From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
Differential Effects of Interleukin-l5 (IL-15) and IL-2 on Human
Neutrophils: Modulation of Phagocytosis, Cytoskeleton Rearrangement,
Gene Expression, and Apoptosis by IL-15
By Denis Girard, Marie-Eve Paquet, Robert Paquin, and Andre D. Beaulieu
Human neutrophils have been shown recently to express
no significant effect of IL-2 was noted.Among the different
both the p and the y chains of the interleukin-2 receptor
proteins that were found to be upregulated by IL-15, one
(IL-2R). IL-15, a cytokine that has recently been cloned and
was identified by microsequencing as the cytoskeletal procharacterized, was found to share many of the biological
tein actin. Finally, we found that IL-15 delays apoptosis of
functions of IL-2 and is known to mediate signals through
neutrophils more efficiently than IL-2 when evaluated by
IL-2Rp and IL-2Ry. In recent studies, we observed that IL-2
both microscopic observations and flow cytometry proceexerts few effects on various neutrophil functions, but infordures. Furthermore, this phenomenon was dose-dependent
mation on IL-15-neutrophil interactions is lacking. In this
( I O to 500 nglmL), and, at 500ng/mL,IL-15
delayed
study, we observed that IL-15, in contrast to IL-2, induces
apoptosis as strongly as granulocyte-macrophage colonyimportant morphological cell shape changesthat are typical
stimulating factor. This study is the firstto show that IL-15
of activated neutrophils. Furthermore, phagocytosis of opso- is a significant neutrophil agonist. Moreover, in view of the
nized sheep red blood cells was significantly increased by
differential effects of IL-15 and IL-2 on
this cell type, our
IL-15 but not by IL-2. However, similar t o IL-2, IL-15 did not
results support the existence of a specific IL-15R compomodulate the oxidative burst response. Furthermore,we obnentls) on human neutrophils.
served that de novo RNA synthesis is increased in neutro0 1996 by The American Society of Hematology.
phils by IL-15along with de novo protein synthesis, whereas
I
NTERLEUKIN-15 (IL-15) is a cytokine that was recently
identified from culture supernatants of the monkey kidney epithelial cell line CV-IEBNA.'.' This cytokine is currently referred to as an IL-2-like cytokine because it was
found to share many biological activities with IL-2.'-' Furthermore, transfection studies showed that IL-15 uses both
the IL-2 receptor P chain (IL-2RP) and IL-2Ry ( y c )chain
of the IL-2R but does not use the a chain.'.' Recently, however, a novel IL-15-binding protein that is structurally related to the IL-2Ra chain was identified on IL-15-responP and y c chains were found to beneeded
sive cellsx Both the
for the transmission of L 1 5 signals. However, the newly
identified a chain was shown to be essential for mediating
high-affinity binding to the receptor.x
Recently, we9 and others'"." have shown that both IL-2RP
and IL-2Ry chains are constitutively expressed on human
neutrophils, whereas the IL-2Ra is undetectable. Therefore,
we decided to investigate IL- 15-neutrophil interactions because neutrophils play important roles in host defense. Furthermore, they were shown to respond to a number of cytokines. We studied a whole array of responses, ranging from
the more traditional roles of neutrophils in phagocytosis and
respiratory burst to the lesswell characterized functions such
as gene expression and protein synthesis in activated cells.
From the Arthritis and In$ammation Research Laboratory, Centre
de Recherche du Centre Hospitalier de L'UniversitC Laval, and the
Department of Medicine, Faculty of Medicine, Laval University. SteFoy, QuPbec, Canada.
Submitted January 25, 1996; accepted June 3, 1996.
Supported by the Medical Research Council (MRC) of Canada.
D.G. is recipient of an Arthritis Society/MRC post-doctoral award.
Address reprint requests to Andre' D. Beaulieu, MD, CHUL, Room
9800,2705 Boulevard Laurier, Ste-Foy (QuCbec), Canada G1V4G2.
The publication costs of this article were defrayed in part by page
charge payment. This article must therefore be hereby marked
"advertisement" in accordance with 18 U.S.C. section 1734 solely to
indicate this fact.
0 1996 by The American Society of Hematology.
0006-4971/96/8808-0010$3.00/0
3176
We also analyzed the effects of 1L-15 on apoptosis because
the regulation of apoptosis in neutrophils by proinflammatory stimuli is an area of study that has just recently been
the subject of investigation and could significantly influence
how weview the role of these cells in host defense responses.
Finally, we considered it important to fully characterize the
effects of IL- 15 on neutrophils because thiscytokine islikely
to be used as a therapy for cancer in humans.""' Furthermore, IL-15 wasfound to use the yL chain to mediate its
effects. It hasrecentlybeen
shown thatmutations in this
chain are associated with X-linked severe combined immunodeficiency.'4"7 Therefore, thediscovery of the presence
of this chain on human neutrophils makes the present study
relevant and of potential importance for future work on this
important clinical entity.
MATERIALS AND METHODS
Neutrophil isolation and incubation conditions. Cells were isolatedfromvenousbloodofhealthyvolunteers
by centrifugation
over Ficoll-Hypaque (Pharmacia Biotech Inc, Quebec, Canada), as
previously described.",""" All cell suspensions contained fewerthan
1 % monocytes as determined by monoesterase staining. Cell viability, as monitored by the ability to exclude Trypan blue, was greater
than 95% immediately after isolation as well as after 4 and 24 hours
of incubation in the presence or absence of agonists. If not specified,
for all experiments. neutrophils were resuspended in RPM1 supplemented with I % human autologous serum.
Agonisrs. Purified recombinanthuman L 1 5 (specific activity,
2.4 X lo5 UIpg)andrecombinanthuman
IL-4 wereprovided by
[L-2 (specific
Immunex Corp (Seattle, WA). Recombinant human
activity, 22 X IOh U/mg) was provided by Cetus Corp (Emeryville,
CA), whereas recombinant human granulocyte-macrophage colonystimulating factor (GM-CSF; specific activityof 9 X 10' U/mg) was
a gift from the Genetics Institute (Boston, MA).
All of the above
recombinant forms of cytokines will be hereafter referred to as IL15, IL-2, IL-4, or GM-CSF. Based on previous published results,
IL-2 was used at 45.5 nglmL, which is equivalent to 1,ooO U/mL,
the amount used to induce some neutrophil responses.'"' GM-CSF
was used at 65 nglmL, which is equivalent to 3 nmol/L, the concentration known to be optimal.q~'x~'O
Microscopic observations of human neutrophils. Cells ( 5 X IO"
cells/mI,) were incubated at 37°C in 5% CO2 in 96-well plates for
Blood, Vol 88, No 8 (October 15), 1996: pp 3176-3184
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
3177
IL-15 AND NEUTROPHILFUNCTIONS
up to 24 hours in the presence of buffer, GM-CSF (65 ng/mL), L15 (1 to 1,OOO ng/mL), or L - 2 (1-4500 ng/mL). Morphological
changes of cells were observed under light microscopy (magnification, X 200).and micrographs were taken after 3 hours (optimal
conditions) using Kodak Tmax 100 ASA film (Eastman-Kodak,
Rochester, NY) for black and white prints.
Phagocytosis of sheep erythrocytes. Sheep erythrocytes were
opsonized with a final U200dilution of rabbit IgG antisheep erythrocyte antibody (Sigma Chemical CO, St Louis, MO) by an incubation
of 45 minutes at 37°C as previously described.%Neutrophils (2.5 X
IO6 cells) pretreated for 15 minutes with buffer, L - 2 (45.5 ng/mL),
IL-15 (10 ng/mL), or IL-4 (10 ng/mL) were incubated with 50 X
lo6 opsonized sheep erythrocytes for 45 minutes, as above. The
samples were centrifuged 200g at 4°C for 10 minutes. Supernatants
were discarded, and an osmotic shock was performed with the pellets
by treating them with 500 pL HzO for 15 seconds followed immediately by the addition of 10 mL ice-cold Hanks' balanced salt solution
(HBSS). The samples were washed twice with ice-cold HBSS, and
the final pellets were suspended in 1 mL HBSS. Duplicate cytocentrifuge preparations were prepared with aliquots of ~ 2 0 pL
0 and were
processed for measuring apoptosis as described below. Phagocytic
activity was measured asthe percentage of neutrophils ingesting
opsonized sheep erythocyte~.'~
Superoxide production. The superoxide production was monitored by the (superoxide dismutase-sensitive) reduction of cytoBriefly, neutrophil suspensions
chrome c as previously rep~rted.'~.'~
(1 X IO6 celYmL) were incubated with 130 pmoVL cytochrome c
(Sigma) for 5 or 30 minutes at 37°C in the presence or absence of
IL-15 (10 or 500 ng/mL) or phorbol myristate acetate (PMA;
mol/L), the latter being used as a positive c o n t r ~ l . Reactions
~~.~~
were stopped by transfemng the tubes to an ice-cold bath. The
absorption of cytochrome c was monitored at 550 and 540 nm, and
the amount of superoxide anions produced was calculated from the
difference between the optical density at the two wavelengths, using
an extinction coefficient of 2 1.I .25.26
RNA synthesis assay. This assay was performed by measuring
the incorporation of [5-3H]-uridine(obtained from Amersham Corp
[Oakville, Ontario, Canada]) into total RNA essentially as previously
described'."?
100 pL of a 5 X IO6 cells/mL suspension was incubated in 96-well microtiter plates in the presence of 1 pCi of [3H]uridine along with agonists for 4 hours (previously shown to be
optimal) at 37°C in 5% CO,. After incubation, the cells were collected onto borosilicate glass fiber paper by a multiple-cell culture
harvester (Skatron Instruments Inc, Sterling, VA), and sections of
the filter corresponding to each microwell were then punched out
and placed in scintillation-counting vials in the presence of 4 mL
of Aquasol-2 (Dupont-NEN, Boston, MA). The results were expressed as stimulation indices representing the ratio of counts per
minute (cpm) obtained with stimulated over unstimulated neutrophils
from several different normal subjects, as indicated inthefigure
legends. All experiments were performed in triplicate.
Metabolic labeling of neutrophils, protein precipitation, and 2dimensional (2-D)gel electrophoresis. Metabolic labeling of neutrophils (200 pL of 50 X lo6 cells/mL) was performed with [35S]
methionine and [35S]cysteine (both used at 125 pCi/107 cells; Amersham), as previously
in the presence of protease inhibitors (60 trypsin-inhibiting U/mL aprotinin, 1 mmol/L phenylmethyl sulfonyl fluoride, 0.5 pg/mL leupeptin, and 200 pmol/L
EDTA). Protein precipitation was performed in Eppendorf tubes,
with a final concentration of 70% ethanol, and cells were treated for
1 hour at -20°C. After centrifugation, the pellet was solubilized
with the lysis buffer (9.5 m o m urea, 2% NP-40, and 5% 8-mercaptoethanol), and 10 pL of each corresponding fraction was loaded in
scintillation-counting vials with 4 mL of Aquasol-2 to determine the
amount of radiolabeled proteins loaded for the migration. High-
resolution 2-D gel electrophoresis was performed using intracellular
neutrophil proteins (from a final number of cells of 5 X lo6) according to the method of 0 ~ ~ using
~ the
1 Millipore
1 ~ Investigator
~
2D Electrophoresis System (Millipore Corp, Bedford, MA). Firstdimension isoelectric focusing was performed using 2% ampholite
(1:4 [voVvol]; pH range, 4 to 8 and 3 to 10). Gels for the second
dimension were 12% acrylamide. The gels were dried and exposed
for 3 to 5 days at -70°C.
Microsequencing. After 2-D gel electrophoresis, proteins were
transferred to polyvinylidene difluoride (PVDF) membranes (Millipore Corp). Two major spots were detected with Coomassie-blue
staining of membranes. They were excised and microsequenced with
an Applied Biosystems gas phase sequencer model 475A with online FTH analysis and data collection (Applied Biosystems, Foster
City, CA). All the chemicals and protocols used were those recommended by the manufacturer.
Assessment of neutrophil apoptosis by cytology and b y f i w cytometry. Cytocentrifuge preparations of neutrophils were performed as
previously described using a Cyto-tek centrifuge (Miles Scientific,
Naperville, FL)." Cells were incubated in the presence or absence
of L-15, L-2,or GM-CSF for 24 hours in RPMI-1640-10% autologous serum and were stained with Diff-Quick stain set (Baxter,
Miami, FL) according to the manufacturer's instructions. Cells were
examined by light microscopy (final magnification, X m),and
apoptotic neutrophils were defined as cells containing one or more
characteristic darkly stained pyknotic nuclei.'' An ocular containing
a 10 X 10 square grill was used to count at least five different fields
(>500 cells in total) for assessment of apoptotic cells. Results were
expressed as percentage of apoptotic cells. We also evaluated apoptotic neutrophils by flow cytometry according to differences in their
stainability with propidium iodide (PI) and Hoechst (HO), essentially
as previously described." Cells were incubated as above, and, after
the 24 hours of incubation, an aliquot of 350 pL of each cell suspension (representing -2.5 X IO6 cells, after 24 hours) waswashed
twice with neutral phosphate-buffered saline followed by an addition
of 100 pL of PI (from a 20-pg/mL solution) for 30 minutes on ice
that was light-protected. Cells were then treated with 950 pL of
25% ethanol and 50 pL of H033342 (from a Il2-pg/mL solution)
and were kept on ice for 12 hours before performing fluorescenceactivated cell sorting (l0,OOO events) analysis using an EPIC 753
(Coulter, Miami Lakes, FL).
RESULTS
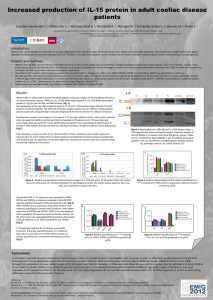
IL-15 induces neutrophil morphological changes. In
previous studies, we observed that GM-CSF, IL-13, and IL4, but not IL-2, induce morphological changes of human
neutrophils in vitro that are typical of activated cells.'' In
the present report, we investigated whether IL-15 can induce
such a response and compared it with that of IL-2 and GMCSF. Figure l shows that cells incubated with IL-2 (500ng/
mL; Fig 1B) remained with a round shape, much like that
of control cells (Fig lA), whereas the shape of cells incubated with E-15 (500ng/mL; Fig 1C) was altered and was
comparable with cells activated with GM-CSF (65 ng/mL;
Fig 1D). This assay was performed with 10 different donors,
and the results were highly reproducible. In previous studies,
we had used amounts as high as 4,500nglmL of IL-2 and
still had observed no effect (data not shown).
IL-15 induces phagocytosis. We next investigated the
effect of E-15 and IL-2 on inducing phagocytosis, knowing
that it was previously reported that IL-2 had no effect on
neutrophil phagocyt~sis.~'
IL-4 was usedas a positive control
because it was shown to increase neutrophil phagocyt~sis.~'
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
GIRARD ET AL
3178
l
I
l
1
I
,
.
.-
-
.._
-
-
As shown in Fig 2. phagocytosis was significantly increased
by IL-4 (n = 4) and IL-IS (n = 6). whereas thisincrease
was not observed with IL-2 (each cytokine was used at 10
ng/mL). The number of erythrocytes ingested per 100 neutrophils was also scored and less than S 8 of cells presented
two or more erythrocytes per cell whether or not they were
treated with cytokines. We then decided to verify potential
dose-response effectsof IL- I S ( 10 to S00 ng/mL) on phagocytosis. The results shown in Fig 3 show that IL-IS increases
phagocytosis in a dose-dependent fashion.
IL-I5 docs not mohrlote respiratot? burst. Superoxide
productionis a well knownclassicalfunction
of neutrophils,?5.'6.>?->Jand, because of the absence of information on
theeffect of IL-IS onthis function, we decided to study
both the direct effect of IL-IS on superoxide production as
well as its potential
role
in priming the response to
the chemoattractant formyl-methionyl-leucyl-phenylalanine
(FMLP) peptide. We used IL-IS at two different concentrations (10 and S00 ng/mL). We found that IL-IS, similar to
IL-2, does not modulatedirectly superoxide production, a
feature previouslyreported by
and others'' with other
cytokines of the IL family. After S minutes of stimulation,
we observedthat superoxide production (meanSEM
of
three different neutrophil donors) from cells incubated with
buffer. IL-15 (10 ng/mL). IL-2 (10 ng/mL), or PMA ( IO"
mol/L), was 0.6 2 0.06, 0.5 2 0.001, 0.7 t 0.003, 13.0 2
1.4 nmol/lOh cells, respectively. PMA was used here as a
positive
After
30 minutes of stimulation, we observed that superoxide production was less than 1 nmoVl0"
cells whether neutrophils were incubated in the presence or
Fig 1. IL-15inducesmorphological
cell
shape
changes.
Freshly
isolated
neutrophils
were incubated in96-well-plates
in the presenceor absence of agonistasdescribed in Materials
and Methods. (A), (B), (C), and
(D)show the morphology of control cells, IL-2-treated (500 ngl
mL), IL-15-treated (500 nglmL).
and GM-CSF-treated 165 nglmL)
cells, respectively, after 3 hours
of incubation. Results arefrom 1
donor and are representative of
10 different donors performedin
triplicate.
absence of IL-IS or IL-2 (data not shown). whereas we
observed that superoxide production from PMA-treated cells
was 42.6 2 S ( n = 3). The results obtained with S O 0 ng/mL
were similar to thoseobtained with 10 ng/mL (data not
shown).We then testedwhether IL-IS canprime the response to FMLP. In these experiments, cells were preincubated for 30 minutes with buffer, IL-IS (S00 ng/mL), IL-2
(S00 ng/mL), or GM-CSF (65 ng/mL: used as a positive
control)".'" followed by stimulation with FMLP (3 X 10""
mol/L) for S minutes. Whereas the superoxideproduction
was 8.9 2 2.0 nmol/106 cells when neutrophils were preincubated with GM-CSF. we observed that it was 1.9 t 0.39,
1.9 2 0.30. and 2.2 2 0.20 nmoVl0" cells for control, ILIS-. and IL-2-treated cells. respectively (n = 3).
IL-15 irlrreosc~sde trove RNA spntllcsi.~. To investigate
the effect of IL-IS on the initiation of
gene expression in
neutrophils, we measured [>H]-uridine uptake into total
RNA. We had previously shown that IL-2 ( 1 to 4.500 ng/mL)
does not significantly increase de novo RNA synthesis.".'x In
the present study, we used the same methodology and observed that. after 4 hours of incubation, IL-IS. unlike IL-2.
increases de novo RNA synthesis (Fig 4).The synthesis of
RNA induced by IL- I S was abolished (up to 9S% inhibition)
in the presence of the transcription inhibitor actinomycin D.
Inductiotr of de 1 1 0 1 ~proteirl synthesis by IL-IS. In view
of the above results, we next decided to study the ability of
1L-IS to induce de novo protein synthesis in neutrophils. To
do so, neutrophils were incubated with 1L-IS (10 ng/mL) or
IL-2 (45.5 ng/mL)for 20 hours in the presence of ["S]
cysteine and ["S] methionine. and cell lysates were analyzed
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
3179
IL-15 AND NEUTROPHILFUNCTIONS
40
1
*
(31
(31
T
ik
T
Ctrl
(31
*T
0
l
50
l00
500
L I L - l 5 added [ n g / m l l i
IL-4
Fig 3. The increase of phagocytosis by IL-15 is dose-dependent.
Phagocytosis was performedas described in the legend to Fig 2, but
cells weretreated with increasing concentrations of IL-15
110 to 500
nglmL). Results are mean ? SEM. The number in parenthesis indicates different neutrophil donors.IL-4(10 nglmLl wasused as a
positive control. *, P < .05 by Student's t-test.
Fig 2. IL-15 increases phagocytosis. Phagocytosis was evaluated
by ingestion of opsonized sheeperythrocytesas describedin Materials and Methods. Results are expressed
as the percentage of phagocytosis (cells that ingested at least 1erythrocytelno. of cells counted
x 100). IL-2 was used at 45.5 nglmL, and both IL-15 and IL-4 were
? SEM (n 2 4 different neutrophil
used at 10 nglmL. Results are mean
donors). Ctrl, control cells; *, P < .05 by Student's t-test.
by 2-D gel electrophoresis and fluorography. Before loading
the gels, the radiolabeled proteins were precipitated, and
total counts (cpm X 1,000) from an equal number of cells
were measured in a scintillation counter. The counts were
415.6 ? 14.7,435.0 ? 15.7, and 499.0 +- 25.0 cpm for
control cells, IL-2-, and IL-15-treated cells, respectively
(n = 3). This result shows clearly that IL-15 significantly
(P < .05, by Student's t-test) increases de novo synthesis
of proteins more efficiently than IL-2. The fluorographs
shown in Fig 5 are representative results obtained from five
different donors and were performed with an equal number
of cells (final number, 5 X lo6 cells). Clearly, this cytokine
was capable of strongly inducing the synthesis of at least
five different proteins or sets of proteins (identified by boxes)
and, to a lesser degree, six other proteins (identified by numbers). No significant effect of IL-2 was observed because
gels were comparable with those of control cells (Fig 5A).
However, the synthesis of other proteins appeared to be inhibited by IL-15, and these are identified by parenthesis (Fig
5).
Identijkation of actin as an [L-15-inducedprotein. Coomassie-blue staining of the gels showed a major spot at the
42-kD molecular weight marker and a PI level of 5.4 (spot
3.0
1
IL-15
+l0
Act D
ng/ml "-I-l
*
500 ng/ml "
I
- - ++"++
Fig4. Induction of de novo RNA synthesis by IL-15. Neutrophils
(5 x 10B/mL) were incubated
for 4 hours in the presence of either IL15 or IL-2 (both at 10 and 500 nglmL1. Total RNA was measured
by [5-3Hl-uridineincorporation. Results are expressed asstimulation
indices (cpm from stimulated over unstimulated cells) and are mean
k SEM obtained from three different blood donors performedin triplicate. Cells weretreated (+l or were not treated (-1 with 5 pglmL of
the transcription inhibitor actinomycin D (Act Dl. *, P < .05 by Student's t-test.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
GIRARD ET AL
3180
B
A
C
l
I
Fig 5. De novo synthesis of actin and other proteins are increased by 11-15. 2-D gel electrophoresis was performed from [mSl-labeled
neutrophils incubated with buffer (A), IL-2 (B; 45.5 ng/mL), or 11-15 (C; 10 ng/mL) as described in Materials and Methods. The synthesis of
several proteins was increased by IL-15 (see boxes), whereas the synthesis ofothers appeared to be downregulated by IL-15(see parenthesis).
Spot no. 2 was microsequenced and identified as actin. Results are paired fluorographs obtained from one donorrepresentativeof five different
donors. Left arrowheads are the 43-kD and 14-kD molecular weight standards.
no.2). This proteinwasprocessed
for microsequencing.
After performing 29 cycles in the sequencer with this protein,
we observed a perfect match (100% homology) withthe
humanprotein actin. According to the l-letter amino acid
code, the sequence was as follows: M-V-G-M-G-Q-K-D-SY-V-G-D-E-A-Q-S-K-R-G-I-L-T-L-K-Y-P-LE.
This sequence corresponds to amino acids 44 + 72 of the human
actinproteinandis
common to both nonmuscle 0 and y
isoforms.”.”’ The synthesis of actin in control cells was comparable with that of IL-2 (see arrow in Fig SA and B).
IL-15 delays apoptosis moreefficientlythan IL-2. IL-2
has been described as a cytokine that can prevent apoptosis
of human neutrophils when used at 1
U/mL (which corresponds in this study to 45.5 n ~ / m L ) Therefore,
.~~
we decided to study the effect of IL-IS on apoptosis. Figure 6
shows that IL- IS. in contrast to 1L-2, significantly decreases
the number of apoptotic cells (35.3% 2 5.1% [ P < .OS] U
45.5% 2 9.2% [ P = 33533, respectively) when compared
with that for control cells (57.8% t 7.3%). Because it had
previously been shown that GM-CSF strongly delays neutrophil apoptosis,’x we usedit in our study as a positive control.
After 24 hours of incubation, we observed up to 75% inhibition of apoptosis with GM-CSF when compared with that
for control cells. We further investigated the effect of IL-15
and IL-2 on neutrophil apoptosis by performing assays with
increasing concentrations of both IL-2 and IL-15 (from 10
to SO0 ng/mL). We observed that IL-IS delays apoptosis in
a concentration-dependent fashion, and, when using 500 ng/
mL of IL- I S, neutrophil apoptosis was delayed as strongly
as it was withGM-CSF, whereas no such effect was obtained
with IL-2 (Fig 7).
We next evaluated apoptosis by flow cytometry to confirm
our microscopic observations. Previous experiments (performed to verify theefficacity of this technique in our hands)
indicated that, after 24 hours of incubation, 43.8% 2 3.9%
of control cells were in apoptosis versus 17.4% -t 2.3% for
O.OO
GM-CSF-treated cells (n = S; P = ,0004; data not shown).
The results obtained by the flow cytometry procedure from
two donors (Fig 8A) show that IL-15 (SO0 ng/mL), but not
1L-2, delays neutrophil apoptosis as strongly as GM-CSF.
Figure 8B shows the corresponding results obtained by the
cytospin preparations from the same two donors, which indicate that the microscopic observation of one or more characteristic darkly stained pyknotic nuclei observed in apoptotic
neutrophils correlates well with the decrease in DNA staining by H0 reagent.
DISCUSSION
It is generally accepted that neutrophils play roles in host
responses that extend well beyond their capacities to function
as phagocytes and cell-releasing cytotoxic compounds. In
particular, they are considered to be capable of synthesizing
de novo, host defense proteins such as cytokines that have
the potential to perform active functions in the afferent and
efferent limbs of the immune re~ponse.”.~”
It is also clear
that ILs and other proinflammatory stimuli are capable of
modulating a number of neutrophil responses, including
apoptosis. Before the present report, little information was
available on the effects of the recently discovered IL-IS
molecule on neutrophil functions. This area of study seemed
promising given the knowledge that this cytokine mediates
its effects through interactions with the IL-2RO and IL-2Ry.
Both of these components were recently shown to be present
on human neutrophils.’”’ It seemed also relevant to study
potential differences in effects between IL-2 and IL-IS, because both cytokines were recently shown to use different
a chains in addition to the IL-2RB and IL-2Ry components
in mice.’ The human IL-2Ra chain isknowntobe absent
on neutrophils7.” and seems to be ~ninducible.~
It remains
to be determined whether the humanequivalent to the murine
1L-1Sa chain is present on neutrophils.
In this study, our first observation was that IL-IS induces
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
3181
IL-15 ANDNEUTROPHILFUNCTIONS
Fig 6. IL-15 delays apoptosis more efficiently than
IL-2. Cells were incubatedin the presence or absence
of IL-2 (45.5 nglmL1. IL-15(10 nglmL), or GM-CSF (65
nglmL) for24 hours. GM-CSF was used as a positive
control because it has been shown t o delay neutrophil apopto~is.'~,'~
Apoptosis was evaluated by microscopic observation of cytocentrifuge preparations. Results are mean 2 SEM from fivedonors and
were performed in duplicate. (Inset) Characteristic
morphological observation of apoptotic
(a) or normal
(NI cells. *, P .05 by Student's t-test.
-=
-M 20
0
*T
Fig 7. The delay of neutrophilapoptosis by IL-l5 is
concentrationdependent. This experiment was performed
after 24 hours of incubation as in the legend t o Fig 6. Results are the mean? SEM from three
different donors, and performed in duplicate. Unlike 11-2, a doseresponse effect was obtained when using IL-15 (from 10 t o 500 n g l
mL). For simplicity, only the results obtainedwith 500 nglmL of IL2 are shown. GM-CSF was also used herein as a control. Ctrl, control
cells; *, P < .05 by Student's t-test.
cell shape changesin neutrophils that are typical of activated
cells. whereas IL-2 exerts no such effects even when used
in very high amounts. The cell shape changes induced by
IL-15 were observed when using as little as 10 ng/mL and
were highly comparable with those observed with GM-CSF
at higher concentrations ( I 00 to S00 ng/mL). At this early
stage of our studies, this tinding appearedto be a clear indication that IL- IS can interact with neutrophils and is capable of
initiating the cellular events that are needed for cytoskeleton
rearrangement.
We next investigated the effects of this cytokine on the
inductionofphagocytosis.
Similar to others, we had previously observed that IL-2 had no effect on the phagocytic
activityof neutrophils'" but had confirmedthepreviously
reported observation that IL-4 is a modulator of phagocytos k 3 'Using IL-2 and IL-4 as negative and positive controls,
respectively, we were able to show clearly that IL-IS must
now be recognized as an IL capable of increasing phagocytic
activity in neutrophils. Others had previouslyshown that
neutrophil chemotaxis is not induced by IL-IS, and we did
not repeat these studies. However, we extended our study to
examine thepotential effects of IL-IS ontherespiratory
burst response and found no effect.At this point, we were led
to conclude that, although IL-15 appears to be a significant
neutrophil agonist in vitro, its effects areselective, dissimilar
to those of molecules such as GM-CSF and FLMP (which
induce a wider range of responses in neutrophils) but more
similar to those of IL-4 and IL-13 (Girard et al'x,").
The results of previous studies from our laboratory had
also shown that. although neutrophils are terminally differentiated cells, they are still capable of undergoing active gene
transcription and de novo protein synthesis.'~'x~'"~4'
Furthermore, we reported that these activities may be modulated by
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
3182
GIRARD ET AL
A
0Ctrl
60
.-
1
v)
v)
0
c
8 40
P
Q
.-c
B
GM-CSF
mIL-15
IL-2
1
0
0
r
20
0
Fig 6. Evaluationof neutrophil apoptosis by flow
cytometry: correlation with cytospinpreparations.
After 24 hours of incubation in the presence or absence of GM-CSF ( 6 5 ng/mL), 11-15 ( 5 0 0 ng/mL), or
IL-2 (500 ng/mL), an aliquot of 2 to 2.5 x 10' cells
was used forflow cytometric procedures. Cellswere
stainedwith PI and H 0 reagent as described in Materials and Methods, and the percentage of cells in
apoptosis was measured by differencesin their
stainability with PI and HO, essentially as previously
described.29Cvtocentrifuae Dramrations were mepared as in Materials and Methods withthe remaining cells from the same corresponding donor.
(A) The results obtained using flow cytometry are
shown. (B) The correspondingresults obtained with
the other methodology are shown. This experiment
was performed with two different donors.
- . .
n
0
Ex; # l
E x p #2
F l o w cytometry)
Exp #l
Exp ry2
(MicroscoPic observations)
a selective group of proinflammatory molecules.*' However,
at that time, IL-15 was not available for study. Therefore,
we were interested in investigating the effects of IL-15 on
both de novo RNA and protein synthesis, knowing that IL2 hadno significant effect. However, we also knew that
another receptor-related cytokine, IL-4, was capable of modulating these activities? Both de novo RNAand protein
synthesis were found to be initiated by IL-15. Induction of
RNA synthesis was inhibited by actinomycin D, showing
that the assay we used reflected transcriptional effects of IL15, and total count measurements as well as 2-D gel analysis
of cell lysates showed significant induction of protein synthesis by IL-15. This was not the case with IL-2 because results
of 2-D gel analysis were comparable with those obtained
with control cells (Fig 5). Although five proteins or sets of
proteins (identified by boxes) were markedly induced by IL15, successful identification of the nature of these proteins
was not made because all were found to be blocked at the
N-terminal and, therefore, were unfit for microsequencing.
Experiments are ongoing to prepare protease-digested fragments of these proteins, a procedure that should circumvent
this difficulty. However, one protein, which wasnot N-terminally blocked, (spot no. 2) and the synthesis of which was
induced by IL-15, was identifiedas actin. Actin isa cytoskeleta1 protein known to play a major role in the basic functions
in cell motility, phagocytosis, cell permeability, movement
of organelles, anchorage of many surface receptors, signal
transduction, cell-cell interactions, and mRNA localization.35.36Recently, we have been able to showa close correlation between induction of actin synthesis and cytoskeleton
rearrangement by IL-13,'* IL-4,and a selective group of
cytokines (D. Girard, unpublished observations).
Finally, when studying the potential modulatory effects
of IL-15 on neutrophil apoptosis, we found that IL-2 has
little effect in delaying apoptosis, whereas IL-15has a
marked effect. In a single recent report, 1L-2 was shown
to significantly prevent human neutrophil apoptosis." This
discrepancy of results with IL-2 may be explained, in part,
by experimental culture conditions. We used fresh, daily
prepared human autologous serum (10%) instead of the commercially available human AB serum (5%). Furthermore, it
has recently been shown that the number of cells undergoing
apoptosis may vary according to the cell concentration
This may also explain our differences of results with
IL-2, because we cultured neutrophils at 10 X lo6 celis/mL
instead of 2 X lo6 cells/mL. It is of particular interest that,
when IL-15 was used at higher concentrations, it was found
to delay apoptosis as strongly as GM-CSF. Results were
confirmed using two different methods of measurement (Fig
8).
It is well accepted that a cytokine may exert biological
functions on various cell types and that different cytokines
can exert common biological functions on thesame cell type.
Such an observation is known as the concept of pleiotropy
and redundancy of ~ y t o k i n e sand
~ ~ ,may
~ be explained by
the recent finding of the presence of a common receptor
component such as y c , which is shared by receptors for IL2, IL-4, IL-7, IL-9, and, more recently, IL-15.'0.".'4.'5.'7.43-47
Before our present study, many investigators were unable to
clearly identify any major differences of biological activity
between IL-2 and IL-15 on various cell type^.^.^ Although
we cannot deny the concept of pleiotropy and redundancy
of cytokines, in this report, using neutrophils as a target
for IL-15, we clearly showed that IL-15 can exert distinct
biological actions when compared with those of IL-2. This
indicates that biological activities of IL-15 are not totally
redundant to those of IL-2.
In light of the results of the present study, which is the
first in-depth analysis on the effects of IL-15 onhuman
neutrophils, we propose that IL-15 is likely to be a significant
neutrophil agonist in vivo and that close monitoring of neutrophil functions will need to be made when using IL-15 as
a therapeutic agent in humans.
REFERENCES
1. Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan
S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M,
Johnson L, Alderson MR. Watson JD, Anderson DM, Giri JG: Clon-
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
IL-15 AND NEUTROPHIL FUNCTIONS
ing of a T cell growth factor that interacts with the p chain of the
interleukin-2 receptor. Science 264:965, 1994
2. Bamford RN, Grant A J , Burton JD, Peters C, Kurys G, Goldman CK, Brennan J, Roessler E, Waldmann TA: The interleukin ( L )
2 receptor p chain is shared by L - 2 and a cytokine, provisionally
designated IL-T, that stimulates T-cell proliferation and the induction
of lymphokine-activated killer cells. Proc Natl Acad Sci USA
91:4940, 1994
3. Gin JG, Anderson DM, Kumaki S, Park LS, Grabstein KH,
Cosman D: IL-15, a novel T cell growth factor that shares activities
and receptor components with IL-2. J Leukoc Biol 57:763, 1995
4. Carson WE, Gin JG, Lindemann MJ, Linett ML, Ahdieh M,
Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA:
Interleukin (IL) 15 is a novel cytokine that activates human natural
killer cells via components of the IL-2 receptor. J Exp Med 180:1395,
1994
5. Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein
KH: IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol 154:483, 1995
6. Seder R A , Grabstein KH, Berzofsky JA, McDyer JF: Cytokine
interactions in human immunodeficiency virus-infected individuals:
Roles of interleukin (1L)-2, IL-12, and IL-15. J Exp Med 182:1067,
1995
7. Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K,
Kumaki S, Namen A, Park LS, Cosman D, Anderson D: Utilization
of the p and y chains of the IL-2 receptor by the novel cytokine
IL-15. EMBO J 13~2822,1994
8. Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM: Identification and cloning of a novel IL-15 binding protein that is structurally
related to the a chain of the IL-2 receptor. EMBO J 14:3654, 1995
9. Girard D, Gosselin J, Heitz D, Paquin R, Beaulieu AD: Effects
of interleukin-2 on gene expression in human neutrophils. Blood
86:1170, 1995
10. Liu JH, Wei S, Ussery D, Epling-Burnette PK, Leonard WJ,
Djeu JY: Expression of interleukin-2 receptor y chain on human
neutrophils. Blood 84:3870, 1994
11. Nakarai T, Roberston MJ, Streuli M,Wu Z , Ciardelli TL,
Smith KA, Ritz J: Interleukin-2 receptor y chain expression on
resting activated lymphoid cells. J Exp Med 180:241, 1994
12. Munger W, Dejoy SQ, Jeyasseelan R, Torley LW, Grabstein
KH, Eisenmann J, Paxton R, Cox T, Wick MM, Kerwar SS: Studies
evaluating the antitumor activity and toxicity of interleukin-15, a
new T cell growth factor: Comparison with interleukin-2. Cell Immunol 165:289, 1995
13. Lewko WM, Smith TL, Bowman DJ, Good RW, Oldham
RK: Interleukin-15 and the growth of tumor derived activated Tcells. Cancer Biother 10:13, 1995
14. Noguchi M, Huafang Y, Rosenblatt HM, Filipovitch AH,
Adelstein S, Modi WS, McBride OW, Leonard WJ: Interleukin2 receptor y chain mutation results in X-linked severe combined
immunodeficiency in humans. Cell 73:147, 1993
15. Puck JM, Deschenes SM, Porter JC, Dutra AS, Brown CJ,
Willard HF, Henthorn PS: The interleukin-2 receptor y chain maps
to Xq13.1 and is mutated in X-linked severe combined immunodeficiency, SCIDXl. Hum Mol Genet 2:1099, 1993
16. Kumaki S, Ochs HD, Timour M, Schooley K,AhdiehM,
Hill H, Sugamura K, Anderson D,Zhu Q, Cosman D, Giri JG:
Characterization of B-cell lines established from two X-linked severe
combined immunodeficiency patients: Interleukin-15 binds to the B
cells but is not internalized efficiently. Blood 86:1428, 1995
17. Matthews DJ, Clark PA, Herbert J, Morgan G, Armitage RI,
Kinnon C, Minty A, Grabstein KH, Caput D, Ferrara P, Callard R:
Function of the interleukin-2 (IL-2) receptor y-chain in biologic
3183
responses of X-linked severe combined immunodeficient B cells to
IL-2, IL-4, IL-13, and IL-15. Blood 85:38, 1995
18. Girard D, Paquin R, Naccache PH, Beaulieu AD: Effects of
interleukin-l3 on human neutrophil functions. J Leukoc Biol59:412,
1996
19. McColl SR, Paquin R, MCnard C, Beaulieu AD: Human neutrophils produce high levels of the interleukin 1 receptor antagonist in
response to granulocyte/macrophage colony-stimulating factor and
tumor necrosis factor a. J Exp Med 176:593, 1992
20. Beaulieu AD, Paquin R, Rathanaswami P, McColl S R Nuclear signaling in human neutrophils: Stimulation of RNA synthesis
is a response to a limited number of proinflammatory agonists. J
Biol Chem 267:426, 1992
21. Djeu JY, Liu JL, Wei S, Rui H, Pearson CA, Leonard WJ,
Blanchard DK: Function associated with L-2R-p on human neutrophils: Mechanism of activation of antifungal activity against Candida
albicans by IL-2. J Immunol 150:960, 1993
22. Wei S, Blanchard DK, Liu JH, Leonard WJ, Djeu JY: Activation of tumor necrosis factor-a production from human neutrophils
by IL-2 via IL-2Rp. J Immunol 150:1979, 1993
23. Wei S, Liu JH, Blanchard DK, Djeu JY: Induction of IL-8
gene expression in humanpolymorphonuclear neutrophils by recombinant IL-2. J Immunol 52:3630, 1994
24. Borish L, Rocklin RE: Effects of leukocyte inhibitory factor
(LIF) on neutrophil phagocytosis and bactericidal activity. J Immuno1 138:1475,1987
25. Naccache PH, Themen S, Caon AC, Liao N, Gilbert C,
McColl SR: Chemoattractant-induced cytoplasmic pH changes and
cytoskeletal reorganization inhuman neutrophils. Relationship to
the stimulated calcium transients and oxidative burst J Immunol
142:2438, 1989
26. Gilbert C, Gaudry M, Naccache PH: Rapid priming of calcium mobilization and superoxide anion production in human neutrophils by substimulatory concentrations of phorbol esters: A novel
role for protein kinase C and tyrosine phosphorylation in the upmodulation of signal transduction. Cell Signal 4511, 1992
27. O’Farrell PH: High resolution two dimensional electrophoresis of proteins. J Biol Chem 250:4007, 1975
28.Lee A, Whyte KB, Haslett C: Inhibition of apoptosis and
prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol 54:283, 1993
29. Darzynkiewicz Z , Bruno S, Delbino G, Gorczyca W, Hotz
MA, Lassota P, Traganos F Features of apoptotic cells measured
byflow cytometry. Cytometry 13:795, 1992
30. Jablons D, Bolton E, Mertins S, Rubin M, Pizzo P, Rosenberg
SA, Lotze MT: IL-2-based immunotherapy alters circulating neutrophil Fc receptor expression and chemotaxis. J Immunol 144:3630,
1990
31. Boey H, Rosenbaum R, Castracane J, Borish L: Interleukin4 is a neutrophil activator. J Allergy Clin Immunol 83:978, 1989
32. Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE: Free
radicals and phagocytic cells. FASEB J 9:200, 1995
33. Clark RA: The human respiratory burst oxidase. J Infect Dis
161:1140, 1990
34. McPhail LC, Harvath L: Signal transduction in neutrophil
oxidative metabolism and chemotaxis, in Ahramson JS, Wheeler JG
(eds): The Natural Immune System: The Neutrophil. Oxford, UK,
Oxford, 1993, p 63
35. Miwa T, Manabe Y, Kurokawa K, Kamada S, Kanda N,
Bruns G, Ueyama H, Kanaga T: Structure, chromosome location,
and expression of the human smooth muscle (enteric type) y-actin
gene: Evolution of six human actin genes. Mol Cell Biol 11:3296,
1991
36. Bershadsky AD, Vasiliev JM: Cytoskeleton. New York, NY,
Plenum, 1988, p 298
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
3184
37. Pericle F, Liu JH, Diaz JI, Blanchard DK, Wei S, Forni G,
Djeu JY:Interleukin-2 prevention of apoptosis in humanneutrophils.
Eur J Immunol 24:440, 1994
38. Brach MA, de Vos S, Gruss HJ, Herrman F: Prolongation of
survival of human polymorphonuclear neutrophils by granulocytemacrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood 80:2920, 1992
39. Lloyd AR, Oppenheim JJ: Poly’s lament: The neglected role
of the polymorphonuclear neutrophil in the afferent limb of the
immune response. Immunol Today 13:169, 1992
40. Cassatella MA: The production of cytokines by polymorphonuclear neutrophils. Immunol Today 16:21, 1995
41. Girard D, Paquin R, Beaulieu AD: Transcriptional events in
interleukin-4 (IL-4)-treated human neutrophils. J Invest
Med
43:196A, 1995 (abstr)
42. Biffl WL, Moore EE, Moore FA, Barnett C Jr: Interleukin6 suppression of neutrophil apoptosis is neutrophil concentration
dependent. J Leukoc Biol 58:582, 1995
GIRARD ET AL
43. Lin J-X, Migone T-H, Tsang M, Friedmann M, Weatherbee
JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, Leonard WJ:
The role of shared receptor motifs and common Stat proteins in the
generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL7, IL-13, and IL-15. Immunity 2:331, 1995
44. Leonard WJ: The defective gene in X-linked severe combined
immunodeficiency encodes a shared interleukin receptor subunit:
Implications for cytokine pleiotropy and redundancy. Curr Opin l m munol 6:631, 1994
45. Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka
N, Munakata H, Nakamura M, Sugamura K: Cloning of the y chain
of the human IL-2 receptor. Science 257:379, 1992
46. Kondo M, Takeshita T, Ishii N, Nakamura M, Watanahe S,
Arai K-l, Sugamura K: Sharing of the interleukin-2 (IL-2) receptor
y chain between receptor for 1L-2 and IL-4. Science 262: 1874, 1993
47. Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M,
Cao X, Leonard WJ: Interleukin-2 receptor y chain: A functional
component of the interleukin-7 receptor. Science 262: 1877, I993
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
1996 88: 3176-3184
Differential effects of interleukin-15 (IL-15) and IL-2 on human
neutrophils: modulation of phagocytosis, cytoskeleton
rearrangement, gene expression, and apoptosis by IL-15
D Girard, ME Paquet, R Paquin and AD Beaulieu
Updated information and services can be found at:
http://www.bloodjournal.org/content/88/8/3176.full.html
Articles on similar topics can be found in the following Blood collections
Information about reproducing this article in parts or in its entirety may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests
Information about ordering reprints may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#reprints
Information about subscriptions and ASH membership may be found online at:
http://www.bloodjournal.org/site/subscriptions/index.xhtml
Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American
Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036.
Copyright 2011 by The American Society of Hematology; all rights reserved.