Supporting Information
advertisement

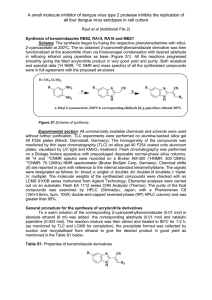
Copyright WILEY‐VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2011. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201102726 Emeraldicene as an Acceptor Moiety: Balanced-Mobility, Ambipolar, Organic Thin-Film Transistors Ali Reza Mohebbi , Jonathan Yuen , Jian Fan , Cedric Munoz , Ming feng Wang , Rahau S. Shirazi , Jason Seifter , and Fred Wudl * Submitted to Supporting Information Balanced-Mobility Ambipolar Organic Thin-Film Transistors Based on the Emeraldicene Acceptor Moiety Ali Reza Mohebbi, Jonathan Yuen, Jian Fan, Cedric Munoz, Ming feng Wang, Rahau S. Shirazi, Jason Seifter, and Fred Wudl* 1. Instrumentation and materials All reactions were carried out under nitrogen (Schlenk conditions). Unless otherwise noted, all chemicals were obtained from commercial sources and used as received without further purification. UV-vis-NIR spectra were recorded with Agilent Technologies G1103A. NMR spectra were recorded on a Bruker DRX-500 and Varian-NMR 600 spectrometers in CDCl3 using TMS as internal standard. Mass spectrometry was performed by UC Santa Barbara Mass Spectrometry Laboratory. Differential scanning calorimetry (DSC) was carried out under nitrogen on a TA Instrument DSC Q10 instrument with a scan rate of 5 °C/min. Thermal gravimetric analysis (TGA) was carried out using a TA Instrument TGA Q50 instrument with a heating rate of 10°C/min. In plane and out of plane X-ray diffraction (XRD) patterns was obtained with a SmartLab Rigaku X-ray Diffractometer system using Cu Ka source (λ = 1.5418 Å) in air. The thin film samples (~10 nm) for XRD measurements were on the OTS-modified SiO2/Si substrate by drop-casting a polymer solution in chlorobenzene (2 mg mL-1), optionally followed by thermal annealing at 80 °C, 160 °C, 240 °C and 320 °C for 15 min in nitrogen. Gel permeation chromatography (GPC) measurements were performed on a Waters 2615 Separations Module using chloroform + 0.25 % TEA as eluent and polystyrene as standards. 2,5-Dihydro-1,4-dioxo-3,6-dithienylpyrrolo[3,4-c]-pyrrole Emeraldicene (EMD) (2)[2] were synthesized according to the reported methods. 1 (1)[1] and Submitted to 2. Synthesis of 3,6-bis-(5-(4,4,5,5-tetramethyl-1,3,2-dioxabrolan-2-yl)thiophen-2-yl)- N,N′-bis((octyldodecyl)-1,4-dioxo-pyrrolo[3,4-c]pyrrole (2): To a solution of DPP (1) (1.5 g, 1.74 mmol) and 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxoborolane (1.3 g, 6.98 mmol) in THF (30 mL) under N2 at -20°C was added dropwise LDA (2M in hexane/THF, 2.6 mL, 5.2 mmol) over 10 minutes. The resulting solution was stirred for 1 hour at 0°C and then quenched with 1 M HCl. The product was extracted by DCM and washed with water and dried over MgSO4. After evaporation of the solvent the residue is dissolved in small amount of DCM and then slowly added to 200 mol of heavily stirred acetone. The precipitate was collected by filtration, washed with acetone and dried under vacuum afford 0.85 g of powder (44%). 1H NMR (CDCl3, 600 MHz): δ ppm 8.90 (d, J = 3.6 Hz, 2H), 7.70 (d, J = 3.6 Hz, 2H), 4.04 (d, J = 8.4 Hz, 4H), 1.89 (m, 2H), 1.36 (m, 24H), 1.24 (br, 68H), 0.85 (m, 12H); 13 C NMR (CDCl3, 600 MHz): δ ppm 161.76, 140.53, 137.66, 136.13, 135.68, 108.75, 84.59, 46.28, 37.79, 31.93, 31.88, 31.31, 31.29, 30.03, 30.02, 29.64, 29.59, 29.53, 29.35, 29.29, 26.36, 26.34, 24.78, 22.69, 22.67, 14.12, 14.12; MS (FD+): m/z (%): 1112.7 (100). 2 Submitted to 3. Synthesis of dibromo-EMD (4): To a solution of EMD (3)(1.12 g, 2.0 mmol) in THF (100 mL) at room temperature was added NBS (0.82 g, 4.6 mmol). The resulting solution was stirred for 2 hours and then quenched with water. The precipitate was collected by filtration, washed with acetone and dried under vacuum afford 1.23 g of powder (86%). 1H NMR (CDCl3, 500 MHz): δ ppm 7.70 (d, 2H), 7.60 (d, 2H), 7.48 (d, 2H), 2.84 (d, 4H), 1.92 (m, 2H), 1.40-1.50 (br, 16H), 0.97 (t, 6H), 0.90 (t, 6H); HRMS (C38H40Br2S2, TOF MS FI+): calculated 718.0938 [M]+, found 718.0948. 4. Additional Figures Figure S1. 1H NMR (600 MHz) spectrum of PDTDPP-alt-EMD in CDCl3. 3 Submitted to Figure S2. GPC elution curves and molecular weights of PDTDPP-alt-EMD. Figure S3. Cyclic voltammograms of the PDTDPP-alt-EMD in chlorobenzene, 0.1 TBBF4. Scan rate = 100 mV/s. Platinum as working electrode, wave at ca 0.7 eV is the internal Fc/Fc+ reference. 4 Submitted to Figure S4. The first and second DSC scan curves of PDTDPP-alt-EMD with a scan rate of 5 °C/min under nitrogen Figure S5. The thermogravimetric analysis (TGA) profile of PDTDPP-alt-EMD with a heating rate of 10°C/min in air. 5 Submitted to X 15 (enlarged picture) Figure S6. XRD data obtain from drop casted PDTDPP-alt-EMD thin films on OTS modified SiO2/Si substrates annealed at different temperatures. [1] Li, Y. U.S. Patent Application 2009/65766 A1, 2009. [2] A. R. Mohebbi, F. Wudl, Chem. Eur. J. 2011, 17, 2642. 6