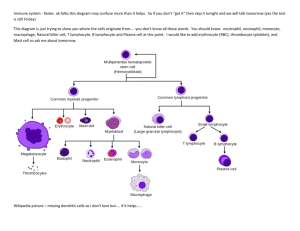
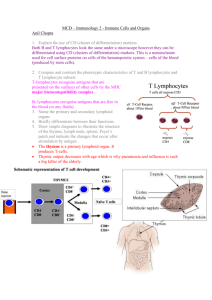
Introduction to Immunology
advertisement

MCD Immunology Alexandra Burke-Smith 1. Introduction to Immunology Professor Charles Bangham (c.bangham@imperial.ac.uk) 1. Explain the importance of immunology for human health. The immune system What happens when it goes wrong? persistent or fatal infections allergy autoimmune disease transplant rejection What is it for? To identify and eliminate harmful “non-self” microorganisms and harmful substances such as toxins, by distinguishing ‘self’ from ‘non-self’ proteins or by identifying ‘danger’ signals (e.g. from inflammation) The immune system has to strike a balance between clearing the pathogen and causing accidental damage to the host (immunopathology). Basic Principles The innate immune system works rapidly (within minutes) and has broad specificity The adaptive immune system takes longer (days) and has exisite specificity Generation Times and Evolution Bacteria- minutes Viruses- hours Host- years The pathogen replicates and hence evolves millions of times faster than the host, therefore the host relies on a flexible and rapid immune response Out most polymorphic (variable) genes, such as HLA and KIR, are those that control the immune system, and these have been selected for by infectious diseases 2. Outline the basic principles of immune responses and the timescales in which they occur. IFN: Interferon (innate immunity) NK: Natural Killer cells (innate immunity) CTL: Cytotoxic T lymphocytes (acquired immunity) 1 MCD Immunology Innate Immunity Depends of pre-formed cells and molecules Fast (starts in mins/hrs) Limited specifity- pathogen associated, i.e. recognition of danger signals Cells involved: - Neutrophils (PMN) - Macrophages - Natural killer (NK) cells Soluble factors involved - Acute-phase proteins - Cytokines - Complement Stimulates the acquired immune response Alexandra Burke-Smith Acquired immunity Depends on clonal selection, i.e. growth of T/B cells, release of antibodies selected for antigen specifity Slow (starts in days) Highly specific to foreign proteins, i.e. antigens Cells involved : - T lymphocytes - B lymphocytes - Dendritic cells - Eosinophils - Basophils/mast cells Soluble factors involved - Antibodies Innate Immunity - Anatomical barriers Skin as a mechanical barrier- keeps out 95% of household germs while IN TACT Mucus membrane in respiratory and GI tract traps microbes Cilial propulsion on epithelia cleans lungs of invading microorganisms - Physiological barriers Low PH Secretion of lysozyme, e.g. in tears Interferons Antimicrobial peptides Complement; responsible for lysing microorganisms Acute-phase inflammatory response An innate response to tissue damage Rise in body temperature, i.e. the fever response This is followed by increased production of a number of proteins (acute-phase proteins), mainly by the liver. Includes: - C-reactive protein - Serum amyloid protein - Mannan-binding lectin C-reactive protein and serum amyloid protein bind to molecules found on the cell wall of some bacteria and fungi- pattern recognition Mannan-binding lectin binds to mannose sugar molecules which are not often found on mammalian cells These molecules are non-specific, but direct phagocytes e.g. macrophages to identify and ingest the infectious agent Cytokines Small proteins that carry messages from one cell to another E.g. to stimulate activation or proliferation of lymphocytes “kick-start”acquired immune response Send messages to other cells, e.g. to kill or secrete 2 MCD Immunology Alexandra Burke-Smith Cells of the innate immune system Granular leukocytes Natural Killer (NK) cells - Identify and kill virus-infected and tumour cells - Complex recognition system- recognise HLA molecule of virus infected cell or tumour, and kill them Macrophages - Mononuclear phagocytes - To main functions: 1. “garbage disposal” - 2. Present foreign cells to immune system Granulocytes Neutrophils Poluymorphonuclear neutrophils (PMN): multilobed nucleus 50-70% of circulating WBC Phagocytic Eosinophils Bi-lobed nucleus 1-3% of circulating WBH Required for immune response to parasites, helminths and allergic responses Basophils <1% of circulating WBC Not phagocytic- release granules containing histamines, serotonin, prostaglandins Important in Th2 responseskick starting acquired immune reponse 3. Define the terms antigen, antibody, B lymphocyte, T lymphocyte, primary and secondary immune responses, and innate and acquired immunity. Acquired/Adaptive Immunity Characteristics Antigen specific Can form memory Requires priming- specific cells help to start the acquired immune response Cellular Immunity: T and B cells Humoral immunity: antibodies Antigens are glycoprotein molecules which react with antibodies or T cells. However not all antigens can induce an immune response in the host: those that can are termed immunogens Antibody molecules can be found in the blood stream and the body fluids and bind specifically to particular molecules termed antigens. They are the acquired component of the humoral immune response.The most basic antibody molecule is bivalent- with two antigen binding sites. Immunoglobulins IgG - 75% of our serum - Crosses placenta, therefore important in protecting newborns - Long serum hal-life - Part of secondary immune respons 3 MCD Immunology Alexandra Burke-Smith - Bivalent- two identical antigen binding sites IgM 10% of total serum Ig Complex of 5 linked bivalent monomeric antibodies, therefore 10 identical binding sites- multivalent Star-like shape Important in primary immune response Slightly lower affinity to antigens compared to IgG, which is compensated for by number of binding sites - IgA 2 basic monomers; dimer with secretory piece Found in body secretions, e.g. mucus membranes in GI tract Contains a secretory component which protects it from digestive enzymes - IgE Involved in allergic response and the response to helminths Binds to basophils and mast cells Triggers release of histamines IgD - Complete function not known A particularly antibody ‘recognizes’ an antigen because that antibody’s binding site it complementary to the EPIPTOPE (region approx 6 amino acids long) on the antigen. This forms the basis of the specificity of antigen recognition. How does an antibody kill a virus? Four important mechanisms: 1. Binds to the virus and prevents attachment to the cell 2. Opsonisation: virus-antibody complex is recognised and phagocytosed by macrophage 3. Complement- mediated lysis of enveloped viruses: cascade of enzymes in the blood which leads to the destruction of cell membranes, and the destruction of the viral envelope 4. Antibody-dependant cell-mediated cytotoxicity (ADCC) mediated by NK-like cells (see earlier for explanation) Cells of the acquired immune system Lymphocytes Agranular leukocytes 20-40% of the circulating WBC 99% of the cells in lymphatic circulation T (thymus-derived) cells - Helper T cells: recognize antigen, help B cells to make antibodies and T cells to kill - Cytotoxic T cells: poisonous to cells,kill cells infected by viruses and intracellular bacteria B (bone marrow-derived) cells - Make antibodies - Have insoluble antigen-binding receptor on its surface. In fact have multiple clones of this receptor; monoclonal antibodies NK (natural killer) cells - See earlier in notes Each subset has distinct cell-surface molecules, e.g. CD4 on helper T-cell which is the receptor for HIV molecules Lymphocyte precursors are produced in the haematopoietic tissue in the bone marrow 4 MCD Immunology Alexandra Burke-Smith T cells are then transported to the thymus, where they undergo THYMAL EDUCATION. Here 95-99% get destroyed as they have the potential to recognise host cells 4. Outline the role of clonal selection in immune responses. Lymphocyte antigen receptors B cell antigen receptor is a membrane-bound antibody, i.e. surface immunoglobulin which binds intact antigens; recognises surface of protein, therefore antigen must be in native conformation Expressed on the T cell surface are 2 protein chains (alpha and beta) which together make the t cell antigen receptor (TCR). This binds to digested antigen fragments. Each antigen receptor binds to an epitope on a different antigen, and is unique to a cell. There are many copies of the receptor on the cell surface The T-cell antigen receptor (TCR) Recognizes complex of antigen peptide and HLA (MHC) molecule HLA (Human leukocyte antigen) binds to little fragments of the pathogen, transports them to the surface so they can be recognized, e.g. so a virus cannot hide inside a host cell. Combination of short peptide from microorganism + HLA = recognition by TCR MHC denotes the Major Histocompatibility Complex (also known as HLA) Generation of clonal diversity in lymphocytes During B and T cell development, random genetic recombinations occur within each cell among multiple copies of immunoglobulin genes (B cells) or TCR genes (T cells). There are parallel genes, but they undergo random splicing and recombination which leads to a large repertoire of antigen receptors These processes generate the diversity of clones of lymphocytes: each clone is specific to a different antigen. Primary Immune Response: clonal selection A typical antigen is recognized by 1 in ~105 naive T cells 98% of T cells are in the lymph circulation and organs; 2% in blood. Antigen binds to surface receptor on the B cell (Ig) or the T cell (TCR) and causes selective expansion of that clone. The receptors which bind with highest affinity to the antigen are selected for, outcompete the other receptors , proliferate and survive to form effector lymphocytes What happens when the antigen is removed? Most lymphocytes that have proliferated recently will die after fulfilling their function (involves 2 or 3 mechanisms) Some survive as memory cells. These are epigenetically modified so that next time the host is infected, the frequency of the receptors will increase. How does the immune response clear a pathogen? 5 MCD Immunology Alexandra Burke-Smith Cytotoxic T lymphocytes (CTLs) kill cells infected by viruses or intracellular bacteria. It recognizes antigen peptide and HLA complex, releases granules of enzymes including proteases which digest DNA. The cell is therefore destroyed- APOPTOSIS Antibodies bind to pathogens: the complex is destroyed or ingested by cells. 5. Understand the role of the physical organization of the immune system in its function. How does a T cell meet its antigen? Antigens are taken up by specialized ANTIGEN-PRESENTING CELLS (class of cells which are capable of taking up particles, ingesting them and presenting proteins on their surface) transported from the tissues into secondary lymphoid organs, where they meet T cells initiate the acquired immune response Antigen-presenting cells include B lymphocytes, macrophages and dendritic cells (which are most efficient) Lymphoid Organs Organized tissue in which lymphocytes interact with non lymphoid cells Sites of initiation and maturation of adaptive immune responses. Primary lymphoid organs produce the lymphocytes, e.g. bone marrow and thymus Secondary lymphoid organs include lymph nodes, spleen, and mucosa-associated lymphoid tissue (MALT) Lymphocytes and antigen-presenting cells circulate continuously blood and lymphatic vessels from tissues via lymph nodes/spleen into the blood T cells spend around 1-2 hours in the blood, but the rest of the day in the lymph The tissues are patrolled by lymphocytes, antibodies and antigen-presenting cells. For example, the skin contains lymphatic vessels that drain into local lymph nodes. Gut lymphoid tissue controls responses in the intestinal tract. Antigens present in the blood are taken to the spleen. 6 MCD Immunology Alexandra Burke-Smith Definitions Lymphocytes are mononuclear cells which are part of the leukocyte (white blood) cell lineage. They are subdivided into B (Bone marrow-derived) and T (Thymus-derived) lymphocytes. Lymphocytes express antigen receptors on their surface to enable recognition of a specific antigen Naïve lymphocytes have never encountered the antigen to which their cell surface receptor is specific and thus have never responded to it. Memory lymphocytes are the products of an immune response, enabling the specificity of their specific receptor to remain in the pool of lymphocytes in the body. Innate immunity An early phase of the response of the body to possible pathogens, characterized by a variety of nonspecific mechanisms (e.g. barriers, acids or enzymes in secretions) and also molecules and receptors on cells which are Pattern Recognition Molecules which recognize repeating patterns of molecular structure found on the surface of microorganisms. The innate immune response does not generate memory. Adaptive immunity is the response of antigen-specific lymphocytes to antigen, and includes the development of immunological memory. Adaptive responses can increase in magnitude on repeated exposure to the potential pathogen and the products of these responses are specific for the potential pathogen. Also known as Specific Immunity or Acquired Immunity. Active Immunity is the induction of an immune response by the introduction of antigen. Passive Immunity is immunity gained without antigen induction i.e. by transfer of antibody or immune serum into a naïve recipient. Primary Response is the response made by naïve lymphocytes when they first encounter their specific antigen. Secondary Response is the response made by memory lymphocytes when they re-encounter the specific antigen. T cells originate in the thymus. They recognize antigen presented at the cell surface by MHC/HLA molecules. Surface markers on T cells are CD3, CD4 & CD8 B cells originate in the bone marrow. They recognize free antigen in the body fluids. Surface markers associated with B cells are CD19, surface immunoglobulin class II MHC 7 MCD Immunology Alexandra Burke-Smith 2. Immune Cells and Organs Dr Keith Gould (k.gould@imperial.ac.uk) Primary lymphoid organs (thymus & bone marrow) for production of lymphocytes Secondary lymphoid organs help antigen to come into contact with lymphocytes expressing appropriate specific receptors Lymphocyte numbers are carefully regulated, and they recirculate T cells express CD3, and recognise processed antigen presented by MHC molecules B cells express CD19 and CD20, and recognise intact, free antigen Important APC are dendritic cells, B cells, and macrophages 1. Name the primary and secondary lymphoid organs and briefly differentiate between their functions. Primary lymphoid organs: organs where lymphopoeisis occurs, i.e. where lymphocytes are produced, including the bone morrow and thymus to produce T and B lymphocytes. Secondary lymphoid organs: where lymphocytes can interact with antigen and with other lymphocytes, including spleen, lymph nodes, mucosal associated lymphoid tissues (MALT) 2. Draw simple diagrams to illustrate the structure of the thymus, lymph node, spleen, Peyer’s patch and indicate the changes that occur after stimulation by antigen. Primary lymphoid Organs: Bone Marrow - Site of haematopoesis, i.e. generation of blood cells - In an embryo, this happens in amniotic sac - In foetus, occurs in all bones, liver and spleen. Marrow is also very cellular - In adults, this occurs mostly in flat bones, vertebrae, Iliac bones, Ribs and the ends of long limbs Thymus - Where maturity of T-cells occurs - Bi- lobed - Medulla and cortex regions - No change during immune response to antigens, continuous development of T cells 8 MCD Immunology - Alexandra Burke-Smith Hassalls’ corpuscle secretes soluble factors, and is important in regulatory T cells Secondary Lymphoid Organs - - Lymphatic System Fluid drained from between tissue cells absorbed into lymph 2 to 3 litres of lymph are returned to the blood each day (via superior vena cava) In the process of draining, lymph can “capture” pathogens Fluid passes through lymph nodes which survey for pathogens LYMPH NODES Kidney shaped organs > 1cm During immune response, swell in size Fluid enters through AFFERENT vessel Fluid leaves via EFFERENT vessel Lymph perculates through all lymphocytes before leaving the node Usually a SUMMATIVE junction, i.e. there are many afferent vessels but one efferent vessel Rich blood supply lets lymphocytes into the lymph nodes via the HIGH ENDOLTHELIAL VENUES T-cell zone: parafollicular cortex B-cell zone: lymphoid follicle- mostly on the periphery of the lymph node During immune response, there is a massive proliferation of B cells, which leads to the formation of a GERMINAL CENTRE Specific chemokines target their respective lymphocytes to their specific areas, e.g. T-cells to the parafollicular cortex The lymph entering lymph nodes may also contain cells such as dendritic cells and macrophages Spleen Filter for antigens in the blood Large organ in the abdomen Separated into white pulp: lymphoid cells around blood vessels, full of lymphocytes red pulp: contains old damaged RBC Any diseases involving RBC, i.e. sickle-cell, often results in an enlargement of the spleen T cell area: peri-arteriolar lymphatic sheath (PALS) B cell area is located further away from blood vessels Not a vital organ: Individuals who do not have a spleen are highly susceptible to infections with encapsulated bacteria Mucosal Associated Lymphoid Tissue (MALT) • • • • Epithelium is the first line of defence mucosae and skin form a physical barrier very large surface area, in large part a single layer of cells heavily defended by the immune system in case it breaks 9 MCD Immunology Alexandra Burke-Smith Gut Associated Lymphoid Tissue - - - Many villi, plus smoother regions Involved in the mesenteric lymphatic drainage system to mesenteric lymph nodes, including intraepithelial lymphocytes PEYER’S PATCH: non-capsulated aggregation of lymphoid tissue- predominantly B lymphocytes and contain germinal centres during immune responses M-CELLS: sample contents of the intestine, surveying for pathogens which they can then deliver to immune cells Cutaneous Immune System - I.e. the skin - Epidermis contains keratinocytes, Langerhans cells and intraepidermal lymphocytes - The dermis heavily guards the epidermis with immune cells, e.g. macrophages, T lymphocytes etc - The demis also consists of venules and lymphatic vessels, providing entry to the blood circulation and drainage to regional lymph node 3. Outline the recirculation of lymphocytes. PROBLEM: There are a very large number of T cells with different specificities There are a very large number of B cells with different specificities There may only be limited amounts of antigen How does the body ensure that the antigen meets lymphocyte with specific receptor? SOLUTION: - - Lymphocyte recirculation Pathogen on mucosal surface Naive lymphocytes leave BM and Thymus and enter the bloodstream Recirculate through peripheral lymphoid tissue Recognition of antigen- massive B cell proliferation in secondary lymphoid tissue (lymphocyte activation) Otherwise the lympcytes die Extravasion of naive T cells into the lymph nodes (occurs during immune response) The naive T cell “rolls” along the epithelium These are then stopped and activated by specific chemokines at a particular place on the epithelium. This “right place” is determined by SELECTINS 10 MCD Immunology - Alexandra Burke-Smith INTEGRINS then increase adhesion of the T cell to the epithelium, leading to arrest of the cell Transendothelial migration of the T cell from the bloodstream into the lymph node then occurs Antigens also enter the lymph nodes via the draining lymphatics Naive lymphocytes recirculate approx once per day -- enter lymph node—high endothelial venue – lymphocyte is activated by antigen – stops recirculatng – massive proliferation of B lymphocytes – reenter the blood via the superior vena cava (via the efferent vessel) – target invading microbes/pathogens Anatomical structure of the immune system 4. Explain the use of CD (cluster of differentiation) markers for discrimination between lymphocytes. Lymphocytes • Small cells with agranular cytoplasm and a large nucleus • Can be subdivided into 2 groups depending on where they were produced - B lymphocytes (Bone Marrow) - T lymphocytes (Thymus) • These express different CD molecules, which are recognised by different antibodies CD Markers • an internationally recognised systematic nomenclature for cell surface molecules • used to discriminate between cells of the haematopoietic system • more than 300 CD markers • clinical importance e.g. CD4 in HIV 5. Compare and contrast phenotypic characteristics of B and T cells. Relative Quantities T cells B cells 9 7.5 x 10 in the blood Blood contains 2% of the total pool, therefore 50 x 7.5 x 109 = 3.75 x 1011 ~ 1012, but mostly in the gut T Lymphocytes • • • • • • all express CD3- antigen specific receptor (TCR) TCR, about 10% in blood TCR, about 90% in blood: ~2/3 express CD4, ~1/3 express CD8. All mature T cells express one or the other CD4+ = T helper cells, regulatory T cells- Secrete cytokines CD8+ = cytotoxic T cells- Lyse infected cells, secrete cytokines Thymic output of naive T cells declines with age, and the thymus atrophies. Therefore older people have a reduced ability to respond to new infections. However the total number of T cells does not change, there are just more memory cells. ANTIGEN RECOGNITION only recognise processed antigen presented at the surface of another cell using T cell receptor antigen is presented by an MHC molecule B lymphocytes • • • • • Produced by and develop in bone marrow Surface antigen receptor (B cell receptor) : immunoglobulin like molecule Express CD markers CD19 & CD20 (not CD3, CD4 or CD8) Express MHC Class II (can present antigen to helper T cells) Effector function is to produce antibodies ANTIGEN RECOGNITION 11 MCD Immunology • • Alexandra Burke-Smith recognise intact antigen free in body fluids (so not presented by another molecule) Use B cell receptor, a membrane anchored form of antibody linked to signalling subunits 6. Give examples of antigen presenting cells (APCs) and their locations. Antigen presenting cells (APC) cells that can present processed antigen (peptides) to T lymphocytes to initiate an acquired (adaptive) immune response: Dendritic cells (DC) - Location: Widely spread e.g. Skin & mucosal tissue - Presents to T cells B lymphocytes - Location: lymphoid tissue - Presents to T cells Macrophages (activated) - Location: lymphoid tissue - Presents to T cells Follicular dendritic cells - Location: lymph node follicles - Presents whole antigens to B cells 12 MCD Immunology Alexandra Burke-Smith 3. Innate Immunity Dr Keith Gould (k.gould@imperial.ac.uk) 1. Briefly describe the functions of the important phagocytic cells: neutrophils, monocytes/macrophages. 2. Define cytokines and describe their general properties. 3. Define complement, list its major functions, and draw a simple diagram of the complement pathways. 4. Describe a typical inflammatory response to a localised infection involving recruitment of neutrophils, and phagocytosis and killing of bacteria. 5. Briefly outline the events involved in a systemic acute phase response. 6. Outline the phenotype and functions of natural killer (NK) cells. Innate Immunity • • • • • Present from birth- “in built” Not antigen specific, but recognizes pathogen-associated molecular patterns (PAMP) Not enhanced by second exposure, i.e. no memory (comes directly from lymphocytes) Uses cellular and humoral components in body fluids Rapid response, cooperates with and directs adaptive immunity Phagocytosis • • • • • • Phagocytic cells can ingest whole microorganisms, insoluble particles, dead host cells, cell debris and activated clotting factors. In the first step, there has to be adherence of the material to the cell membrane. Finger-like projections called pseudopodia engulf the material, and a membrane-bound structure called a phagosome is formed. This then fuses with a lysosome to form a phagolysosome, mixing the contents of the lysosome with the engulfed material. Lysosomes contain hydrogen peroxide, oxygen free-radicals, and various hydrolytic enzymes which can digest and break down the engulfed material. Finally, any waste products are released from the cell. Phagocytic Cells Neutrophils - (POLYMORPHONUCLEAR LEUKOCYTE) 13 MCD Immunology Alexandra Burke-Smith - 50-70% of leukocytes short lived cells, circulate in blood then migrate into tissues; first cells to be recruited to a site of tissue damage/infection - ~1011 produced per day in a healthy adult, but this can increase approx ten-fold during infection Macrophages - less abundant - dispersed throughout the tissues - signal infection by release of soluble mediators Neutrophils To fight infection, neutrophils: 1. Migrate to site of infection (Diapedesis and Chemotaxis) - Neutophil rolls along normal endothelium - At site of damage/when antigen is presented by macrophage, a change in the nature of the endothelium occurs - Integrin activation by chemokines- This leads to a change in adhesion molecules into high affinity state- they flatten out and undergo migration through endothelium - Chemotaxis- directed migration along chemokine concentration gradient towards area of high concentration 2. - Bind pathogen- Opsonisation Coating of pathogen with proteins to facilitate phagocytosis Opsonins are molecules that bind to antigens and phagocytes Antibody and complement function as opsonins NEUTROPHIL BINDING TO OPSONINS Bacterium-antibody complex complement activation Fc receptor on phagocyte binds to antibody, CR receptor to complement opsonins bound to pathogen signal activation of phagocyte 3. - Phagocytose Key component of host defence May result in pus-filled abscess Much more effective after OPSONISATION 4. Kill pathogen - Neutrophil Killing Mechanisms OXYGEN-INDEPENDENT Uses enzymes: - Lysozyme - Hydrolytic enzymes Uses antimicrobial peptides (defensins) OXYGEN-DEPENDENT Uses Respiratory burst: Toxic Metabolites - Superoxide anion - Hydrogen perozide - Signlet oxygen - Hydroxyl radical Reactive Nitrogen Intermediates: - Nitric oxide Phagocyte Deficiency Associated with infections due to extracellular bacteria and fungi Bacteria - Staphylococcus aureas - Pseudomonas aeruginosa 14 MCD Immunology Alexandra Burke-Smith - Escherichia coli Fungi - Candida albicans - Aspergillus flavus • Deep skin infections, impaired would healing • Poor response to antibiotics • E.g. chronic granulomas disease Phagocytes - Monocytes Circulate in blood Smaller than tissue macrophages Precursor to tissue macrophages Macrophages Express pathogen recognition receptors (e.g. toll-like receptors TLR, NOD-like receptors NLR, RIG-I: viral genomes) for many bacterial constituents Bacteria bind to macrophage receptors- initiate a response release of cytokine (soluble mediators SIGNAL INFECTION) Phagocytosis then occurs: Engulf and digest bacteria Cytokines • • • • • Small secreted proteins Cell-to-cell communication Generally act locally Powerful at low concentrations Short-lived INTERLEUKINS (IL-x) Between leukocytes approx 35 different types INTERFERONS (IFN) Anti-viral effects approx 20-25 different types CHEMOKINES Chemotaxis, movement approx 50 different types GROWTH FACTORS development of immune system CYTOTOXIC Tumor necrosis factor (TNF) Mechanism • • Inducing stimulus – transcription of gene for soluble protein in cytokine-producing cell – cytokine binds to receptor on target cell -- Binding generates signal – changes in gene transcription and gene activation – biological effect Cytokines are usually released in a mixture, therefore have a wide range of effects on a range of different target cells 15 MCD Immunology Alexandra Burke-Smith Autocrine Action same cell e.g. Interleukin 2 Paracrine Action nearby cell e.g. interferon Endocrine action circulate in bloodstream distant cell e.g. interleukin 6 Important Cytokines IL-1 alarm cytokine fever TNF- alarm cytokine IL-6 acute phase proteins liver IL-8 chemotactic for neutrophils IL-12 directs adaptive immunity activates NK cells Bacterial Septic Shock • • • • • Systemic infection Bacterial endotoxins cause massive release of the TNF- and IL-1 by activated macrophages Increased vascular permeability Sever drop in blood pressure 10% mortality Dendritic Cells • • • Network of cells located at likely sites of infection, in the skin and near mucosal epithelia Recognise microbial patterns, secrete cytokines engulf pathogens, and migrate to local lymph node to present antigens to adaptive immune system Complement “describe the activity in serum which could complement the ability of specific antibody to cause lysis of bacteria” Ehrlich (1854-1915) • • • • • • major role in innate and antibody-mediated immunity complex series of ~30 proteins and glycoproteins, total serum conc. 3-4 mg/ml triggered enzyme cascade system; initially inactive precursor enzymes, and as a few enzymes are activated, they catalyse the cleaving of secondary components etc rapid, highly amplified response very sensitive components produced mainly in the liver, but also by monocytes and macrophages 16 MCD Immunology Alexandra Burke-Smith Activation The Classical Pathway initiated by antigen-antibody complexes The Alternative Pathway direct activation by pathogen surfaces The Lectin Pathway antibody-independent activation of Classical Pathway by lectins which bind to carbohydrates only found on pathogens, e.g. MBL and CRP • • • • • • Classical & Alternative Pathways converge at C3 C3 leads to the final Common Pathway late phase of complement activation Ends with the formation of the Membrane Attack Complex (MAC) As a bi-product of the classical pathway, fragments cleaved are pro-inflammatory molecules Principle opsonin is C3b Control Mechanisms Acheieved by: • Lability of components, i.e. their short half-life • Dilution of components in biological fluids • Specific regulatory proteins: - Circulating/soluble, eg C1-inhibitor, Factor I, Factor H, C4-binding protein - membrane bound, eg CD59 (interferes with MAC insertion) and DAF (competes for C4b) Function 1. Lysis 2. Opsonisation 3. Inflammation/chemotaxis Mast Cells • Secrete histamine and other inflammatory mediators, including cytokines Mucosal mast cell lung Connective tissue mast cells skin and peritoneal cavity near blood vessels • Recognise, phagocytose and kill bacteria • activated to degranulate by complement products (ANAPHYLATOXINS) leading to vasodilation and increased vascular permeability. Local Acute Inflammatory Response 17 MCD Immunology Alexandra Burke-Smith • tissue damage trigger cascades: • invasion of pathogens recognition by macrophages phagocytosis release of soluble cytokines + chemokines Diapedesis and Chemotaxis (slowing down of neutrophils in blood vessels and migration towards site of infection) complement activation mast cell degranulates release of pro-inflammatory fragments + histamines endothelial damage change in nature of endothelium signals site of infection to neutrophils • • Systemic “Acute-Phase” Response • May accompany local inflammatory response 1-2 days after • Fever, increased white blood cell production (LEUKOCYTOSIS) • Production of acute-phase proteins in the liver • Induced by cytokines ACUTE PHASE PROTEINS Required to enhance immune response C-reactive protein (CRP) - C polysaccharide of pneumococcus - Activates complement - Levels may increase 1000 fold Mannan Binding Lectin (MBL) - Opsonin for monocytes - Activates complement Complement Fibrinogen - clotting Importance of Cytokines Signal liver: - produce acute-phase proteins Signal bone marrow: - Increase Cerebrospinal fluid (CSF) by stromal cells and macrophages - Increase leukocytosis (WBC production) Signal Hypothalamus: - Prostaglandins production – fever - Via pituitary gland and adrenal cortex, release corticosteroids – signals liver again Natural Killer (NK) cells • • • • • • • Large granulated lymphocytes Cytotoxic: lyse target cells ad secrete INTERFERON- 5-10% peripheral blood lymphocytes No antigen-specific receptor Complex series of activating and inhibitory receptors Have receptors which bind to antibody-coated cells (ADCC- ANTIBODY DEPENDENT CELL-MEDIATED CYTOTOXICITY) Important in defence against tumour cells and viral infections, especially Herpes Target Cell Recognition - Missing self recognition Ligation of inhibitory NK receptors = inhibition of target cell killing Involves recognition of lack of MHC molecules Induced self recognition Ligation of activating NK receptors = target cell killing Involves stress-induced molecules 18 MCD Immunology Alexandra Burke-Smith 4. Antibodies Dr Keith Gould (k.gould@imperial.ac.uk) 1. Describe with the aid of a simple diagram the immunoglobulin molecule, identifying the antigen-binding site (Fab) and Fc portions of the molecule. 2. Briefly describe the properties of the antigen-binding site. 3. Distinguish between antibody affinity and avidity. 4. List the immunoglobulin classes and sub-classes in man. Describe their functions and relate these to their individual structure. Overview What is an antibody? • A protein that is produced in response to an antigen • Binds specifically to the antigen • Form the class known as IMMUNOGLOBULINS • Large family of soluble GLYCOPROTEINS • Produced by B lymphocytes • Found in serum • >107 different types • Deficiency is life threatening • After binding antigen, initiate secondary effector functions - Complement activation - Opsonisation - Cell activation via specific antibody-binding receptors (Fc receptors) Structure • symmetrical • Two light (25kDa) chains, two heavy (50kDa) chains • Each chain has amino and carboxyl terminal • Chains heald together by disulphide bridges • Electrophoresis of globulins found in serum: - Relative amounts (decreasing): A, γ, α, β - Electrophoretic mobility- towards +ve electrode: A, α, β, γ • Different antibodies therefore have different charges The discovery of antibody structure • Rodney Porter • Limited the digestion of gamma-globulin with purified papain, which produced 3 fragments in equal amounts • 2 fragments had antigen binding activity (Fab) • The third did not, but formed protein crystals (Fc) Flexibility • There is a hinge in the antibody which allows flexibility between the two Fab 19 MCD Immunology • Note: • • • • Alexandra Burke-Smith This allows the angle between the two antigen binding sites to change angle depending on the proximity of cell surface determinants, i.e. how close together antigens are Both light and heavy chains can be divided into variable (where the sequences are different) and constant (same sequence) regions Each IG (immunoglobulin/antibody) domain, e.g. variable light, has INTRAMOLECULAR DISULPHIDE BONDS to maintain their specific 3D structure required for antigen binding Many cell surface proteins also have IG-like domains, and are said to belong to the IG super family The constant region binds to Fc receptors, which can lead to cell activation, e.g. NK cells (secondary effector functions in immune response) Antigen-binding site • • • Antigen binding occurs at 3 HYPERVARIABLE regions, known as COMPLEMENTARITY DETERMINING REGIONS (CDR’s) These have specific residue positron numbers The region of binding is a large undulating 3D structure (~750A = 10-10m), so is highly specific and there are a significant number of interactions between the antibody and antigen surface Forces involved • Hydrogen bonds • Ionic bonds • Hydrophobic interactions • Van der Waals interactions Are non-covalent, therefore are relatively weak. This means that in order to have a HIGH AFFINITY, there can only be a short distance between the antigen and antibody, highly complementary nature, and a significant number of interactions. Antibody Affinity The strength of the total non-covalent interactions between a single antigen binding site and a single epitope on the antigen. The affinity association constant K can be calculated: K varies from 104 to 1011 L/mol Antibody Avidity The overall strength of multiple interactions between an antibody with multiple binding sites and a complex antigen with multiple epitopes • • • • This is a better measure of binding capacity in biological systems Monovalent interactions have a low affinity Bivalent interactions have a high affinity Polyvalent interactions have a very high affinity Cross-Reactivity Antibodies elicited in response to one antigen can also recognise a different antigen, for example: 1. Vaccination with cowpox induces antibodies which are able to recognise smallpox 20 MCD Immunology Alexandra Burke-Smith 2. ABO blood group antigens are glycoproteins on red blood cells. Antibodies made against microbial agents on common intestinal bacteria may cross-react with the glycoproteins, which poses a problem for blood transfusions. Isotypes and Allotypes • Isotypes are antibodies who are present in everybody, with a constant region. • Allotypes are antibodies that contain single amino acid mutations, giving allelic polymorphisms which vary in the population Immunoglobulin Classes Different classes of antibodies differ in the constant regions of their heavy chains Class IgG IgA IgM IgD Heavy chain γ α µ δ CH Domains 3 3 4 3 Light Chain κ/λ κ/λ κ/λ κ/λ IgG and IgA have subclasses Class Subclass H chain IgG IgG1, IgG2, IgG3, IgG4 γ1, γ2, γ3, γ4 IgG • γ heavy chain • most abundant • monomer • 4 subclasses- variability mainly located in hinge region and effector function domains • Actively transported across the placenta- protection from mother to newborn • Found in Blood and extracellular fluids • Major activator of classical complement pathway (mainly IgG1 and IgG3) • Subclasses decrease in proportion from 1-4 • • • • • IgA IgA1, IgA2 Α1, α2 IgA • heavy chain • Second most abundant • monomer (blood) • dimer (secretions) • Major secretory immunoglobulin • Protects mucosal surfaces from bacteria, viruses and protozoa • Secretory IgA: joined by J chain and secretory component. Plasma cell secretes dimeric form without secretory. This bonds to poly-Ig receptor and is endocytosed and secreted into lumen. The poly-Ig receptor is cleaved and becomes the secretory component • IgE ε 4 κ/λ IgM • µ heavy chain • pentameric • 5 monomers joined by J chain (10 x Fab) • mainly confined to blood (80%) • first Ig synthesised after exposure to antigen (primary antibody response) • multiple binding sites compensate for low affinity • efficient at agglutination of bacteria • activates complement The secretory component protects IgA from being degraded in the lumen, by proteases etc IgD δ heavy chain extremely low serum concentrations least well characterised surface IgD expressed early in B cell development involved in B cell development and activation • • • • IgE heavy chain present at extremely low levels produced in response to parasitic infections and in allergic diseases binds to high affinity Fc receptors of mast cells and basophils 21 MCD Immunology Alexandra Burke-Smith • cross-linking by antigen triggers mast cell activation and histamine release Selective Immunoglobulin Distribution • • • • • • IgG and IgM in blood IgG in extracellular fluid Dimeric IgA in secretions across epithelia, including breast milk Maternal IgG in foetus via placental transfer IgE with mast cells below epithelium Brain devoid of antibodies Antibody effector functions Effector Function Activity Example Neutralization of toxins Neutralization of viruses Neutralization at body surfaces Agglutination Inhibits toxicity Inhibits infectivity Inhibits infectivity of bacteria & viruses Ag-Ab complexes/ Lattice formation Promotes phagocytosis Classical Pathway Tetanus toxin Measles Polio Salmonella Bacteria & RBC Antibody Class Mainly IgG Mainly IgG Secretory IgA IgM, IgG Bacteria, fungi IgG Ag-Ab complex IgM, IgG Parasites Pollen Virus infected cells IgE Opsonization Complement activation Mast Cell sensitisation & Expulsion triggering Hypersensitivity NK cell Cytotoxicity ADCC Mainly IgG Summary Antibodies: In defence - targeting of infective organisms - recruitment of effector mechanisms - neutralisation of toxins - removal of antigens - passive immunity in the new born In medicine - levels used in diagnosis and monitoring - pooled antibodies for passive therapy/protection In laboratory science - vast range of diagnostic and research applications 22 MCD Immunology Alexandra Burke-Smith 5. B Lymphocytes Dr Ingrid Muller (i.muller@imperial.ac.uk) 1. Describe the process of stimulation of individual B cells to divide and secrete antibody such as to generate immunity to a particular antigen (clonal selection) 2. Briefly outline the principles of immunoglobulin (Ig) gene rearrangement in the generation of diversity 3. Outline the differences in antibody production during primary and secondary immune responses 4. Differentiate between monoclonal and polyclonal antibody Adaptive Immune response B lymphocytes operate during the adaptive immune response Develops after encounter of antigem Takes 4-7 days to develop and become effective Elicited antibody production specific to encountered antigen 2 types: Humoral- B cells -- antibodies Cell Medicated- T cells -- cytokines, lysis of pathogens B Lymphocytes • • • White blood cells Derived from haemopoietic stem cells Are effector cells of humoral immunity; they secrete antibodies and form memory cells Where do they come from? • Derived in the bone marrow in the absence of antigens • Mature in the bone marrow, whereby they express specific B cell receptors (BCR) • Migrate into the circulation (blood, lymphatic system) and into lymphoid tissues • Antibody production requires antigen-induced B cell activation and differentiation- this occurs in peripheral lymphoid organs B cell Maturation • Pro-B Cell Pre-B Cell Immature B Cell Mature B Cell • Occurs in the bone marrow in the absence of antigen • Mature B cells are specific for a particular antigen- their specificity resides in B cell receptor (BCR); a membrane bound immunoglobulin B cell Receptor (BCR) • Transmembrane protein complex composed of: mIg - central larger immunoglobulin molecule - cytoplasmic tail too short so is not involved in signalling Igα/Igβ - di-sulfate linked heterodimers - contain immunoglobulin-fold structure - cytoplasmic tails of Igα/Igβ is long enough to interact with intracellular signalling molecules • has a unique binding site- binds to ANTIGENIC DETERMINANT or EPITOPE -made before the cell ever encounters antigen • large monoclonal population on surface of the B lymphocyte 23 MCD Immunology Alexandra Burke-Smith Antigen and BCR diversity • For the immune system to respond to the large number of antigens we are exposed to, we need to have a large REPERTOIRE of specific BCR on different B cells that can recognise the huge array of antigens • 1010 different antibody molecules can be generated by B cells with specific BCR • Functional BCR genes do not exist until they are generated during lymphocyte development • Each BCR chain (κ & λ light chains, and heavy chain) is encoded by separate MULTIGENE FAMILIES ON DIFFERENT CHROMOSOMES • During maturation, these gene segments are rearranged and brought together to form the BCR – IMMUNOGLOBULIN GENE REARRANGEMENT • There are a number of VARIABLE; V, DIVERSITY;D and JOINING;J gene segments that may be responsible for each chain. The Diversity segment is only associated with the heavy chain. There is also a CONSTANT REGION associated with each chain • This generates the diversity of the lymphocyte repertoire Prototypical Membrane Protein Synthesis • Genomic DNA – (transcription) – Primary transcript RNA/pre-mRNA – (Splicing) – Mature mRNA – (translation) – Membrane protein • Intracellular; Amino terminus of protein and protein domains relating to specific exons • Transmembrane; relates to specific exon/s • Extracellular; cytoplasmic tail- consists of exons and carboxyl terminus Light Chain Synthesis • Germline DNA– (rearrangement of V and J segments involving VDJ RECOMBINASE) – B cell DNA – (Transcription) – Primary transcript RNA/pre-mRNA – (Splicing) – Mature mRNA – (translation) – Light chain polypeptide (Kappa or Lamda) • During joining of gene segments the unused DNA is looped out and removed (Germline DNA – B cell DNA) Heavy Chain Synthesis • Germline DNA– (rearrangement of V and J segments involving VDJ RECOMBINASE) – B cell DNA – (Transcription) – Primary transcript RNA/pre-mRNA– (Alternative Splicing) – Mature mRNA – (translation) – Heavy chain polypeptide • ALTERNATIVE SPLICING; results in different mature mRNA, as the mRNA express different genes (e.g. they may have different constant region genes present) BCR rearrangement Required for B cell maturation Adaptive Immune Response • • Antibody production is a highly regulated process after activation by epitope If a B cell does not meet an antigen – death 24 MCD Immunology • • • Alexandra Burke-Smith Antibodies may keep specificity but change class During immune response, the first antibody produced is IgM, but this can change The adaptive immune response is characterised by: 1. Specificity 2. Diversity 3. Memory Clonal Selection • Basis of adaptive immunity • Non-self reactive mature lymphocytes then migrate to the periphery • Our immune system is usually exposed to multiple antigens, therefore multiple cells will be activated • Each lymphocyte (T or B) expresses an antigen receptor with a unique specificity, • Binding of antigen to its specific receptor leads to activation of the cell, causing it to proliferate into a clone of cells • All of these clonally expanded cells bear receptors of the same specificity to the parental cell • Lymphocytes expressing receptors that recognize self molecules are deleted early during lymphocyte development and are phagocytosed/lysed • Result: Plasma Cells, Antibodies, Memory cells Antibody production • Naive antigen-specific lymphocytes cannot be activated by antigen alone; they require accessory signals either from: - Microbial Constituents- Thymus Independent - Helper T cells- Thymus Dependent - Thymus Independent Microbial Consistuents Only IgM is produced No memory cells formed Antigens directly activate B cells without the help of T cells This can induce antibodies in people with no thymus and no T cells (Di-George syndrome) The second signal required is either provided by the microbial constituent or by an accessory cell - - - Thymus Dependent Helper T cells All Ig-classes produced Memory is formed Membrane bound BCR binds with antigen and is internalised and delivered to intracellular sites Antigen is degraded into peptides Peptides associated with Self- MHC Class II, forming a complex which is expressed at the cell surface T lymphocytes with a complementary T cell receptor (TCR) recognises the complex T helper cells then secrete LYMPHOKINES B cell then enters the cell cycle, forming a clone of cells with identical BCRsdifferentiating into plasma and memory cells T-B cell collaboration • Antigen cross link with BCR induces signal 1-- ↑MHC II, ↑B7 • Antigen is internalised and degraded, and the peptide-MHC II complex is presented 25 MCD Immunology • • • • • • Alexandra Burke-Smith T cell recognises complex and co-stimulation by B7and CD28 interaction activation of T cells B7(expressed by B cell) CD28(expressed by TH cell) Activated T cell expresses CD40L The interaction between CD40L and CD40 (expressed by B cell) induces signal 2 Activated B cells (CENTROBLAST) express cytokine receptors T cell derived cytokines bind to receptors on B cells B cells proliferate and differentiate into antibody secreting plasma cells Cytokines Certain cytokines help to produce certain Ig classes during differentiation of CENTROCYTES into plasma cells Class switching • During class switching, the variable region (and hence the specificity) remains constant • However the constant region changes from the original IgM 26 MCD Immunology Alexandra Burke-Smith Example of Ig class switching above Immunological Memory • • • • • • • • • Consequence of clonal selection and antigen recognition Memory responses are characterised by a more rapid, heightened and more efficient immune reaction that serves to eliminate pathogens fast and prevent diseases Can confer life-long immunity Initial antigen contact induces a PRIMARY RESPONSE Subsequent encounter with the same antigen will induce a SECONDARY RESPONSE which is more rapid and higher The secondary response reflects the activity of the clonally expanded population of MEMORY B CELLS The primary response consists of mainly IgM, whereas the secondary response will involve other Ig classes Immunological memory forms the basis for immunisation B cell memory: Increase in antibody amount and antigen affinity Property Responding B cell Lag period Time of peak response Magnitude of peak antibody response Isotype produced Antigens Antibody affinity Primary Response Naive 4-7 days 7-10 days Varies depending on antigen Secondary Response Memory 1-3 days 3-5 days 100-1000x greater Predominantly IgM Thymus independent and thymus dependent Lower Predominantly IgG Thymus dependent higher Polyclonal and Monoclonal antibodies • • • Polyclonal antiserum- all antigenic epitopes induce an immune response many different B cells activated different antibodies produced Invading microorganisms have multiple antigenic epitopes A mixture of antibodies directed to several antigenic determinants will be produced which are derived from many different clones of B cells = polyclonal response Monoclonal antibodies are derived from a single B cell clone, which can be extracted after first combining the plasma cells with myeloma cells to form hybridomas. Monoclonal antibodies are used to quantify CD4 count in HIV patients 27 MCD Immunology • • Alexandra Burke-Smith Myeloma = cancerous plasma cells that divides permanently without antigenic stimulation and secretes antibodies which are indistinguishable from normal antibody = myeloma proteins. They confer immortality when hybridised with another cell Plasmacytoma - clone of malignant plasma cells 28 MCD Immunology Alexandra Burke-Smith 6. T lymphocytes and Antigen recognition Dr Keith Gould (k.gould@imperial.ac.uk) 1. Outline the origins and functions of T lymphocyte subsets. 2. Briefly describe the structure and distribution of major histocompatibility complex (MHC) class I and class II molecules. 3. Outline the mechanisms by which antigen presenting cells (APCs) process and present antigens. 4. Compare and contrast antigen recognition by B and T lymphocytes and by CD4+ and CD8+ T lymphocytes T lymphocytes • • Destroy intracellular pathogens T cell receptor (TCR) recognizes small peptide fragment of antigen presented by MHC molecule on the surface of host infected cell T cell receptor (TCR) • Analogous to membrane bound Fab portion of antibody • The variable region is towards the N terminus • The constant region is towards the membrane • The cytoplasmic tail is too short for signaling, so the polypeptides associate with CD3 POLYPEPTIDES with longer CYTOPLASMIC DOMAINS- this is critical for signaling. • CD3 polypeptides may consist of GAMMA, DELTA, EPSILON and ZETA subsets Antigen Recognition • 2 major populations of T cells: - CD4+: use CD4 co-receptor, see peptides on MHC class II- “class II restricted” - CD8+: use CD8 co-receptor, see peptides on MHC class I- “class I restricted” • CO-RECEPTOR molecules bind to the relavent MHC, increasing the avidity of T CELL-TARGET CELL INTERACTION • Important in signalling Target Cell Destroying CD8 (Tc or CTL) - most are cytotoxic and kill target cells - also secrete cytokines - Induce apoptosis in the target cell (programmed cell death, suicide) CD4 (T helper cells, Th) - secrete cytokines - Recruit effector cells of innate immunity - help activate macrophages - Amplify and help Tc and B cell responses • MHC molecules present antigen fragments at cell surface • CD8+ CTL- kill target cells, e.g. viruses • CD4+ TH1- activate macrophages • CD4+ TH2- amplify antigen-specific B cell response 29 MCD Immunology Alexandra Burke-Smith The Thymus • • • • Full of lymphocytes, but no immune response to infection T cell precursors; PROGENITOR CELLS, develop in the bone marrow and migrate towards the thymus in the circulation Maturation of the Thymocytes occurs from the CORTEX to the MEDULLA Mature THYMOCYTES/T cells then are transported out of the thymus and around the body via the circulation Development 1. T cells are CD4- and CD8- (they express neither; double negative) 2. In the cortex, the T cells express a TCR precursor (pre TCR; β + “surrogate” αTCR) 3. In the medulla, ~1010 different αβTCR’s created by gene rearrangements. The generated TCRs will only express either CD4 or CD8 • Due to these random gene rearrangements, many of the generated T cells will be “SELF-REACTIVE”, therefore these must be destroyed Selection Occurs during interaction with macrophages and dendritic cells within the thymus. Only useful cells leave the thymus. Pre TCR checkpoint - Is the new β chain functional? - No: Death by APOPTOSIS - Yes: Survival and development to CD4+ CD8+ αβ TCR+ Post TCR checkpoint - Is the αβ TCR functional? - Is the αβ TCR dangerous/autoreactive? - Useless: cannot see MHC – die by apoptosis - Dangerous: see “self”, i.e. host molecules – receive signal to die by apoptosis, i.e. NEGATIVE SELECTION - Useful: binds weakly to MHC molecule – receive signal to survive, i.e. POSITIVE SELECTION - Note: only 5% of thymocytes survive selection 30 MCD Immunology Alexandra Burke-Smith Major Histocompatibility Complex (MHC) Discovery • Tumour propagation in mice, i.e. tissue transplants -acceptance = HISTOCOMPATIBLE - rejection = HISTOINCOMPATIBLE • Inbred mouse strains - all genes are identical • Transplantation of skin between strains showed that rejection or acceptance was dependent upon the genetics of each strain • Skin from an inbred mouse grafted onto the same strain of mouse = acceptance • Skin from an inbred mouse grafted onto a different strain of mouse = rejection • Transplantation antigens: MHC Class I • Gene mapping of the same locus shows second class of MHC molecule. This controls the ability to mount an antibody response; celled IMMUNE RESPONSE GENE- MHC class II Overview • Group of tightly linked genes important in specific immune responses • Found in all vertebrates • Present antigens to T lymphocytes MHC Class I • Consists of two NON-COVALENTLY ASSOCIATED polypeptide chains: - Heavy; α1, α2¸ α3 – these are transmembrane polypeptides with a peptide binding, immunoglobulin like and cytoplasmic region - Light; β2-microglobulin – this only consists of an immunoglobulin like region • α1 and α2 are joined by PEPTIDE BINDING GROOVE • CD8 interacts with the alpha-3 domain MHC Class II • Consists of 2 transmembrane polypeptides of equal length • Each polypeptide (alpha and beta) have two domains • CD4 interacts with the beta-2 domain Cleft Geometry MHC class I - accommodate peptides of 8-10 amino acids - Peptide buried within structure - Peptides all same length MHC class II - accommodate peptides of >13 amino acids - peptides stick out from MHC molecule • individuals have relatively few MHC, but need to present many peptides, so present SUBSETS of peptides using BINDING MOTIFS • BINDING POCKET: certain residues (anchor residues) are directly associated with the peptide due to their specific sequence • Binding pockets are useful in order to predict which peptides will be presented 31 MCD Immunology Alexandra Burke-Smith Human Leukocyte Antigens (HLA) • Human MHC molecules • Base DNA sequence- 3.6 million • 128 functional genes, only 40% immunerelated Gene expression • Polygenic: there are several gene loci • Co-dominant: both paternal and maternal MHC expressed • MHC Class I: present in nearly all nucleated cells, and levels may be altered during infection or by cytokines • MHC Class II: normally only on professional APC, and may be regulated by cytokines Polymorphism • Large number of alternative different versions of the same gene within the population- termed an ALLELE • Each group of MHC alleles linked on one chromosome is termed MHC HAPLOTYPE • Different MHC Haplotypes lead to different immune responsiveness • >4200 HLA proteins in human population • Most polymorphic: Class I- HLA B, Class II- HLA DR β • In reality MHC alleles are NOT randomly distributed in the population: some alleles are much rarer than others, and alleles segregate with race. • This poses a problem for tissue transplants tissue typing Antigen Processing and Presentation • • • • • T lymphocytes recognize only processed antigens presented on cell surfaces by MHC molecules ENDOGENOUS antigen: synthesised within cell (taken to CD8) EXOGENOUS antigen: synthesised outside the cell, and can be taken up by macrophage etc (taken to CD4) Antigens in different locations require different responses Different pathways present antigens from different locations to different T cell CLASS II CLASS I subsets Class 1: - Antigen cleaved by proteasome, taken TAP into RER by TAP (transporter associated TRANSPORTER with antigen presenting) ASSOCIATED WITH ANTIGEN - Bind with MHC class I PROCESSING - Shaperones, e.g. calnexin, help protein folding - Then trafficked by golgi to surface Class 2: - Antigen endocytosed - Cleaved by proteases - MHC II migrates into RER- associates with INVARIANT chain - The MHC II –invariant complex is migrated into the golgi in ENDOSOME - Invariant chain is digested by CLIP (Class II associated invariant chain peptide) - CLIP is then exchanged for the antigenic peptide, which is then presented at the surface CLIP CLASS II ASSOCIATED INVARIANT CHAIN PEPTIDE 32 MCD Immunology Alexandra Burke-Smith 7. Effector T-lymphocytes Dr Ingrid Muller (i.muller@imperial.ac.uk) 1. Outline the importance of antigen presenting cells in the induction of T lymphocyte responses 2. Describe effector functions of T lymphocytes including cell-mediated cytotoxicity, macrophage activation, delayed type hypersensitivity and T/B lymphocyte cooperation 3. Briefly outline the function of T helper cells in relation to the cytokines they produce 4. Explain the different requirements for activation of naive and memory T lymphocytes T-cell mediated immunity Different pathogens require different immune defence strategies (intra/extracellular bacteria, virus, parasites, worms and fungi) Detects and eliminates intracellular pathogens Eliminates altered cells, i.e. tumour cells Location of antigen determines immune response: o Phagocytes with ingested microbes microbial antigens in vesicles CD4+ effector T cells (TH 1) o Infected cell with microbes in cytoplasm CD8+ T cells (CTLs) CD4+ cytokine secretion macrophage activation killing of ingested microbes (also leads to inflammation) CD8+ killing of infected cell CD4+ produce IFN-γ, IL-2, TNF-β CD8+ secrete granules Naive T-lymphocytes Activated in secondary lymphoid organs Lymphocytes re-circulate in the blood lymph lymphoid organs Enter lymph node through specialized areas in postcapillary venues called HIGH-ENDOTHELIAL VENUES (HEV) Advantage: recirculation increases likelihood to encounter antigen Only effector cells can enter non-lymphoid tissue (not naive T cells) as they have to have undergone differentiation process in response to antigen Naive T cells migrate through secondary lymphoid organsthis is mediated by receptors on recirculating cells Encounter with antigen in secondary lymphoid organs activate naive T cells The migration of naive and effector/memory T cells differs Dendritic cells, macrophages and B cells are all professional ANTIGEN PRESENTING CELLS (APC) – they all have MHC Class II molecules and lead to the activation of T cells into effector cells 1. Immature DC take up antigen (INNATE IMMUNITY) in the peripheral tissues 2. Immature DC activated – leave tissue – migrate to secondary lymphoid tissue 3. In the lymph node, the DC matures – expresses high levels of peptide/MHC complexes and COSTIMULATORY molecules therefore leads to more efficient APC 33 MCD Immunology Alexandra Burke-Smith Induction and Effector Phases of CMI Initial Activation T cells enter HEV in cortex T cells monitor for antigens presented by APC: encounter proliferation and differentiation into EFFECTOR CELLS non-encounter leave lymph nodes Both antigen and costimulation is required for T-cell activation Costimulatory molecules e.g. CD28 (requires CD80 or CD86 ligand) Lack of costimulation unresponsive T cells and can lead to tolerance in peripheral T cells After Recognition and Costimulation Recognition proliferation/differentiation effector function T-cells secrete IL-2 and IL-2 receptor (required for proliferation); DIRECT RESPONSE = AUTOCRINE ACTION This leads to the cell activation and multiplication Effector function: APOPTOSIS- destroy infected target cell Effector T cells are less dependent on costimulation Resting T-cell: moderate affinity receptor (IL-2Rβ + γ chains only) Activated T-cell: high affinity receptor (β + γ + α chains) and secretion Binding of IL-2 and its receptor signals the T cell to enter the cell, which induces proliferation IL-2 DEFINITIONS Naïve T cells: mature recirculating T cells that have not yet encountered antigen Effector T cells: encountered antigen, proliferated and differentiated into cells that participate in the host defense Target cells: Cells on which effector T cells act T-effector cells CD8: peptide + MHC class I- cytotoxic cells CD4: Th1 cells- interact with macrophages- phagocytosis intracellular bacteria Th2 cells – interact with antigen-specific β cell – antibody production CTLs Naive T cell = CTLp CTLp is essential a precursor, and must differentiate before it can kill CTLp does not express IL-2 receptor Require helper T cells for activation and proliferation (Th1) 34 MCD Immunology Alexandra Burke-Smith When binding to their specific antigenic peptide:self-MHC complexes, TCRs and their associated coreceptors cluster to the site of cell-cell contact. Clustering of TCRs then signals a reorientation of the cytoskeleton that POLARIZES the effector cell to focus the release of effector molecules at the site of contact with the target cell. CTLs contain lytic granules which contain cytotoxic molecules. In the polarized T cells the secretory apparatus becomes aligned toward the target cell and the content of the lytic granules is secreted. The lytic granules induces APOPTOSIS CTLs can kill multiple targets Early apoptosis: chromatin becomes condensed Late apoptosis: nucleus very condensed, mitochondria visible, cell loses much of cytoplasm and membrane Granules PERFORIN: polymerises to form pore of Target cell GRANZYMES: serine proteases, activate apoptosis in cytoplasm GRANULYSIS: induce apoptosis Cell Death - Granule Exocytosis Pathway Perforin Granzymes Cascades FAS Pathway Interaction expression of Fas ligand on T cell – binding initiates cascades apoptosis CTL are re-used after dissociation with target cell Cytotoxicity Apoptosis characterised by fragmentation of nuclear DNA CTL store PERFORIN, GRANZYMES, GRANULYSIN Granules released after target recognition Also release of soluble mediators that contribute to host defence: - IFN- γ; inhibits viral replication and activates macrophages - TFN α and TNF β synergise with IFN-γ TH cells The type of TH activated depends on environment e.g. APC and cytokines The signals the precursors receive correspond to the type of TH: IL-12 TH1 and IL-4 TH2 TH1 and TH2 correspond to MHC II TH1 activate macrophages in a very regulated and coordinated manner and are involved in opsonisation and phagocytosis - involving IFN- γ TH2 coordinate mast cell degranulation involving IL-4 and release IL-5 eosinophil activtion 35 MCD Immunology Alexandra Burke-Smith TH cells coordinate immune responses to infections with intracellular pathogens Cytokine mediated interactions are also very important T Helper subset differentiation, cytokine profile and effector functions SEE POWERPOINT FOR MORE DIAGRAMS Delayed Type Hypersensitivity (DTH) reaction Mediated by pre-existing antigen specific T cells, mainly by Inflammatory Th1 cells CD4+ Th1 cells release inflammatory cytokines that affect blood vessels (TNF-β), recruit chemokines and activate macrophages (IFN-γ) Can be protective as well as pathological Primary role in defence against intracellular pathogens DTH inducers: - intracellular parasites (Leishmania) - intracellular bacteria (Mycobacteria) - intracellular fungi (candida) - intracellular viruses (Herpes simplex) If the source of the antigen is not eradicated – chronic stimulation granuloma formation If the antigen is not a microbe, DTH produces tissue injury without protection – HYPERSENSITIVITY The DTH response consists of two phases: Sensitization phase Effector phase 36 MCD Immunology Alexandra Burke-Smith Clinical and histological appearance Tuberculin-type hypersensitivity Reaction characterised by an area of red firm swelling of the skin Maximal 48-72 hrs after challenge Histologically there is a dense dermal infiltrate of leukocytes and macrophages T-B cell collaboration Immunoglobulin+ B cells bind specific antigen Ig-antigen complex is internalised, processed and antigenic peptides are presented on the B cell surface in context with MHC class II T helper cells with specific TCR recognise antigen-MHC complex The T-B interactions trigger expression of CD40 ligand on T cells CD40 ligand will interact with CD40 expressed by B cells T cells secrete cytokines and B cells express cytokine receptors The activated B cell then differentiates into antibody secreting plasma cell T cell Functions Recognition of antigenic peptides results in T cell activation and: 1. Clearance of pathogen - antigenic peptide derived from foreign pathogen Pathological reactions can be caused by T cells 2. Autoimmunity - antigenic peptide derived from self protein 3. Rejection (transplants) - antigenic peptide derived from self protein of transplant donor T helper cells in relation to their cytokines Th1 associated functions in cell-mediated immunity Macrophage activation DTH reaction Cytokines involved IFN-γ, TNF-α IL-2, IFN-γ, TNF-α, IL-3, GM-CSF 37 MCD Immunology Help for CD8 cells Down-regulation of Th2 responses Th2 associated functions in humoral immunity B cell proliferation B cell differentiation and Ig class switching Down-regulation of Th1 responses Alexandra Burke-Smith IL-2 IFN-γ Cytokines involved IL-2, IL-4, IL-5 IL-2, IL-4, IL-5, IFN-γ, TGF-β IL-4, TGF-β, IL-10 Regulator T Cells Some T cells differentiate into regulatory cells in the thymus or in peripheral tissue Regulatory T cells inhibit the activation of naive and effector T cells by CONTACT-DEPENDENT INHIBITION or by CYTOKINE-MEDIATED INHIBITION Regulate activation and effector functions of other T cells Natural; 5-10% in body; from thymus and important in autoimmunity Down-regulate immune response; both cell-to-cell and cytokine mediated Antigen specific induced Immunological Memory Adaptive immune response in which the immune system remembers subsequent encounters with the same pathogen Memory responses are characterised by a faster and stronger immune response that serves to eliminate pathogens and prevent diseases Can confer life-long immunity to many infections, and is the basis for successful vaccination Memory cells show qualitatively different and quantitatively enhances responses upon re-exposure T cell memory T cells do not undergo isotype switching or affinity maturation CD45RA expression allows to differentiate between naïve memory cells Expression of the chemokine receptor CCR7, which controls homing to secondary lymphoid organs, allows a further subdivision of human memory T cells CCR7- CD45RA- memory cells = effector memory T cells = TEM CCR7+CD45RA- memory cells = central memory T cells = TCM TEM: display immediate effector function TCM: lack immediate effector function, differentiate into CCR7- effector cells after secondary stimulation 38 MCD Immunology Alexandra Burke-Smith Following Leishmania infection (a) The initial events that trigger development of the most effective CD4+ T-cell response for controlling Leishmania infection require a primary infection with live parasites. The combined stimulus provided by activated macrophages and their interaction with naïve CD4+ T cells leads to the development of an effector T-cell response. The effector T cells produce cytokines and interact with macrophages and/or monocytes, increasing their capacity to present antigen (Ag), activate other T cells and kill intracellular parasites. During this process, effector T cells leave the node and also home to infection sites. The majority of effector T cells die and, through a series of poorly defined signals, memory T cells are generated. (b) At least two populations of memory T cells are generated during the immune response – CM T cells (i) and EM T cells (ii) – each of which can be defined by a group of functional characteristics and phenotypic markers. It is unclear what the specific signals are that induce the formation and maintenance of each subpopulation, CM T cells are maintained in the absence of live parasites, whereas EM T cells require the presence of live parasites. (c) Following secondary stimulation, CM and EM T cells are poised to respond to the challenge, albeit with key differences. EM T cells can home immediately to infected lesion sites and produce effector cytokines, whereas CM T cells must first pass through a phase of activation and differentiation to generate effector cells. The importance of this delay on inducing rapid protection, and the extent to which CM T cells are influenced by the EM T-cell compartment are unclear. Because the most effective protection is provided when both CM and EM T cells are generated and maintained, a balance of both subsets is important for maximal protection. 39 MCD Immunology Alexandra Burke-Smith 8. Host Defence Overview Professor Peter Openshaw (p.openshaw@imperial.ac.uk) Immunity Protection from infection Immune response: reaction to a threat (antigen) Immune system: cells and molecules leading to protection Role: to defend against viruses, bacteria, fungi, parasites, i.e. dangerous but not SELF things Our cells are outnumbered by our bacteria 10:1 Modes of transmission of disease Respiratory GI tract Venereal Zoonoses (vectors) Defences Coughing Sneezing Mucus Cilia Rapid cell turnover Antimicrobial peptides produced by phagocytes and epithelial cells General Surface Defence - Mechanical Epithelial tight junctions Skin- waterproofed by fatty secretions Social conditioning, e.g. wahsing Chemical Fatty acids- skin Enzymes: lysozyme (saliva, sweat and tears), pepsin (gut) Low pH (stomach, sweat) Antibacterial peptides (Paneth cells in intestine) Microbiological: Normal flora compete for nutrients/attachment sites Production of antibacterial substances Overview of the Immune Response - Pre-infection—“first line” Avoidance Small Taste Mucus Physical barriers 40 MCD Immunology - Alexandra Burke-Smith Surface environment Early infection—“second line” Phagocytes Opsonins Some lymphocytes Interferons Acute phase proteins Toll-like receptors Late infection—“specific” - T cells - Antibody responses General Trend: Increase in learning and specificity, decrease in breadth of response Innate Immune System Innate Sensing Stranger Model - PAMPs (pathogen associated molecular patterns) are recognised by dendritic cells - DC maturation and migration to lymph node - Danger Model Necrotic cell death DAMPS (damage associated molecule patterns) released, which bind to receptor on DC DC maturation and migration to lymph node Phagocytes Cells that engulf invaders Antigen is destroyed in intracellular vesicles Includes macrophages, neutrophils 41 MCD Immunology Alexandra Burke-Smith Antimicrobial Defence Mechanisms Involves neutrophils, Eosinophils, basophils and mast cells Toxic oxygen, e.g. superoxide O2, H2O2 Toxic nitrogen oxides, e.g. NO Enzymes, e.g. lysozyme Antimicrobial peptides, e.g. defensins DNA nets Virus Recognition Pathways Chemical Signals Interferons - TYPE I/III: a/b/l o activates NK cells o upregulates MHC, Mx proteins o activates RNase L, PKR o induces anti-viral state - TYPE II: IFNg o proinflammatory o Th1 cytokine o “immune interferon” 42 MCD Immunology Alexandra Burke-Smith Chemokines Cytokines Innate Cellular Defences Natural Killer Cells - kill host cells that are: o Infected o Transformed o ‘Stressed’ - Important in viral infections. o Viruses evade NK cell killing o NK deficiency leads to increased infections - Important early source of cytokines - Shape adaptive immune responses The acquired Immune System B cells There are 1014 potential different antibodies (VDJ combinations) Each antibody recognises one specific shape/charge combination Each B cell expresses one unique antibody Antibody binds antigen Antibody is membrane bound or secreted Role of antibodies: neutralisation, opsonisation, complement activation Antibodies may trigger cell mediated cytotoxicity (ADCC) T cells Each T cell expresses one TCR There are potentially 10^18 different TCRs Each TCR sees a specific combination of MHC and peptide at high affinity Antigen processing and presentation Protection against specific microbes Defence against bacteria Surface defences (mechanical and chemical) Antibody opsonisation 43 MCD Immunology Alexandra Burke-Smith Complement (alternative pathway) causing lysis/opsonisation Phagocytosis Release of inflammatory mediators and acute phase proteins (also opsonins) etc. Fever Mucosal defences Mannan binding proteins Antimicrobial peptides Enzymes e.g. lysozyme Mucosal lymphocytes Secretory IgA Special antigen sampling o Waldeyer’s ring o Peyer’s patches o Dendritic cell networks Defences against viruses Surface defences Interferons Inflammatory mediators and acute phase proteins/opsonins etc. NK cells Antibody, complement, ADCC T cells Flu Pathogenesis Factors that affect severity of infection RNA sequence Viral load Environment DNA of host Viral Strategies block IFN induction decoy IFN receptors perturbation of IFN signaling downregulate ISGs How infection causes disease - Normal response Good immune response Appropriate regulation Pathogen defeated Immune defect Poor immune response Poor control of infection High pathogen load Poor T regulatory cells Defective regulation 44 MCD Immunology - Alexandra Burke-Smith Normal viral load Uncontrolled immune response Sustained, enhanced response LECTURER’S NOTES Why do we have an immune system? It can be argued that the immune system has developed to provide us with a survival advantage against infection, and that all other functions are a by-product. Our internal and external surfaces are bathed in microbes. We inhale potentially lethal microbes with every breath that we take, and our cells are outnumbered by our bacteria by 10:1, which form about 3% of our body mass. The essential challenge of the immune system is to remain indifferent to non-pathogenic microbes, while responding rapidly and appropriately to the constant microbial onslaught. The physical and chemical barriers: innate defence A vital barrier to the entry of pathogens is the so-called ‘wall of death’, made up of surface layers of skin that are dead or dying and constantly being shed. Unless the skin is broken by trauma or biting insects, it is very unlikely that infection can gain access except through the lung or gut. The lung and gut are organs specialised to provide a large area of contact with the environment, necessary for gas exchange and absorption of food and water. The mucosal surfaces turn over at a very fast rate, with all the superficial cells being sloughed within a few hours or days. Any microbe that attaches to these cells is soon lost along with the dead and dying cells. The mucocilliary system in the lungs clears microbes from the lung; Cystic fibrosis patients cannot form mucus normally and suffer from recurrent respiratory infections. Mechanical defence should not be underestimated. There are also chemicals (fatty acids, enzymes etc.) that bathe the skin and other body surfaces: Lysozyme in our tears digests bacterial cell walls and the acid in our stomachs kill many of the microbes we ingest. The normal flora in our gut prevents other bacteria from gaining a foothold, so affecting susceptibility to gut infections following antibiotic treatment. Detection of Pathogens by the Innate Immune System The innate immune system is our first line of defence against infection. Its components are generally innate, i.e. preformed, and rapidly react to pathogen invasion. Classically, the innate system does not ‘adapt’ and therefore shows no memory response. Recognition is based on the sensing of common molecular patterns on the surface of pathogens, a signal that is contingent on whether or not that particular foreign component is normally present at the site concerned. Therefore, a molecular pattern may be sensed at the surface and lead to no response, whereas the same molecular pattern sensed in the cytosol may induce a vigorous reaction. The toll-like receptors (TLR) are an excellent example of this pathogen sensing system. 204 The complement system is a pre-formed protein cascade which can rapidly punch holes in the outer membrane of microbes, coat them for phagocytosis (‘opsonisation’) and produces chemoattractants which recruit cellular components of the immune system. Chemical signals: interferons, chemokines and cytokines The production of interferons is also crucial to host defence. Interferons are soluble low molecular weight mediators released by cells in response to infection, that act both on the cell that releases them (autocrine action) and on other neighbouring cells (paracrine) to induce an antiviral state and increase defence. The type 1 interferons activate natural killer (NK) cells and increase the expression of molecules involved in processing and presenting viral proteins 45 MCD Immunology Alexandra Burke-Smith on the cell surface. The importance of the interferon system to viruses is shown by the very large number of viruses that have evolved mechanisms to block the synthesis and actions of interferons. Low molecular weight mediators are also very important in recruiting other cells to the site of information. Cells that circulate in the blood and lymph migrate out into the tissues in response to infection, particular combinations of mediators attracting particular cells (by secretion of chemoattractants called ‘chemokines’). Neutrophils, for example, are attracted by a chemokine called interleukin 8 (IL-8). The eosinophils, on the other hand respond to eotaxin or RANTES. Cytokines are chemical signals used for communication by the immune system. They may have local and systemic effects and direct the extent and the nature of the immune response. For example, Interferon-gamma can be produced by T cells to enhance activation of macrophages. The cytokine TNF-alpha has many systemic effects associated with infection, including fever and weight loss. Innate cellular defences Once in the tissues, the inflammatory cells produce additional chemoattractive or activating mediators, and may themselves be phagocytic (e.g. they take up particles that are degraded by vesicles within the cells). Macrophages are important phagocytes which may be tissue resident or be recruited during infection. Neutrophils, which make up the majority of circulating leukocytes are rapidly recruited to sites of infection. Phagocytes can use their surface receptors to directly recognise the outer surface of microbes or to recognise other components of the immune system, including complement and antibody, that have coated or opsonised the microbe surface. The phagocyte’s defence mechanisms include toxic enzymes, reactive radicals and defensins that are produced in the phagosome once the pathogen has been taken up. Natural killer (NK) cells are regulated by a combination of inhibitory and stimulatory receptors. Surface receptors on NK cells that recognise a normal cell (e.g. one displaying class I major histocompatibility complexes or ‘MHC I’) stops the NK cell from becoming active. Lack of MHC on the surface of the cell may indicate that a virus is trying to hide in the cell. On the other hand, a stimulatory receptor may be triggered by recognition of cell surface proteins on other cells that signify an abnormal state of infection or transformation, leading to NK activity. NK cells form a bridge between the innate and the acquired immune system. They kill abnormal or infected cells; if they are defective (e.g. in rare inherited deficiency states), common virus infections tend to be severe and can be fatal. They are an important source of some of the mediators produced by classic T-cells (see below). By producing different combinations of T-cell cytokines, they can help to shape the adaptive immune response. T and B cells (the acquired immune system) Acquired immune responses are highly specific to each antigen and result in memory of that antigen. The acquired immune system can be divided into T-cells and B-cells. Both of these originate from the bone marrow and circulate in the blood, but T-cells need to pass through the thymus to mature. T and B cells recognise specific antigens using their surface receptors; each T or B cell will recognise only one antigen. When these cells mature cells with a huge diversity of receptors are generated by random reassortment of the genes encoding the receptors. Because this process is random, there is a risk that ‘autoreactive’ T and B cells are produced. These cells are usually eliminated or regulated but autoimmune disease can result, if these processes fail. B-cells have antibody on the surface as their receptor, and secrete soluble antibody that is able to bind to an almost infinite variety of protein or non-protein ‘antigens’. Each B-cell represents a clone, able to produce only one exact variety of antibody. The antibody can bind directly to the surface of pathogens so helping the pathogen to be engulfed by a phagocyte or punch holes in the membrane of the pathogen using the complement system. Alternatively, antibodies may ‘neutralise’ a pathogen, blocking its surface receptors and preventing it from attaching to or infecting host cells. 46 MCD Immunology Alexandra Burke-Smith T-cells are quite different. They do not recognise the molecular surface, shape and charge of antigen, but instead recognise sequences of peptide from digested antigens presented by antigen presenting cells. The T-cell receptor locks on to MHC surface proteins which are of two types. MHC I is present on all nucleated cells, under normal circumstances. There is a cleft on the external tip of this protein that holds a short peptide ‘signature’, representing a digest fragment of internally synthesised proteins. If this is a normal host protein, T-cells detect the presence but are selected not to respond strongly. If it is a novel sequence, the T-cells recognise it as foreign and respond strongly. On the other hand, the MHC II has an external cleft that bears a digestion fragment of protein that has been picked up from outside a professional antigen presenting cell. These ‘professionals’ include dendritic cells, macrophages and some B-cells. MHC II is not present on ordinary cells. T-cells with a helper function (those recognising peptide presented by MHC II) are often subdivided according to the soluble mediators that they produce. ‘Th1’ cells classically make interferon gamma and tumour necrosis factor (TNF). On the other hand ‘Th2’ cells make IL-5, IL-4, IL-9 and IL-13. These are mostly involved in allergic responses and lead to eosinophil recruitment. However, the situation is getting ever more complex; it has recently been shown that there are cells specialised to produce IL-17 (‘Th17’) and various types of regulatory T-cell that make combinations of inhibitory cytokines. Regulatory T cells can also dampen immune responses by depriving other cells of the immune system of vital factors (like IL-2) or by acting on dendritic cells to inhibit activation. Protection against specific microbes The key defences against bacteria are the intact surfaces of the body, antibody, complement, phagocytosis and the acute phase proteins (which are opsonins, and bind in a relatively non-specific way to bacteria). On the other hand, defences against viruses include the surface defences, interferons, inflammatory mediators, NK-cells, antibody and T-cells. We can exploit the ability of the immune system to remember previous exposure to specific antigens in developing protective vaccines. If the immune system encounters a foreign substance for the first time, it is required to recognise the material as foreign and then to expand the number of cells that recognise that specific antigen before it can mount a full T and B-cell response. However, on second encounter the response is very much more rapid and vigorous (more antibody is produced) and better (antibody is of higher affinity). Indeed, 206some vaccines lead to very long lasting antibody that may be enough alone to protect against infection. Since vaccination was popularised by Edward Jenner, many different vaccines have been introduced to protect against the majority of the severe life threatening infections that were so prevalent only a century ago. It is very rare to see cases of tetanus, diphtheria, typhus, anthrax or measles, except in people who have not received the benefit of vaccination. However, these medical marvels are only available to people living in well-resourced parts of the world, and about 3 million children die every year because they have not been given standard vaccines that would have otherwise have saved their lives. Perhaps good, stable political systems should be regarded as a crucial component of our defence against the microbial world. Major challenges in the future are to control the immune response when it causes disease (in allergy, autoimmunity, in toxic shock and transplantation, for example). We also need a better understanding of why the immune system fails to protect us against some infections (such as HIV) and in cancer, and design novel strategies for enhancing protective immune responses. 47