Questions 1-5
advertisement
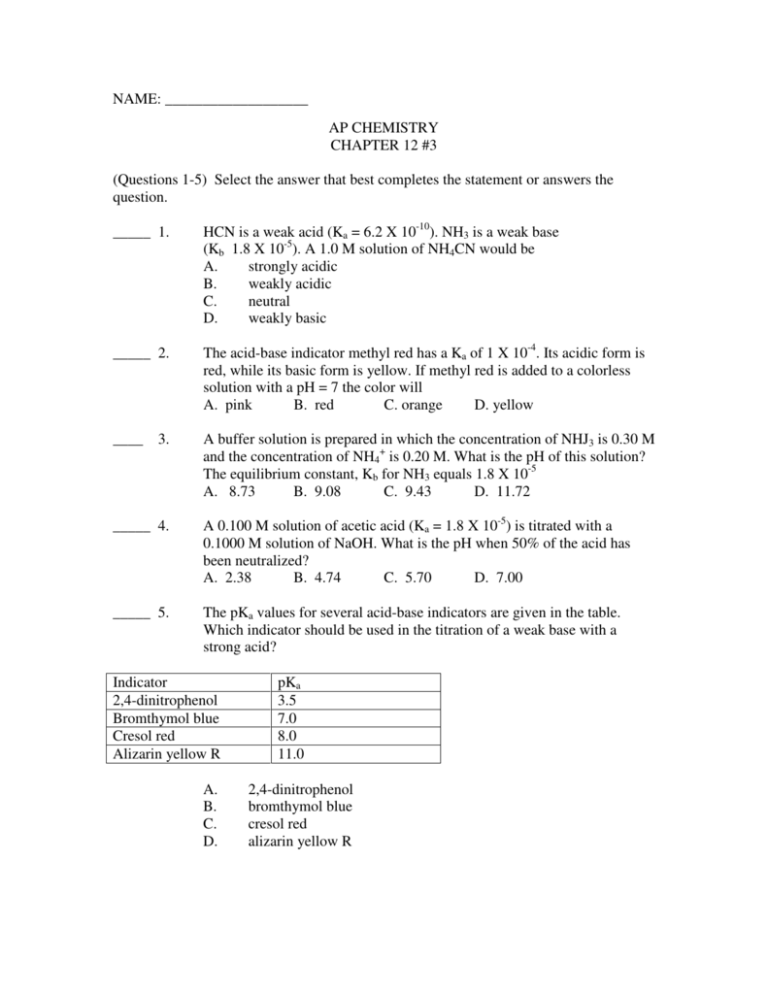
NAME: ___________________ AP CHEMISTRY CHAPTER 12 #3 (Questions 1-5) Select the answer that best completes the statement or answers the question. _____ 1. HCN is a weak acid (Ka = 6.2 X 10-10). NH3 is a weak base (Kb 1.8 X 10-5). A 1.0 M solution of NH4CN would be A. strongly acidic B. weakly acidic C. neutral D. weakly basic _____ 2. The acid-base indicator methyl red has a Ka of 1 X 10-4. Its acidic form is red, while its basic form is yellow. If methyl red is added to a colorless solution with a pH = 7 the color will A. pink B. red C. orange D. yellow ____ A buffer solution is prepared in which the concentration of NHJ3 is 0.30 M and the concentration of NH4+ is 0.20 M. What is the pH of this solution? The equilibrium constant, Kb for NH3 equals 1.8 X 10-5 A. 8.73 B. 9.08 C. 9.43 D. 11.72 3. _____ 4. A 0.100 M solution of acetic acid (Ka = 1.8 X 10-5) is titrated with a 0.1000 M solution of NaOH. What is the pH when 50% of the acid has been neutralized? A. 2.38 B. 4.74 C. 5.70 D. 7.00 _____ 5. The pKa values for several acid-base indicators are given in the table. Which indicator should be used in the titration of a weak base with a strong acid? Indicator 2,4-dinitrophenol Bromthymol blue Cresol red Alizarin yellow R A. B. C. D. pKa 3.5 7.0 8.0 11.0 2,4-dinitrophenol bromthymol blue cresol red alizarin yellow R _____ 6. Which one of the following is the solubility product constant for Mn(OH)2? A. Ksp = [Mn2+][OH-]2 B. Ksp = [Mn2+][2OH-] C. Ksp = [Mn2+]2[OH-]2 D. Ksp = [Mn2+]2[OH-] E. Ksp = [Mn2+]2[2OH-]2 _____ 7. Which expression best describes the relationship between solubility product, Ksp, and the solubility, x, of MgF2? A. Ksp = 2x B. Ksp = x2 C. Ksp = 2x2 D. Ksp = 4x2 E. Ksp = 4x3 Answer the following questions. 8. Consider the titration of 50.0 mL of 0.200 M HONH2 (Kb = 1.1 X 10-8) by 0.100 M HNO3. Calculate the pH of the resulting solution after the following volumes of HNO3 has been added. a. How many mL of HNO3 are required to reach the equivalence point? b. 0.00 mL c. 25.0 mL d. 50.0 mL e. 75.0 mL f. 100.0 mL g. 125.0 mL h. Sketch the titration curve for the data above (be certain to label the axes and the equivalence point). 9. Calculate the Ksp value for Ca3(PO4)3 if the solubility is 1.6 X 10-7 mol/L. 10. What is the concentration of a saturated silver acetate solution? Ksp(AgC2H3O2) = 1.94 x 10-3. 11. What is the concentration of a saturated lead chloride solution? Ksp(PbCl2) = 1.17 x 10-5. 12. I have discovered a new chemical compound with the formula A2B. If a saturated solution of A2B has a concentration of 4.35 x 10-4 M, what is the solubility product constant for A2B? 13. Answer the following questions that relate to solubility of salts lead and barium. (a) A saturated solution is prepared by adding excess PbI2(s) to distilled water to form 1.0 L of solution at 25oC. The concentration of Pb2+(aq) in the saturated solution is found to be 1.3 X 10-3 M. The chemical equation for the dissolution of PbI2(s) in water is shown below. PbI2(s) <-------> Pb2+(aq) + 2I-(aq) (i) Write the equilibrium-constant expression for the equation. (ii) Calculate the molar concentration of I-(aq) in the solution. (iii) Calculate the value of the equilibrium constant, Ksp. (b) A saturated solution is prepared by adding PbI2(s) to distilled water to form 2.0 L of solution at 25oC. What are the molar concentrations of Pb2+(aq) and I-(aq) in the solution? Justify your answer. (c) Solid NaI is added to a saturated solution of PbI2 at 25oC. Assuming that the volume of the solution does not change, does the molar concentration of Pb2+(aq) in the solution increase, decrease, or remain the same? Justify your answer. (d) The value of Ksp for the salt BaCrO4 is 1.2 X 10-10. When a 500. mL sample of 8.2 X 10-6 M Ba(NO3)2 is added to 500. mL of 8.2 X 10-6 M Na2CrO4, no precipitate is observed. (i) Assuming that volumes are additive, calculate the molar concentration of Ba2+(aq) and CrO42-(aq) in the 1.00 L solution. (ii) Use the molar concentrations of Ba2+(aq) ions and CrO42-(aq) ions as determined above to show why a precipitate does not form. You must include a calculation as part of your answer.








