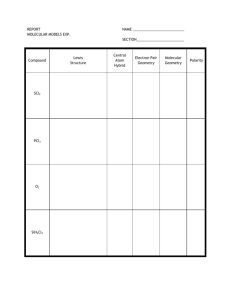
Question of the Week 13 (Lewis Structures) Print a copy of this
advertisement

Question of the Week 13 (Lewis Structures) Print a copy of this assignment from my website. 1. Complete the following table. Read the instructions!! Lewis Structure Draw the 3-D shape (molecular geometry) and name both the Identify the total number of valence electrons and draw electron and molecular geometry. the electron dot structures. Draw resonance structures if appropriate. a. AsF3 Number of Valence e- = ___ Is this species polar, non-polar or an ion? Explain. Identify all of the IFA(s) present unless the species is an ion. Electron Geometry _________________________ Molecular Geometry _________________________ b. NO2Number of Valence e- = ___ Electron Geometry _________________________ Molecular Geometry _________________________ c. CHF3 Number of Valence e- = ___ Electron Geometry _________________________ Molecular Geometry _________________________ d. NH3 Number of Valence e- = ___ Electron Geometry _________________________ Molecular Geometry _________________________ AP Chemistry 2006 (Baverstock) 26 Lewis Structure Identify the total number of valence electrons and draw the electron dot structures. Draw the 3-D shape (molecular geometry) and name both the electron and molecular geometry. Draw resonance structures if appropriate. Is this species polar, nonpolar or an ion? Explain. Identify all of the IFA(s) present unless the species is an ion. e. BrCl4+ Number of Valence e- = ___ Electron Geometry _________________________ Molecular Geometry _________________________ f. XeOCl4 Number of Valence e- = ___ Electron Geometry _________________________ Molecular Geometry _________________________ 2. a. Draw the structural diagram and electron dot structure for: Propanoic acid. (2) Structural Diagram Electron Dot Structure b. In your structural diagram number each carbon (from 1 to 3) and oxygen (1 to 2). Name both the electron geometry and molecular geometry for each. (5) Atom Electron Geometry Molecular Geometry Carbon 1 Carbon 2 Carbon 3 Oxygen 1 Oxygen 2 3. a. Consider the structural diagram for the following molecule. Complete the electron dot structure. (1) Structural Diagram Electron Dot Structure O 4 1 NH2 3 2 1 OH 1 b. Name the molecular geometry for each atom numbered in the structural diagram. (5) Atom Molecular Geometry Atom Molecular Geometry Carbon 1 Oxygen 1 Carbon 2 Nitrogen 1 Carbon 3 Carbon 4 AP Chemistry 2006 (Baverstock) 27