to impart an odor of orange blossoms to perfumes, and is also prescribed as a sleep-producing drug under the generic name hypnone. Charles Friedel forst prepared acetophenone in 1857 by distilling a mixture of calcium benzoate and calcium acetate. Another fragrant phenone, benzophenone, is a white solid with a geranium-like odor that has been used as a fixative for perfumes and as a starting material for the manufacture of drugs and insecticides. Theodor Zinke first prepared benzophenone by heating benzoyl chloride with a metal in benzene. This was essentially a FriedelCrafts reaction, but Zincke did not know it because that reaction had not been discovered yet! O O Acetophenone Benzophenone The natural and synthetic musks are powerfully odoriferous substances that supply a long-lasting, musky “end note” characteristic of some perfumes, deodorants, and aftershave lotions. The large-ring ketone called muscone (3-methylcyclopentadecanone) is the major constituent of natural musk, which is a secretion from the musk pod of the male musk deer. Muscone is very costly and its use threatens the existence of the deer, so it has been largely replaced by synthetic musks. Among these are the phenones known as musk ketone and Celestolide. O O O O2N NO 2 Muscone 1 Musk Ketone Miles, W. H.; Nutaitis, C. F.; Anderton, C. A. J. Chem. Educ. 1997, 74, 272. Celestolide Musk ketone is prepared from m-xylene by two Friedel-Crafts reactions: alkylation with t-butyl chloride and acylation with acetyl chloride- followed by nitration of the aromatic ring (Figure 1). O O t-BuCl CH 3COCl AlCl 3 AlCl 3 O2N HNO 3 NO 2 m-Xylene Musk Ketone Figure 1. Synthesis of Musk Ketone Like benzophenone and the synthetic musks, most phenones can be prepared by a Friedel-Crafts reaction of an aromatic compound with an appropriate acylating agent. The Friedel-Crafts reaction might well have been named the “Zinke reaction” if Theodore Zinke had understood the significance of the experiment that failed. In 1869 Zinke tried to synthesize 3-phenyl propanoic acid by combining benzyl chloride and chloroacetic acid in the presence of metallic silver – a variation of the Wurtz reaction. While carrying out the reaction with benzene as the solvent, to his surprise, Zinke observed that a great deal of hydrogen chloride was evolved and that the major product was diphenylmethane instead of the expected carboxylic acid. O Ag O Cl + Cl OH OH benzene Zinke’s Attempted Synthesis Cl Ag + + HCl The Actual Reaction (“Zinke Reaction”) was a Friedel-Crafts Alkylation About four years later, a Frenchman named Charles Friedel was watching a student in Wurtz’s laboratory perform a “Zinke reaction” using (appropriately) powdered zinc as the catalyst. When the reaction suddenly became violent, Friedel helped the student separate the solution from the zainc powder, thinking that removing the catalyst would moderate the reaction. To the astonishment of both, the reaction was just as violent in the absence of zinc. Although there is no record of his thought processes after this event, Friedel must have recognized its significance. In 1877, he and his collaborator, an american named charles Mason Crafts, published a paper that marked the inception of the Friedel-Crafts reaction as one of the most important synthetic procedures in the history of organic chemistry. Friedel and Crafts’ basic discovery was a simple one – it was a chloride of the metal, and not the metal itself, that catalyzed the reaction of organic halides with aromatic compounds. In Zinke’s experiment, traces of silver chloride had formed during the reaction as a result of the oxidation of the metal. Friedel and Crafts found that anhydrous aluminum chloride was the most effective catalyst of those then available. It is still the catalyst of choice for most Friedel-Crafts reactions. Understanding the Experiment The Friedel-Crafts reaction is not a single reaction type, although the term has often been applied to alkylations and acylations of aromatic compounds using aluminum chloride (or another Lewis acid catalyst) and a suitable alkylating or acylating agent. A typical Friedel-Crafts acylation reaction uses a carboxylic acid chloride as the acylating agent and anhydrous aluminum chloride as the catalyst. The aluminum chloride, a Lewis acid, removes a leaving group from the acylating agent, forming an acylium ion, as illustrated in step 1 of the following mechanism (Figure 2) for the reaction of benzene with an acyl chloride. The acylium ion then is then attacked by one of the π-bonds of benzene to form an arenium ion (step 2), which loses a proton to re-establish the aromatic ring and regenerate the catalyst (step 3). When the acyl group is acetyl (CH3CO), acetic anhydride is often used as the acylating agent rather than acetyl chloride. The anhydride is safer to work with and it usually provides better yields and a simpler work-up. More catalyst is needed with acetic anhydride however, because some of the aluminum chloride complexes with the acetic acid produced during the reaction, making it ineffective as a catalyst. As a rule, 2-3 moles of AlCl3 are used per mole of acetic anhydride. O O + AlCl4 + AlCl3 Step 1 R R acylium ion Cl O H O R Step 2 R arenium ion O H O R R Step 3 + H AlCl 4 HCl + AlCl 3 Figure 2. Mecahnism for the Friedel-Crafts Acylation of Benzene In this experiment you will use acetic anhydride as the acylating agent and dichloromethane as the reaction solvent. The reaction is highly exothermic so it will be carried out by slowly adding the acetic anhydride to the other reactants, then heating to reflux to complete the reaction. Pouring the product into ice water will decompose the Lewis acid complex of the product and transfer inorganic salts into the aqueous phase. The product can then be recovered by evaporating the organic solvent and distilling the residue. On cooling to room temperature, the product should crystallize. Its infrared and NMR spectrum as well as a GC-MS can then be obtained. In principle, acylation of a monosubstutited benzene can yield any or all of three different disubstuted products. From the melting point and spectra of your product, you should be able to determine whether it is predominantly a single compound or a mixture of isomers, and if it is a single compound, establish its identity. Disubstituted benzenescan be distinguished by the location of their out-of-plane C-H bending bands in their IT spectrum. -1 These bands occur at frequencies (expressed in wavenumbers) below 850 cm . The frequency of such a band decreases with the number of adjacent hydrogens on the ring, as shown in Table 1. Thus a para-disubstituted -1 benzene, with two sets of two adjacent hydrogens, should show an absorption band in the 840-810 cm region; -1 meta compounds with three adjacent ring hydrogens absorb in the 810-750 cm region; and ortho compounds -1 with four adjacent ring hydrogens, absorb in the 770-735 cm region. Absorption by the isolated hydrogen of a meta compound is usually very weak, and its frequency may vary. Monosubstituted and meta-disubstituted -1 benzenes have an additional band in the 710-680 cm region, which arises frfom a vibration involving the entire benzene ring. No. of Adjacent Hydrogens Frequency Range, cm 1 900-860 (weak) 2 840-810 3 810-750 4 770-735 5 770-730 -1 Table 1. Frequencies of C-H Out-of-Plane Bending Bands in Aromatic Hydrocarbons O O O MeO MeO OMe ortho-Methoxyacetophenone meta-Methoxyacetophenone para-Methoxyacetophenone Possible Products from the Friedel-Crafts Acylation of Anisole Your product will be an ether as well as a ketone, so its infrared spectrum will contain bands characteristic of both -1 functional groups. The carbonyl band of a phenone generally appears in the 1685-1665 cm region, and a weak carbonyl overtone band may be observed at twice the frequency of the fundamental band. Aryl alkyl ethers -1 display an asymmetrical C-O-C stretching band at 1275-1200 cm and a symmetrical C-O-C band near 1075-1 1020 cm . The NMR spectrum of your product will also give information regarding the number of adjacent hydrogens on the aromatic ring. The 1,4-disubstituted product (para) would have the simplest spectrum. the symmetry of the molecule results in two doublets in the aromatic region of the NMR spectrum. The ortho product would have a more complex coupling pattern in the aromatic region and the meta product could be distinguished by the singlet due to the lone hydrogen between the substituent groups. Experimental Section Reaction: Wear gloves and eye protection! work under a hood. Anhydrous aluminum chloride is deactivated by water, so protect it from atmospheric moisture and be sure to dry your glassware thoroughly. Weigh 22.0 mmol of powdered aluminum chloride and add it to a 25 mL round bottom flask. Quickly but carefully add 10 mL of dichloromethane and swirl to mix. Add a stirbar, and 10 mmol of anisole then attach the flask to a water cooled condensor. Be sure to clamp the condensor securly and make sure the condensor and flask are snug. Mix 1.0 mL of acetic anhydride with 3-5 mL of dichloromethane in a small flask. SLOWLY, add the acetic anhydride solution in small portions through the top of the condensor. The reaction is exothermic and it is alright for the reaction to boil but do not let it boil vigorously. When the reaction is complete, heat the reaction to a gentle reflux for 30 minutes. Separation and Purification: Pour the warm reaction mixture slowly with vigorous stirring onto about 10 g of crushed ice in a large (250 mL) beaker. Use a little ice water to rinse any residue out of the reaction flask into the beaker. Separate the dichloromethane layer in a separatory funnel and wash it with 5 mL of 3 M NaOH solution followed by 5 mL of saturated aqueous NaCl solution. Dry the dichloromethane layer over MgSO4. Use this dried solution to prepare a sample for GC-MS analysis. Decant the dichloromethane solution away from the solids into a 100 mL round bottom flask and rinse the solids with a small volume of fresh dichloromethane. Decant thid second portion of dichloromethane into the round bottom flask. Use the Rotovap to concentrate the dichloromethane solution to a volume of about 3-4 mL. Transfer the concentrated solution back into 10 mL round bottom flask and set-up the apparatus for a simple microscale distillation. Gently heat to collect the remaining dichloromethane then increase the heat to distill the o product. Collect everything that distill over at around 240 C. Stop heating if the contents of the flask start smoking. If the distillate solidifies in the reciever, melt it by gentle heating with a heat gun. Transfer the product to a pre-weighed vial. Get the mass of product, then collect the IR spectrum, prepare a new GC-MS sample, and collect an NMR of the product. Questions: 1. If your product was a single compound, explain why it was that compound rather than another isomer. 2. Write a mechanism for the Friedel-Crafts reaction of anisole with acetic anhydride. 3. Write an equation for the reaction of aluminum chloride with a large excess of water. 4. Write equations for one or more reactions that would account for the production of HCl during the acylation reaction. 5. Explain why Friedel-Crafts reactions are usually carried out by adding the alkylating or acylating agent to the aromatic compound rather than vice versa.